Abstract
Aims
We quantified the impact of type 2 diabetes on incidence of non-fatal cardiovascular disease (CVD) and mortality, considering CVD as a continuum from occurrence of diabetes to the end point, and estimated the 15-year life expectancy with and without CVD.
Methods
A total of 7239 Iranian adults (3246 men), aged ≥ 30 years, were followed from 1999 to 2014. We applied a multi-state semi-Markov model with three transitions including CVD-free to non-fatal CVD, CVD-free to death and non-fatal CVD to death, and studied the influence of diabetes on each transition rate, stratifying by sex and adjusting for confounders.
Results
Diabetes was significantly associated with increased risk of non-fatal CVD in men [hazard ratio, 1.70 (1.36–3.53)] and women [2.19 (1.74–2.77)], and of all-cause death [2.72 (2.03–3.63) and 1.92 (1.37–2.67) in men and women, respectively]. An increased risk of mortality was found only among diabetic men, when non-fatal CVD was occurred [2.19 (1.36–3.53)]. Men with diabetes experienced first non-fatal CVD and death without CVD 1.7 and 1.4 years, respectively, earlier than those without diabetes; the corresponding values were 1.4 and 0.7 years for women. Moreover, diabetic men lived 1.3 years less than non-diabetic counterparts when non-fatal CVD was occurred.
Conclusions
Diabetes increased the risk of non-fatal CVD events and all-cause mortality and consequently decreased the number of years lived without CVD. A decrease in LE was found only among diabetic men compared to non-diabetics after non-fatal CVD occurred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a major and fast-growing health problem worldwide [1,2,3], increasing the risk of early mortality and both macrovascular and microvascular complications [4]. Current studies indicate that long-term glycemic control is an important predictor not only of microvascular disease, but also macrovascular complications [5]. Excess mortality in diabetes is multi-factorial, but the major cause of death remains cardiovascular disease (CVD) [6, 7]. Among patients with diabetes, the impact of CVD on mortality has previously been evaluated after acute myocardial infarction (MI) [8,9,10]. On the other hand, increased risk of all-cause death among diabetic patients has been evaluated without considering CVD as a continuum from occurrence of diabetes to the end point [1, 5, 11,12,13]; these studies considered the transition from the initial state to a one primary endpoint. However, more than one endpoint may exist in the path between the initial and final point. In fact, separate analyses are usually conducted for each of the endpoints which are not completely satisfying, since they fail to demonstrate the relations between different types of events.
Recently, multi-state methods have been developed in which a number of states are defined and the focus is on the process of going from one state to another [14, 15]. Multi-state models have widely been studied and clinical applications becoming more common in medical research [16, 17], because they can provide a detailed insight into effects of exposures on each state. Additionally, during the course of the disease, predictions for outcome can be adjusted when additional information, e.g., the occurrence of intermediate events is available [18].
In the present study, we used multi-state model to (1) ascertain how diabetes were associated with all-cause mortality with and without CVD, (2) estimate the 15-year life expectancy (LE) of a large population in different states and to compare it among the populations with and without diabetes, and (3) estimate the number of years of life lost due to diabetes with and without CVD.
Methods
Data sources and population
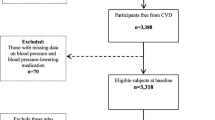
We used data from the Tehran lipid and glucose study (TLGS), a large prospective cohort of Iranian population. The study has been described in detail elsewhere [19]. For the present analysis, we selected all subjects aged ≥ 30 yr from the first (1999–2001) and second (2002–2005) phases as baseline population (n = 9553) and excluded 602 participants with prevalent CVD at baseline, 385 individuals without information on diabetes status, and 747 individuals without any follow-up data until end of the study (March 20, 2014). We also, excluded 580 individuals with missing data on potential confounders. Thus, the final sample included 7239 participants (3246 men and 3993 women) (Online Resource 1). This study was approved by the ethics committee of the Research Institute for Endocrine Sciences of the Shahid Beheshti University of Medical Sciences according to the principles of the Declaration of Helsinki. All participants were informed of the study protocol, and their written informed consents were obtained before inclusion.
Data collection
At baseline, information on age, sex, education, history of CVD, medication use, smoking habits and anthropometric measures including weight and height was obtained [19]. BMI was calculated as weight in kilograms divided by the square of height in meters. Blood pressure was measured twice, and the mean of two consecutive measurements was documented. Fasting plasma glucose (FPG) and serum total cholesterol (TC) were measured using standard laboratory techniques [19]. In first phase, physical activity level (PAL) was assessed by the lipid research clinic questionnaire [19]. It was replaced by the Modifiable Activity Questionnaire from the second phase, for obtaining the quantitative measure of PAL [20].
