Abstract
Purpose
Long bone defects due to fractures resulting from high-energy trauma, infections and tumor resections are problems that orthopedic surgeons commonly face. We investigated the effects of a titanium mesh cage on bone healing with an induced membrane technique.
Methods
Three groups, each composed of eight rabbits, were formed. Extraarticular diaphyseal bone defects were created. Femora of the first group were fixed with an empty titanium mesh cage and two K-wires. After formation of the defect, polymethylmethacrylate was inserted and fixed with a K-wire in the second group. At the third week, the cement was removed, a sterilized cancellous graft-filled titanium mesh cage was placed into the defect, and the membrane that was previously formed over the cement was placed on the cage and repaired. In the third group, sterilized cancellous grafts were filled into the titanium mesh cage, and the titanium mesh cage was fitted into the bone defect area.
Results
At the end of the third month, all subjects were killed. Radiological data revealed that the healing of the bone in the second and third groups was significantly better than that in the first group. There was no difference between the second and third groups. A histological evaluation of the healing status, such as fibrous tissue, cartilage tissue and mature or immature bone formation, was performed. Histological healing in the second and third groups was also significantly better than that in the first group.
Conclusion
We concluded that the combination of membrane-induced bone healing and graft-filled titanium mesh cages expedites osteogenesis in extraarticular bone defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Massive bone defects are mostly seen in fractures following high energy trauma, infections and resections of bone tumors [1]. Intercalar endoprosthesis, distraction osteogenesis, the Masquelet technique, otogenous bone grafts or vascular bone grafts are frequently used techniques to overcome these problems [2, 3]. Autogenous bone grafts are highly osteoconductive and osteoinductive materials that are predominantly used. However, donor site morbidity restricts the amount that is used [4].
Distraction osteogenesis is generally used in the treatment of segmental bone loss and nonunion cases, and promising results have been reported [5]. Vascular pedicular bone grafts have been popularized in the last era. However, this is a technically and time-consuming technique that necessitates a long period before weight bearing following surgery [6]. The Masquelet technique is a two-stage surgical procedure for the treatment of intercalar defects. Despite its two stages, the provision of effective local infection control, increased vascularity and precursor mediators of bone healing in the surrounding membrane are the main advantages of this technique [7].
The cage system both works as a mechanical support during axial loading and permits neoangiogenesis with its fenestrated structure in the treatment of bone defects. The titanium mesh cage (TMC) was first described for bone defects in the spine; however, usage in defects of long bone diaphyses and metaphyses was also stated [8,9,10].
A wide variety of surgical techniques have been developed for the treatment of long bone defects. However, selection of the optimal technique both biologically and mechanically is the main objective of the treatment. Therefore, we aimed to investigate the behavior of bone healing both radiologically and histologically with a combination of membrane-induced grafting and titanium mesh cages in a segmental bone loss model.
Method
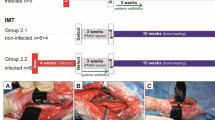
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval for this study was obtained from the Ethics Committee of Cukurova University before the initiation of the study (27.10.2016/9). Twenty-four New Zealand rabbits weighing 2500–3000 g were chosen. There were three groups, and each was composed of eight rabbits. All of the subjects underwent surgery for critical segmental bone loss formation. Critical bone loss is described as a defect that is larger than twice the diameter of bone at that level and that cannot be healed during the lifetime of the animal. The value for critical bone defects in New Zealand rabbits was calculated as 15 mm by Yang et al. [11]. Therefore, we also induced a 15 mm intercalar bone defect in the mid-diaphyseal region of the femur. Subjects were killed on the 90th day of surgery. All of the interventions were formed by the same experimenter.
Surgical technique
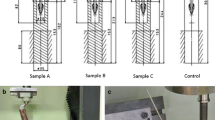
Cefazoline sodium prophylaxis (50 mg/kg, iv administration) was applied before surgery in all of the experiments. Rabbits were anesthetized with intramuscular ketamine hydrochloride (40 mg/kg) and xylazine hydrochloride (5 mg/kg). Surgical exposure was performed in the lateral decubitus position. Legs were shaved and swabbed with povidone iodine solution for sterilization of the surgical site. A lateral longitudinal incision was used. The vastus lateralis was retracted anteriorly, and the femur was reached through the intermuscular septum. The periosteum was dissected with a sharp incision, and the mid-third of the bone was exposed. Then, 15 mm of bone was cut by an oscillating blade (Fig. 1a). In clinical applications, locked intramedullary nails or plates are used in addition to a TMC to fix reconstructions of segmental bone loss. We try to imitate this procedure with two intramedullary K-wires. We also attempt to obtain rotational stability by penetrating the distal cortex of the bone to imitate distal locking screws, in contrast with intramedullary nails.
