Abstract
Purpose
Introducing a suture repair technology, endoscopic double line suture repair technique, for iatrogenic dural injury during Percutaneous Endoscopic Lumbar Discectomy (PELD) surgery.
Methods
A patient with dural injury and cauda equina herniation during PELD surgery was treated with endoscopic double line suture repair technique.
Results
A patient with dural injury and cauda equina nerve herniation during PELD surgery was successfully treated using double-line suture technique. After the repair, no obvious cerebrospinal fluid leakage and cauda equina nerve re-herniation was seen. During the postoperative observation period, the wound healed well and there were no complications related to cerebrospinal leakage. During the follow-up period (1 year), the patient reported significant symptom relief and no complications.
Conclusion
This novel dural repair technology is safe and effective and can be used to treat dural injuries during PELD surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over time, Percutaneous Endoscopic Lumbar Discectomy (PELD) has emerged as the primary treatment for degenerative spinal lesions in China. Statistical data indicates an annual occurrence of approximately 50,000–60,000 cases of endoscopic spinal surgery in Chinese hospitals, constituting around 10% of the total spinal surgeries, and this percentage continues to rise [1]. The iatrogenic dural tear stands out as a common injury in PELD, presenting early complications like low cranial pressure headache, wound infection or delayed healing, and intracranial infection. Additionally, long-term complications encompass meningeal pseudocyst formation, cutaneous sinusoid formation, acquired Chiari syndrome, and prolonged bed rest leading to pneumonia with hypostatic pneumonia, pressure sores, and deep vein thrombosis [2,3,4]. The presence of severe complications is deemed unacceptable for both clinical follow-up treatment and care, given their adverse impact on health and the resultant financial burden on patients.
Owing to operational space limitations, performing endoscopic dural suture repair proves to be a challenging procedure. As a result, the established gold standard for addressing iatrogenic dural tear in PELD involves an immediate transition to open surgery for direct repair [5]. Nevertheless, the transition from local anesthesia to general anesthesia and additional surgical trauma, could result in a highly awkward situation. Certainly, certain sealants, including fibrin glue, hydrogel, and bioabsorbable staples, may be employed for dural repair in PELD [6]. However, these sealants not only pose the risk of serious complications, including neurotoxicity, allergic reactions, and viral transmission, but they also contribute to an additional financial burden in terms of medical costs [7]. Hence, a direct endoscopic suture of the dura seems to be the best choice.
Presently, there exists an absence of detailed technical descriptions pertaining to endoscopic dural suture repair. Park et al. reported the use of non-penetrating titanium clips for dural repair in a two-channel procedure and achieved favorable results [8]. Shin et al. and Bergamaschi et al. introduced a comparable endoscopic dural suture technique, where the primary procedure involves using double needles sutures to pass sequentially through the broken edges on both sides, tying a knot and then feeding the knot into the lesion with an endoscopic curette [9, 10]. The main difference lies in Bergamaschi et al.‘s introduction of a novel knotting method. Regrettably, there is no more reports on endoscopic dural suture repair techniques beyond those previously mentioned in the existing literature.
To fill the gap in this field, our team has developed a novel endoscopic double line suture repair technique. The efficacy of this technique has been demonstrated in the presented case report, and, and we hope that this technique will bring a new treatment regimen to surgeons.
Case report and technique description
A 74-year-old female presented with low back pain accompanied by numbness and radiating pain in the left lower extremity (L2) without apparent triggers. A lumbar spine MRI was conducted, revealing a herniated disc in L1/L2 (Fig. 1). The patient’s surgical indications were clear. Given her compromised general condition (advanced age and a history of cardiovascular stent implantation), our team opted for endoscopic disc removal through the intervertebral foramen approach following a thorough discussion.