Definition of diabetes
At baseline, diabetes was defined as FPG ≥ 7 mmol/L or 2 h post-challenge plasma glucose (2 h-PCPG) ≥ 11.1 mmol/L or taking anti-diabetic medications.
Definition of confounders
We considered multiple potential confounders at the design stage of study and based on both our previous studies [13, 21] and other literature available [1, 22]; these included: age, TC, systolic BP (SBP) and BMI as continuous variables, and the others as categorical, including sex, education (< 9, 9–11 and ≥ 12 years of education), smoking status (current, former, or never) and family history of CVD (FH-CVD). Active participants were identified as those who participated in a vigorous physical activity at least 3 days per week or achieving a minimum of at least 600 MET (metabolic equivalent task)-minutes per week [23].
Assessment of outcomes
Details of the collection of outcomes in TLGS have been published elsewhere [19]. In brief, participants were followed annually for any medical conditions from the date of the baseline examination until the date of first documented outcome; data collected were then evaluated by an outcome committee to code the events according to the International Classification of Diseases, 10th edition. The main outcome measures were incident non-fatal CVD and all-cause mortality. Non-fatal CVD was defined as definite MI, probable MI, unstable angina pectoris, angiographic proven CHD, heart failure and stroke. All-cause mortality was determined as death from all causes including CVD and non-CVD death. Details are presented in Online Resource 2.
Statistical analysis
Continuous and categorical variables at baseline were compared using the independent sample t tests and χ2 test, respectively. To minimize type I error related to multiple comparisons, we considered the Bonferroni correction; accordingly, two-tailed P values < 0.006 were considered statistically significant. Incidence density rate of events and respective 95% confidence intervals (CI) were calculated by dividing the number of events by the person-years at risk.
Multi-state model
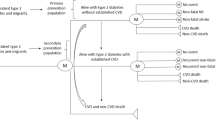
We defined a three-state model for our data analysis: CVD-free (state 1), non-fatal CVD (state 2) and all-cause death (state 3 or absorbing state); in Fig. 1, boxes represent the states and arrows symbolize the possible transitions. All individuals start free of CVD in state 1 at time t, and move to either a non-fatal CVD event in state 2 or death of any cause at state 3. Participants are not allowed to move back from state 2 to state 1, and only the first entry into a state is considered [15]. We used the time homogeneous semi-Markov model for our analysis [14, 15]. Cox’s proportional hazards model (Cox PH) was applied for estimation of each transition hazard. Analyses were performed separately in the total population, men and women. Model 1 was adjusted for age/sex, and Model 2 was adjusted for age/sex, smoking status, PAL, education level, FH-CVD, TC, SBP and BMI. Details are presented in Online Resource 2.
Mean survival time
To estimate the mean survival years in 15 years of follow-up in each transition, we used restricted mean survival time (RMST) which is defined as the area under the curve of the survival function up to a truncation time point (a pre-specified time horizon):
where S(t) is the survival function for the time t. Interpreting this quantity is straightforward; for example, if t is equal to 15 years, then, µ is interpreted as 15-year LE, i.e., “what is the LE for the next 15 years” [24, 25] (details are shown in Online Resource 2).
Results were presented in the following ways: (1) RMST for diabetic and non-diabetic individuals, (2) differences between diabetic patients’ RMST and the RMST of the non-diabetic population which is interpreted as the number of years of life lost due to the diabetes [24,25,26], (3) restricted mean time lost (RMTL) is defined as the average survival time lost during the follow-up time, and (4) the ratio of RMTL of diabetic and non-diabetic participants.
All analysis was performed in R (https://CRAN.R-project.org) and completed in 2017. We estimated unadjusted RMST and RMTL using survRM2 package. To obtain adjusted estimation for RMST, we used the pseudo-package [27]. The multi-state Markov model and the PH Cox model were fitted using the mstate [28] and survival packages, respectively. Two-tailed P values < 0.05 were considered statistically significant.