Surgical technique a formation of 15 mm length bone defect. b preparation of bone cement equal to the defect. c K-wire was passing through the cement for central hole. d Cement was inserted into the defect. e Vastus lateralis retracted and induced membrane was seen above the cement. f Induced membrane was dissected sharply and seen with clamp at the tip of the black arrow. g TMC filled with cancellous bone graft. h TMC was inserted into the gap
Irrigation with isotonic NaCl solution was applied to the surgical site. The fascia and skin were closed in layers with nylon sutures. For pain control, ketoprofen (2 mg/kg/d, i.v.) was injected during the first three days following the surgery.
All experiments were conducted in a room with a standard temperature of 22 degrees Celsius and were illuminated to imitate 12-h day and 12-h night conditions. None of the subjects exhibited infections at the end of the study.
First group
A previously prepared and sterilized 15 mm length and 6 × 8 mm2 empty titanium mesh cage was inserted into a defect area. Then, two K-wires were added for intramedullary fixation from the trochanter major. The fascia and skin were closed, and wires were bent over the trochanter major where they had been inserted.
Second group
Following the formation of the bone defect, PMMA was mixed with N–N dimethyl-p-toluidine, and a 15 mm length cylindrical formed bone cement (Fig. 1b,c) was prepared and inserted into the defect. One intramedullary K-wire was inserted through the bone cement (Fig. 1d, e). The fascia and skin were closed. At the end of the 3rd week, the experiments were performed again. The K-wire and bone cement were removed through the membrane (Fig. 1f) formed around the bone cement (Fig. 1g). Previously sterilized grafts were filled into the TMC (Fig. 1h, i), and the TMC was fitted into the defect (Fig. 1j). Two K-wires were sent from the trochanter major. The induced membrane was sutured around the cage, and the fascia and skin were closed.
Third group
Following the formation of bone defects in the third group, sterilized grafts were filled into the TMC (Fig. 1h, i), and the TMC was fitted into the defect (Fig. 1j). Two K-wires were sent from the trochanter major. The fascia and skin were closed, and the wires were bent over the trochanter major.
Radiologic assessment
Anteroposterior and lateral radiographic views were obtained under sedoanalgesia on the 3rd week of the surgery. Then, the animals were killed, and all femora were disarticulated from hip joints on the 12th week. AP, lateral views and computerized tomography (CT) were obtained. Bone healing was assessed according to the scoring system described by Lane et al. [12] A CT scan was obtained with a slice thickness of 1.5 mm and an interspace of 3 mm. Axial images were evaluated in terms of the presence of callus and newly formed bone.
Mechanical assessment
Two randomly selected killed femoral materials from each group were placed on a platform at a vertical angle. Controlled axial loading was applied to the materials. The procedure was stopped when the bone appeared to break. Testometric M500-50 CT was used to perform axial loading and force measurements. The control value for a normal femur that was gathered from the same species of rabbit was determined to be 70 kgf. After the radiologic and mechanical processes, femora were sent to the pathology department for microscopic evaluation of bone healing.
Histological assessment
Femora were placed in a 10% formaldehyde solution and ethylenediaminetetraasetic acid. Following decalcification, five mm of histologic specimens from the newly formed bone around the TMC were gathered and placed into paraffin blocks. Hematoxylin–eosin staining was applied. Histopathologic findings were classified according to the scale that was described by Huo et al. A microscopic evaluation was performed by a pathologist who was blinded to the group assignments [13].
Statistical analysis
Statistical analysis was performed by the NCSS (Number Cruncher Statistical System, 2007 Statistical Software [Utah, USA] program. Descriptive statistical methods were used for the evaluation of continuous variables. The Kruskal–Wallis test was used for comparisons of multiple groups, and the Mann–Whitney U test was used for comparisons of two groups. The results were accepted as significant if the p value was < 0.05.
Results
No experiments were lost during the study, and no infection was observed.