In this operation, the patient took the prone position with 30° of lumbar flexion. Following the identification of the L1/L2 intervertebral space by the robot-assisted surgical system, an entrance point 5 cm to the left of the median line was selected. Subsequently, local anesthesia was administered using 2% lidocaine (20 ml) and 0.9% sodium chloride injection (40 ml). Next, a robot-assisted guide needle was utilized for puncture, and a working sleeve was placed after positioning to the lateral margin of the intervertebral foramen. Subsequently, hemostasis and intervertebral foramen enlargement and molding operation were performed using a plasma scalpel and power grinder drilling system. After observing the clearly prolapsed disc, using the nucleus pulposus forceps to remove the nucleus pulposus. While cleaning the remaining wrinkled tissue, the attending surgeon observed an approximately 1-cm-long dural tear with cauda equina herniation on the anterolateral aspect of the dural sac (Fig. 2). Following a brief discussion, the decision was made to utilize the endoscopic double line suture repair technique for repairing the broken dura mater.
The endoscopic double line suture repair technique is segmented into three primary phases: pre-suture preparation, suture repair and post repair inspection. The primary objective of the pre-suture preparation was to expand the surgical operating space and expose both edges of the rupture. The main operations include further enlargement of the intervertebral foramen (resection of the anterior edge of the L2 superior articular process, part of the ligamentum flavum, and soft tissues) and recovery of the herniated cauda equina (accomplished by increasing the water pressure in the rupture).
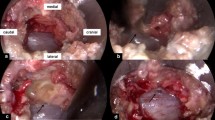
Suture repair commenced once the breach was fully exposed, and the general procedure is illustrated in Fig. 3. Initially, an annulus fibrosus suture device (white line) was employed to establish a stable anchor point at the disc. Subsequently, another annulus fibrosus suture device (blue line) was utilized to traverse both edges of the torn dura mater from the outside to the inside, with gentle pulling to close the breach (Fig. 4A). It is important to note that, during the placement of the white and blue anchor points, the spacing between the “T”-shaped ends of the two lines should be minimized to mitigate the pull on the dura mater during knotting. Next, the blue and white lines were tied in a surgical knot and then an endoscopic curette was used to send the knot along the white line to the breach, with this process repeated 3 times (Fig. 4B). Excessive traction must be avoided during the operation, and our technique by setting the first anchor point at the intervertebral disc can greatly avoid secondary injury to the dura mater. Finally, endoscopic scissor was used to cut off the remaining white and blue line tails (Fig. 4C, D). The lines used in this case are all 0.18 mm in diameter, and the schematic diagram of the annulus fibrosus suture device and the “T”-shaped line tail was shown in Fig. 5.
Technical route of double line suture. A: First, a white line is secured to the disc as the first anchor point; subsequently, a blue line is passed through the edge of the broken dura mater from the outside to the inside and is set up as the second anchor point; finally, the double lines are knotted and pressed down to the broken area with an endoscopic curette. B: Sample diagram of the spine (including spinal cord, intervertebral discs, and vertebral structures)
After the repair was completed, the patient was instructed to cough for observing whether there was any re-herniation of the cauda equina and rupture re-opening to test the repair effect. After observing no obvious abnormalities, the incision was closed, and the patient was transferred to the ward for 24-hour bed rest observation. Throughout the observation period, the attending doctor did not observe noticeable discharge from the patient’s wound, and there were no symptoms of radiating lower extremity pain or complications related to cerebrospinal fluid leakage. The postoperative MRI also did not reveal any evident abnormalities (Fig. 6). The patient was discharged from the hospital on the third postoperative day, and during the follow-up period (1 year), the patient feedback that the symptoms were relieved significantly and did not have any other discomfort.
Discussion
Iatrogenic dural tear represents a common complication of PELD, with an incidence of approximately 1.1%, and this probability is higher in revision cases [5, 11, 12]. PELD-associated dural injuries usually present with neurological impairment and intractable radiating pain [11]. Meanwhile, prolonged postoperative bed rest also increases the risk of venous thrombotic events (VTE) as well as pulmonary, gastrointestinal and wound complications [13, 14]. Additionally, Tamaki et al. also reported a rare complication of lumbar disc protrusion into the dura mater via the defect after PELD [12]. A study demonstrated that medical costs, length of hospitalization, and the probability of secondary admission were significantly higher in patients with dural tears compared to conventional patients [15]. Therefore, a considerable number of scholars concur that intraoperative one-stage repair of the dura mater is the best choice for surgeons in order to avoid postoperative complications [16, 17].