Results
Baseline characteristics and incidence of outcomes
The study sample included 3246 men and 3993 women, aged ≥ 30 yr with mean (SD) ages of 47.5 (12.9) and 45.9 (11.3) yr, respectively. Among both genders, diabetic individuals, had higher mean TC, SBP and BMI (P < 0.001), and were less educated compared with non-diabetic counterparts (P < 0.001) (Table 1). During the study period, 7239 individuals were at risk of transition from the state 1 to states 2 and 3. Of these, 812 (469 men and 343 women) experienced a transition to the state 2, and 398 (234 men and 164 women) to state 3. Of 812 individuals who experienced first non-fatal CVD, 124 (81 men and 43 women) died of all causes (Table 2). Overall 522 cases of all-cause mortality were documented, 398 cases from transition 2 and 124 from transition 3.
Incidence density rate of non-fatal CVD was 12.4 (95% CI: 11.3–13.6) and 6.9 (6.2–7.7) per 1000 persons years in men and women, respectively. Incidence density rate of mortality was 6.2 (5.4–7.0) and 3.3 (2.8–3.8) in transition 2, and 3.1 (2.5–3.9) and 2.4 (1.8–3.2) in transition 3 in men and women, respectively.
Diabetes and risk of CVD and mortality
Results of the multi-state model are summarized in Table 3. Among diabetic patients in the total population and in males, increased risk of developing non-fatal CVD, of mortality among those with non-fatal CVD, and of mortality among those free of CVD was observed compared to their non-diabetic counterparts (Table 3). However, diabetic women had increased risk of developing non-fatal CVD and of mortality among those free of CVD compared to their non-diabetic counterparts. Figure 2 illustrates the estimated cumulative hazards for the three transitions. In all transitions, the cumulative hazards were higher for diabetic participants. Online Resource 3 shows stacked probability transition curves for participants.
Fifteen-year RMST
In transition 1, from the initial state, non-diabetic men had an RMST of 13.9 years over the next 15 years for experiencing the non-fatal CVD, while RMST of men with diabetes was 12.2 years (Table 4). Therefore, non-fatal CVD-free LE was 1.7 years lower for diabetic compared to non-diabetic men on average (P < 0.001). Diabetic women had 1.4 years lower non-fatal CVD-free LE compared to non-diabetic women (13.1 vs. 14.5) (P < 0.001). In transition 2, for both men and women, diabetes was associated with fewer years lived (1.4 and 0.7 years for men and women, respectively). In transition 3, differences in number of years lived with CVD between non-diabetic and diabetic men were 1.3 years (P < 0.05), not however, significant for women.
Years of life lost
The average years of life lost (RMTL) of diabetic men and women without CVD (transition 2), were 3.9 and 4.1 times higher, respectively, compared to their non-diabetic counterparts (P < 0.001); however, the RMTL with non-fatal CVD (transition 3) was 1.8 times higher in diabetic compared to non-diabetic men (P < 0.05) (Table 4). The RMTL ratio of diabetic and non-diabetic women with non-fatal CVD was not significant. Online Resource 4 shows the results of adjusted RMST; diabetic individuals had the shorter RMST, namely 0.83, 0.83 and 1.07 years in transitions 1, 2 and 3, respectively, than their non-diabetic counterparts.
Discussion
In this study, we found that among the total population, having diabetes at age ≥ 30 years was associated with higher risk of developing non-fatal CVD and mortality with and without CVD. Individuals with diabetes in total population experienced first non-fatal CVD 1.5 years earlier than those without diabetes. Also, LE with and without CVD was decreased by about 11 months in diabetic compared to non-diabetic participants.
Although diabetes mellitus has been known as an established risk factor for CVD [29, 30], there is uncertainty concerning the extent of its associated with CVD [31, 32]. In 2009, a systematic review reported that predictive role of FPG for CHD was inconsistent even in good quality cohort studies [33]. Then, a meta-analysis of 102 prospective studies found that diabetes conferred about a twofold excess risk of CHD, major stroke subtypes, and deaths attributed to other vascular causes [32].
We found that diabetic men and women had 1.92 and 2.19 times higher risk of developing non-fatal CVD, respectively, compared to their non-diabetic counterparts.
Current evidence shows that heart diseases death rates among adults with diabetes are 2–4 times higher than the rates for those without diabetes [34]. Furthermore, in a collaborative meta-analysis from 97 prospective studies, diabetes was associated with a 80% higher risk of all-cause mortality [35]. In line with current evidence, we found that the risk of all-cause mortality was 2.72 and 1.92 times higher in CVD-free diabetic men and women, respectively, than for their non-diabetic counterparts.