Radiological findings
Radiographs taken at the 3rd (Fig. 2a) and 12th (Fig. 2b) weeks were evaluated. Radiological views revealed that radiologic bone healing at the 12th week seemed to be significantly different in favor of the second and third groups compared with the first group (Table 1). There was no significant difference between the second and third groups. CT views (Fig. 3) showed significant bone healing at the proximal and distal ends of the bone and cage junction in the second group (Fig. 3b). Bridging of callus formation throughout the defect was also observed (Fig. 3d). The radiopacity of the callus was noted to be similar to that of normal bone.
CT view: Axial sections were taken from the proximal, middle and distal parts of the defect. a and c indicate that there was callus in the proximal, middle and distal parts, but the bridging callus was deficient in the first and second groups. b The second group had better bone healing than the first (a) and third group (c). d second groups’ healing and bridging callus around the cage on 3D CT view
Histological findings
According to the scoring system described by Huo et al., the median values of the groups were 5.4 (1–9), 8.7 (8–9) and 7.4 (3–9) consecutively (Fig. 4; Table 2). Statistical analysis showed that the second group had significantly better bone healing than the first group. There were no significant differences between the second and third groups or between the first and third groups (Table 2).
Mechanical findings
The median values of force that led to axial loading and femoral fracture were 2.2 (2–2.5), 52.5 (50–55) and 32 (30–34), respectively. Although there were no significant differences between groups, the first group appeared to be weaker than the other groups (Table 3).
Discussion
The treatment of bone defects is a clinical challenge for orthopedic surgeons. Reconstruction with a TMC is a treatment option that has been used for the treatment of long bone defects for the last two decades. Since bone defects larger than 3 cm are not suitable for treatment with cancellous bone grafts alone, filling of the space with a TMC containing cancellous bone grafts was developed as a new technique for mechanical stability [8].
Cobos et al. first described this technique for the reconstruction of two cases of defective tibia fractures with TMC-filled grafts and intramedullary fixation [14]. At the first year of follow-up, patients could stand without crutches, and functional results were reported to be excellent. One patient showed bone healing at the posterolateral aspect of the bone, and the other showed complete healing inside the mesh cage.
Another multicentric study that included the results of 17 patients who had segmental long bone diaphyseal defects and were treated with TMC filled with cancellous bone grafts combined with intramedullary nailing and plate osteosynthesis revealed favorable results [10]. Sixteen of 17 patients showed radiological healing of bone.
In addition to the clinical usage of TMC, for the assessment of biological behavior and mechanical features of mesh cages, experimental studies in animal models were also conducted. Lindsay, Gagula and colleagues performed an experimental study of dog femurs and investigated the effect of mesh cages [15]. They evaluated bone healing with CT views that revealed the presence of a bridging callus throughout the reconstructed area and interpreted the quality of newly formed bone with the comparison of the radiopacity of normal bone. The researchers also performed SPECT and showed biological activity in the reconstruction site, both at the center and at the bone-cage junction site. They concluded that the titanium mesh cage could promote bone healing not only at the bone-cage junction site but also at the center.
Fujibayashi et al. performed a study on rabbit femurs and reported significant lamellar bone formation after treatment of segmental bone loss with a bioactive TMC at the 8th week [16]. In our study, we formed the first group of only mesh cages and two K-wires. Titanium is a well-known biological inert material, and we aimed to observe whether it would contribute to bone healing. In this group, the healing yielded the worst histological and radiological scores. The third group was composed of reconstructed materials with graft-filled TMCs. Bone healing in this group was significantly better than that in the first group, which was confirmed by both radiographs and CT scans. Histological investigation revealed better bone healing in the third group than in the first group. We concluded that cancellous bone grafts promoted bone healing in the defective area in addition to the TMC. However, healing was mainly prominent outside of the bone. This could be attributed to peripheral vascularization and biological support of graft material. Therefore, the second group was formed via a two-stage operation to apply highly vascularized and biologically active surrounding soft tissue. The technique was first described by Masquelet based on the formation of a peripheral highly vascularized and osteoinductive membrane [2]. Bone cement in the defective area stimulates the surrounding soft tissue, and an inductive pseudosynovial membrane forms. Successful results have been reported in the literature for the reconstruction of bone defects. Masquelet et al. shared the clinical results of 35 patients [17]. They reported that the patients had segmental bone defects measuring between 5 and 25 cm, and after treatment, bone healing was obtained in a mean time of 8.5 months. The technique was also clinically applied to complex wrist fractures primarily by Flamans et al. [18]. They inserted bone cement into the fracture site and performed soft tissue reconstruction. Removal of the cement and reconstruction with a bone graft were performed following soft tissue healing.
Viateau et al. reported that the membrane formed around the bone cement was a rich source of fibroblast-like cells, type 1 collagen and endothelial-like cells [19]. Hypothetically, the membrane can promote bone healing as well. In our study, the second group had better (although statistically insignificant) radiological and histological scores than the third group in terms of bone healing. This could point to the viability of the surrounding soft tissue and its contents, such as osteoprogenitor cells and growth factors, which had a noteworthy effect on the healing of the bone. Similar healing patterns were reported by the authors.
Reynerds et al. reported the treatment of a patient with segmental bone defects of the femur that successfully achieved bone healing [20]. They also emphasized that the healing was prominent at the surface of the cage and could not document the osseous integrity of the bone inside the cage, which was similar to our findings. We could not determine the healing inside of the cage radiologically because of the presence of artifacts due to the two K-wires inside the cage. Histological samples were also obtained from the surface of the TMC. However, we concluded that the TMC provided a surface for the newly formed bone, the graft material played a role as osteoinductive material, and peripheral soft tissue sustained a blood supply that contained oxygen and adjacent growth factors. However, the key factor for healing could not be determined. A novel technique was described for defective tibial nonunion by the authors [21]. In this technique, the defective area was spared during the procedure, the proximal and distal sides of the nonunion were exposed, and the graft was implemented. Then, the fibula adjacent to these areas was decorticated, and osteoinductive support was obtained. Twenty-three of 24 patients achieved healing without any additional intervention. This clinical study also showed that under appropriate circumstances, healing is available even through the soft tissue. This was consistent with our finding that the second group had the best histological as well as radiological bone-healing results.
One weakness of our study was the choice of graft material. To eliminate the surgical comorbidity of graft harvesting, we preferred to use human-based cancellous grafts. We did not perform mechanical tests on all specimens. We believed that fracture could affect and disturb the histological evaluation of bone healing. The sample size that was used for statistical analysis was reduced.
However, this was an animal experimental study that aimed to show the effect of the combined Masquelet and TMC technique on bone healing. Studies with larger sample sizes and longer-term follow-ups should be performed to obtain more definitive results.
Conclusion
Many techniques have been described for the treatment of segmental bone loss. However, this is still a challenging clinical situation for new techniques to be investigated and described to reduce complications and costs and increase patient compliance. The Masquelet technique seems to be one of the most preferred methods. Combining this method with a TMC could both increase mechanical stability and shorten the time required for bone healing. This study showed that bone healing was the best in the combined-procedure group. However, this study should be supported by clinical studies.
Availability of data and materials
Not applicable.
References
Wiese A, Pape HC (2010) Bone Defects caused by high-energy injuries, bone loss, infected nonunions, and nonunions. Orthopedic Clin 41:1–4. https://doi.org/10.1016/J.OCL.2009.07.003
Pelissier P, Masquelet AC, Bareille R, Mathoulin Pelissier S, Amedee J (2004) Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res 22:73–79. https://doi.org/10.1016/S0736-0266(03)00165-7
Reichert JC, Saifzadeh S, Wullschleger ME, Epari DR, Schütz MA, Duda GN, Schell H, van Griensven M, Redl H, Hutmacher DW (2009) The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30:2149–2163. https://doi.org/10.1016/J.BIOMATERIALS.2008.12.050
Giannoudis P, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 3:20–27. https://doi.org/10.1016/j.injury.2005.07.029
Tsuchiya H, Tomita K (2003) Distraction osteogenesis for treatment of bone loss in the lower extremity. J Orthop Sci 8:116–124. https://doi.org/10.1007/S007760300020
Hu QT, Jiang QW, Su GL, Shen JZ, Shen X (1980) Free vascularized bone graft. Chin Med J (Engl) 93:753–757
Masquelet AC (2003) Muscle reconstruction in reconstructive surgery: Soft tissue repair and long bone reconstruction. Langenbeck’s Arch Surg 388:344–346. https://doi.org/10.1007/S00423-003-0379-1
Ayvaz M, Bekmez S, Yucekul A (2018) Titanium mesh cage as an alternative reconstruction method for epiphyseal-sparing tumour resections in children. J Pediatric Orthopaedics B 27:350–355. https://doi.org/10.1097/BPB.0000000000000482