A number of studies have also investigated the impact of diabetes on mortality in patients with CVD [9, 10]. A recently published study reported that among those with non-fatal MI at baseline, there was a 48% increased risk of death in those with diabetes compared to those without it [9]; however, we could not find any study to estimate the impact of diabetes on the CVD and mortality simultaneously in a multi-state Markov model. In 2006, Franco et al. [22] built a multi-state life tables to estimate the associations of diabetes with total LE and total life years with and without CVD at the age of 50 years using data from the Framingham Heart Study (the 1950s to the 1980s). They showed that diabetic men and women, compared with their non-diabetic counterparts, had more than double the risk of developing CVD and a 1.7 and 2.22 times higher risk of death, respectively, once CVD was present. A similar result was found in our study for men, although we found no significant difference in risk of mortality between diabetic and non-diabetic women once CVD had occurred. The contradictory results found in our study might be attributable to the relatively small numbers of events among women and consequently inadequate power to reach statistical significance. Lower occurrence of events observed among women in our study might be due to increased medication adherence among women living with heart disease, compared to men in our country. Non-adherence to cardiovascular medications is a global threat which is associated with adverse health outcomes [36]. According to a meta-analysis, approximately 50% of patients with CVD had poor adherence to their prescribed medications [37]. Moreover, the contradictory results are most likely due to the sex differences in the occurrence of various manifestations of CVD among diabetic individuals. Among individuals with diabetes, a range of 17–59% have been reported for prevalence of myocardial ischemia, and 46% for carotid arterial disease [38]. Current evidence also shows that women with diabetes have the highest mortality after MI [39]. Women in our study had a lower incidence of definite non-fatal MI (8.5 vs. 18.5%) than men; hence, sex differences in mortality risk after non-fatal CVD may be due to the lower incidence of MI among women, compared to men. Additionally, in the present study we included participants aged ≥ 30 years, whereas Franco et al. [22] investigated a population aged ≥ 50 years. It has been reported that MI imposes higher risk of coronary artery disease and worse outcomes only after menopause in diabetic women than men [40]. Further studies are needed to clarify the sex difference in risk of mortality after CVD events among individuals with diabetes.
We found that over 15 years follow-up, potential lifetimes were about 11 and 17 months less among CVD-free diabetic individuals in total population and males, respectively, compared to their non-diabetic participants. Gu et al. [41] previously showed that median LE was 8 years lower for diabetic individuals aged 55–64 years compared to their non-diabetic counterparts. Using cross-sectional data, Narayan et al. [42] estimated loss of 8 years in LE among 50-year-old non-Hispanic men with diabetes. However, the above studies did not assess the association of diabetes with LE. Using the multi-state life table, Franco et al. [22] showed that at age ≥ 50 years, women and men with diabetes were expected to live 8.4 and 7.8 years less, free of CVD, respectively. However, they found no significant difference between the years spent with CVD between diabetic and non-diabetic populations. Unlike their study, we found significant difference between LE of diabetic and non-diabetic men once a non-fatal CVD event had occurred. Some of the differences in conclusions regarding differences in LE with CVD are most likely due to the methodology we used to calculate LE, i.e., we estimated LE over 15 years of follow-up using RMST, whereas, Franco et al. [22] estimated total lifetime expectancy using life table analysis. Secondly, this difference could be due to date of study; Franco et al. [22] analyzed data from the 1950s to the 1980s, whereas we selected participants starting from 1999 and followed them until 2014, when there had been significant improvement in healthy life expectancy worldwide [43] compared to 1950–1980.
Interestingly, we did not find any significant differences in the years spent with CVD between diabetic and non-diabetic women, unlike men; the gender difference in LE with CVD is most likely due to the lower incidence of non-fatal MI among women compared to men, as previously mentioned. On the other hand, we included participants aged ≥ 30 years. Evidence shows that at younger ages, men have a higher risk of CHD than women [44]; this gender difference could also be explained by the higher LE of females than males in Iran. In 2010, global healthy life expectancy at birth was 59.0 and 63.2 years for Iranian males and females, respectively [43].
The strengths of the present study include use of data from a prospective cohort with a relatively long-term follow-up. We used a combination of glycemia tests (FPG and 2 h-PCPG) and pharmacological treatment of diabetes rather than self-reported diabetes status for diagnosis of diabetes. We estimated associations between diabetes and LE with and without CVD using the multi-state Markov model which is an ideal method for establishing life expectancies [45]. Our study has some limitations. First, as inherent to any prospective study, the level of some confounders such as smoking at the baseline examination might change during the follow-up. Second, we conducted our investigation among Persian ethnicities with relatively high incidence of diabetes and CVD events [3, 13]; therefore, results of our study are not expected to be similar across the populations with different incidence rates of CVD.