Attias N, Lehman R, Bodell LS (2005) Surgical management of a long segmental defect of the humerus using a cylindrical titanium mesh cage and plates: a case report. J Orthop Trauma 19:211–216. https://doi.org/10.1097/00005131-200503000-00011
Attias N, Thabet AM, Prabhakar G, Dollahite JA, Gehlert RJ, DeCoster TA (2018) Management of extra-articular segmental defects in long bone using a titanium mesh cage as an adjunct to other methods of fixation. Bone Joint J 100B:646–651. https://doi.org/10.1302/0301-620X.100B5.BJJ-2017-0817.R2
Yang J, Chen HJ, Zhu XD, Vaidya S, Xiang Z, Fan YJ, Zhang XD (2014) Enhanced repair of a critical-sized segmental bone defect in rabbit femur by surface microstructured porous titanium. J Mater Sci Mater Med 25:1747–1756. https://doi.org/10.1007/S10856-014-5202-8
Lane J, Sandhu H (1987) Current approaches to experimental bone grafting. Orthop Clin North Am 18:213–225. https://doi.org/10.1016/S0030-5898(20)30385-0
Huo MH, Troiano NW, Pelker RR, Gundberg CM, Friedlaender GE (1991) The influence of ibuprofen on fracture repair: Biomechanical, biochemical, histologic, and histomorphometric parameters in rats. J Orthop Res 9:383–390. https://doi.org/10.1002/JOR.1100090310
Cobos J, Lindsey R, Gugala Z (2000) The cylindrical titanium mesh cage for treatment of a long bone segmental defect: description of a new technique and report of two cases. J Orthop Trauma 14:54–59. https://doi.org/10.1097/00005131-200001000-00011
Lindsey RW, Gugala Z, Milne E, Sun M, Gannon FH, Latta LL (2006) The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. Wiley Online Library 24:1438–1453. https://doi.org/10.1002/jor.20154
Fujibayashi S, Kim H, Neo M, Uchida M (2016) Repair of segmental long bone defect in rabbit femur using bioactive titanium cylindrical mesh cage. Biomaterials 24:3445–51. Doi: https://doi.org/10.1016/s0142-9612(03)00221-7
Masquelet A, Fitoussi F, Begue T (2000) Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir 45:346–353
Flamans B, Pauchot J, Petite H, Blanchet N (2010) Use of the induced membrane technique for the treatment of bone defects in the hand or wrist, in emergency. Chirurgie de lamain 29:307–314. https://doi.org/10.1016/j.main.2010.06.008
Viateau V, Ve Guillemin G, Bousson V, Oudina K, Hannouche D, Sedel L, Logeart-Avramoglou D, Petite H (2007) Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. Wiley Online Library 25:741–749. https://doi.org/10.1002/jor.20352
Reynders P, Broos PLO (2003) The use of cylindrical titanium mesh cages in the treatment of post-traumatic segmental bone loss of the femur. Osteosynthesis Trauma Care 11:99–104. https://doi.org/10.1055/s-2003-42524
Ryzewicz M, Morgan SJ, Linford E, Thwing JI, de Resende GVP, Smith WR (2009) Central bone grafting for nonunion of fractures of the tibia: A retrospective series. J Bone Joint Surg Ser B 91:522–529. https://doi.org/10.1302/0301-620X.91B4.21399
Acknowledgements
This research funded by Çukurova University Scientific Research Projects Coordination Unit. Project number: TTU-2017-8360. Corresponding author Onur KAYA has received research support from Çukurova University Scientific Research Projects Coordination Unit. The other authors declare they have no financial interests.
Funding
We would like to thank Çukurova University Scientific Research Projects Coordination Unit for funding the study.
Author information
Authors and Affiliations
Contributions
KO performed the animal experiments, funding acquisition,processed samples, analyzed and interpreted the data, and wrote the manuscript. MA processed samples, analyzed and interpreted the data, and wrote the manuscript. ÖSB, MAD, and MT supported in sample processing and supported in data analysis and interpretation. CÖ designed the study, guided the animal experiments, interpreted the data, and edited the manuscript. KEA analyzed pathological evaluation. All authors have revised the manuscript for important intellectual content and have approved the submission of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Animal study design
This study was performed in line with the principles of the Declaration of Helsinki.
Ethical approval
Ethical approval for this study was obtained from Ethics Committee of Cukurova University before the initiation of the study (27.10.2016/9).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaya, O., Mirioglu, A., Ozkan, C. et al. Reconstruction of critical size segmental femoral diaphyseal defects of New Zealand rabbits by using combined titanium mesh cage and induced membrane technique. Eur J Orthop Surg Traumatol 33, 629–637 (2023). https://doi.org/10.1007/s00590-022-03330-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-022-03330-y