Conclusions
We found that diabetes was associated with higher risk of CVD and all-cause mortality which can be translated to a 1.5 years early onset of CVD among diabetic subjects. Furthermore, about 11 months decrease was shown in lifetime years spent with and without CVD among diabetic compared to non-diabetic patients. However, this decrease was influenced mainly by CVD events among diabetic men.
References
Marzona I, Avanzini F, Lucisano G et al (2017) Are all people with diabetes and cardiovascular risk factors or microvascular complications at very high risk? Findings from the Risk and Prevention Study. Acta Diabetol 54(2):123–131
Guariguata L, Whiting D, Hambleton I, Beagley J, Linnenkamp U, Shaw J (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2):137–149
Derakhshan A, Sardarinia M, Khalili D, Momenan AA, Azizi F, Hadaegh F (2014) Sex specific incidence rates of type 2 diabetes and its risk factors over 9 years of follow-up: Tehran Lipid and Glucose Study. PLoS ONE 9(7):e102563
Barutta F, Bruno G, Matullo G et al (2017) MicroRNA-126 and micro-/macrovascular complications of type 1 diabetes in the EURODIAB Prospective Complications Study. Acta Diabetol 54(2):133–139
Anjana RM, Shanthirani CS, Unnikrishnan R et al (2015) Regularity of follow-up, glycemic burden, and risk of microvascular complications in patients with type 2 diabetes: a 9-year follow-up study. Acta Diabetol 52(3):601–609
Tadic M, Cuspidi C, Vukomanovic V et al (2016) The influence of type 2 diabetes and arterial hypertension on right ventricular layer-specific mechanics. Acta Diabetol 53(5):791–797
Mozaffarian (2016) Heart Disease and Stroke Statistics-2016 Update: a Report From the American Heart Association (vol 133, pg e38, 2016). Circulation 133(15):E599–E599
Hudzik B, Szkodziński J, Hawranek M, Lekston A, Poloński L, Gąsior M (2016) CHA2DS2-VASc score is useful in predicting poor 12-month outcomes following myocardial infarction in diabetic patients without atrial fibrillation. Acta Diabetol 53(5):807–815
Patel PA, Cubbon RM, Sapsford RJ et al (2016) An evaluation of 20 year survival in patients with diabetes mellitus and acute myocardial infarction. Int J Cardiol 203:141–144
Rousan TA, Pappy RM, Chen AY, Roe MT, Saucedo JF (2014) Impact of diabetes mellitus on clinical characteristics, management, and in-hospital outcomes in patients with acute myocardial infarction (from the NCDR). Am J Cardiol 114(8):1136–1144
Tasci I, Basgoz BB, Saglam K (2016) Glycemic control and the risk of microvascular complications in people with diabetes mellitus. Acta Diabetol 53(1):129–130
Tancredi M, Rosengren A, Svensson A-M et al (2015) Excess mortality among persons with type 2 diabetes. N Engl J Med 373(18):1720–1732
Sardarinia M, Akbarpour S, Lotfaliany M et al (2016) Risk factors for incidence of cardiovascular diseases and all-cause mortality in a Middle Eastern Population over a decade follow-up: Tehran lipid and glucose study. PLoS ONE 11(12):e0167623
Andersen PK, Keiding N (2002) Multi-state models for event history analysis. Stat Methods Med Res 11(2):91–115
Putter H, Fiocco M, Geskus R (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26(11):2389–2430
Meier-Hirmer C, Schumacher M (2013) Multi-state model for studying an intermediate event using time-dependent covariates: application to breast cancer. BMC Med Res Methodol 13(1):80
Gillam MH, Ryan P, Salter A, Graves SE (2012) Multi-state models and arthroplasty histories after unilateral total hip arthroplasties: introducing the Summary Notation for Arthroplasty Histories. Acta Orthop 83(3):220–226
Eulenburg C, Mahner S, Woelber L, Wegscheider K (2015) A systematic model specification procedure for an illness-death model without recovery. PLoS ONE 10(4):e0123489
Azizi F, Ghanbarian A, Momenan AA et al (2009) Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials 10(1):5
Delshad M, Sarbazi N, Rezaei_Ghaleh N, Ghanbarian A, Azizi F (2012) Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med 15(5):279
Afsharian S, Akbarpour S, Abdi H et al (2016) Risk factors for cardiovascular disease and mortality events in adults with type 2 diabetes-a 10-year follow-up: Tehran Lipid and Glucose Study. Diabetes Metab Res Rev 32(6):596–606
Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W (2007) Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 167(11):1145–1151
Committee IR (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)–short and long forms. Retrieved September 17:2008
Royston P, Parmar MK (2011) The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat Med 30(19):2409–2421
Royston P, Parmar MK (2013) Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 13(1):152
Uno H, Claggett B, Tian L et al (2014) Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol 32(22):2380–2385
Klein JP, Gerster M, Andersen PK, Tarima S, Perme MP (2008) SAS and R functions to compute pseudo-values for censored data regression. Comput Methods Programs Biomed 89(3):289–300
De Wreede LC, Fiocco M, Putter H (2010) The mstate package for estimation and prediction in non-and semi-parametric multi-state and competing risks models. Comput Methods Progr Biomed 99(3):261–274
Spencer EA, Pirie KL, Stevens RJ et al (2008) Diabetes and modifiable risk factors for cardiovascular disease: the prospective Million Women Study. Eur J Epidemiol 23(12):793
Schramm TK, Gislason GH, Køber L et al (2008) Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk. Circulation 117(15):1945–1954
Janghorbani M, Hu FB, Willett WC et al (2007) Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes. Diabetes Care 30(7):1730–1735
Collaboration ERF (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375(9733):2215–2222
Helfand M, Buckley DI, Freeman M et al (2009) Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the US Preventive Services Task Force. Ann Intern Med 151(7):496–507
Control CfD, Prevention (2011) National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention 201 (1)
Collaboration ERF (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011(364):829–841
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353(5):487–497
Naderi SH, Bestwick JP, Wald DS (2012) Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 125(9):882–887
Poulsen MK, Henriksen JE, Dahl J et al (2009) Myocardial ischemia, carotid, and peripheral arterial disease and their interrelationship in type 2 diabetes patients. J Nucl Cardiol 16(6):878–887
Farhan S, Baber U, Vogel B et al (2017) Impact of diabetes mellitus on ischemic events in men and women after percutaneous coronary intervention. Am J Cardiol 119(8):1166–1172
Kim BJ, Lee S-H, Kim CK et al (2011) Advanced coronary artery calcification and cerebral small vessel diseases in the healthy elderly. Circ J 75(2):451–456
Gu K, Cowie CC, Harris MI (1998) Mortality in adults with and without diabetes in a national cohort of the US population, 1971–1993. Diabetes Care 21(7):1138–1145
Narayan KV, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF (2003) Lifetime risk for diabetes mellitus in the United States. JAMA 290(14):1884–1890
Salomon JA, Wang H, Freeman MK et al (2013) Healthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet 380(9859):2144–2162
Leening MJ, Ferket BS, Steyerberg EW et al (2014) Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 349:g5992
Tang W, Tu XM (2013) Modern clinical trial analysis. Springer, Berlin
Acknowledgements
The authors wish to acknowledge Ms Niloofar Shiva for critical editing of English grammar and syntax of the manuscript. The authors also wish to thank Dr Hajime Uno from Harvard Medical School, who introduced the correct methods for estimating the restricted mean survival time.
Funding
This study was supported by Grant No. 121 from the National Research Council of the Islamic Republic of Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
This study was approved by the ethics committee of the Research Institute for Endocrine Sciences of the Shahid Beheshti University of Medical Sciences according to the principles of the Declaration of Helsinki.
Informed consent
All participants were informed of the study protocol, and their written informed consents were obtained before inclusion.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
592_2017_1083_MOESM3_ESM.pdf
Online Resource 3 Stacked probability transition curves for participants with diabetes (A) and without diabetes (B); the distance between two curves represent the probabilities of the different events (PDF 65 kb)
592_2017_1083_MOESM4_ESM.pdf
Online resource 4 Adjusted restricted mean survival time (RMST) of total population; Tehran Lipid and Glucose Study (1999-2014) (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Ramezankhani, A., Azizi, F., Hadaegh, F. et al. Diabetes and number of years of life lost with and without cardiovascular disease: a multi-state homogeneous semi-Markov model. Acta Diabetol 55, 253–262 (2018). https://doi.org/10.1007/s00592-017-1083-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-017-1083-x