Existing techniques for dural tears are using autologous tissue (muscle or fat) and biomaterials to repair and fill the lesion out, but their efficacy is still debatable, especially in cases with large lesions [5, 16, 18, 19]. Meanwhile, abnormal tissue proliferation due to autogenous/ biomaterials, free grafts entering the dura mater, and allergic reactions due to grafts can have unbearable consequences, which are unacceptable in clinical work. Due to the limited operating space, the endoscopic dural suture repair is technically difficult. Consequently, scholars generally concur that a one-stage conversion to open surgery for suture repair may be the only treatment option [5]. However, the usual anesthesia for PELD is local anesthesia. The additional risks and cost burden associated with transitioning from local anesthesia to general anesthesia are deemed unacceptable to patients and their families, and this may impact the positive relationship between doctors and patients [20]. Taken together, we believe that endoscopic dural suture repair is the optimal treatment option.
The endoscopic double line repair suture technique is an exceptionally valuable technological breakthrough. Firstly, in both our case and reported cases, patients did not experience cerebrospinal fluid leakage or other complications after surgery. Secondly, the instruments we employed were all standard instruments for PELD, eliminating the necessity to acquire or design new surgical tools. Additionally, increasing the water pressure at the breach can prevent the nerve root and cauda equina from drifting outward; endoscopy facilitates detailed observation of the anatomical structure between the nerve root, dura mater, and arachnoid membrane; and under local anesthesia, in the event of nerve root binding or cauda equina lassoing, the patient can inform the doctor in time, which controls the potential dangers from the source. These advantages can significantly reduce the incidence of serious medical malpractice due to nerve root bundling and cauda equina lassoing [21]. Lastly, patients in the awake state can cough and cooperate with us to observe the suture effect.
The porosity formed during the suturing process contributes in part to the failure of the one-stage dural repair and carries a certain probability of causing persistent leakage of cerebrospinal fluid [22,23,24]. Hence, it is crucial to select the needle entry point and anchor point in a single step during endoscopic dural suturing to prevent additional damage to the dura mater caused by multiple sutures. Simultaneously, it is essential to minimize excessive pulling between anchor points during the suturing process. In comparison with reported cases, our endoscopic double line suture repair technique set the primary anchor point on the intervertebral disc and employs this anchor point as the main force point for knotting the suture, which could avoid the pulling of the dura mater to a great extent. And, the “T”-shaped ends of the suture line could also have a filling effect on the holes created by the suture needle. Of course, according to the actual intraoperative situation, the attending surgeon could opt to use a small amount of sealant to fill the pores at the anchor point after completing the suture [5]. Regarding injuries with large breaks and uneven edges, it is advisable to use a small amount of sealant to reinforce the repair of the breach after completing the suture [22,23,24].
Although the endoscopic double line suture repair technique is a difficult operation for most surgeons, the level of skill will continue to advance as the volume of procedures rises. We are confident that with the guidance of senior physicians, repetitive training on animals or body models, and the development of new surgical instruments or apparatus, surgeons will eventually overcome this challenge. In addition, we think that this repair technique is mainly applicable to cases of anterolateral dural sac injuries in transforaminal approaches. Meanwhile, it is also necessary to verify the repair effect in more subsequent cases.
Conclusion
Addressing intraoperative dural injury in PELD poses significant challenges, and we introduce a novel endoscopic dural suture repair technique—the endoscopic double line suture repair technique. This method offers a fresh solution for surgeons dealing with intraoperative dural breaches.
References
Zhang X, Du J, Yeung A (2017) Development of percutaneous endoscopic lumbar discectomy (PELD)technology in China. J Spine 06. https://doi.org/10.4172/2165-7939.1000376
Bosacco SJ, Gardner MJ, Guille JT (2001) Evaluation and treatment of dural tears in lumbar spine surgery: a review. Clin Orthop Relat Res. (389):238–247
Jones AA, Stambough JL, Balderston RA et al (1989) Long-term results of lumbar spine surgery complicated by unintended incidental durotomy. Spine (Phila Pa 1976) 14(4):443–446
Khan MH, Rihn J, Steele G et al (2006) Postoperative management protocol for incidental dural tears during degenerative lumbar spine surgery: a review of 3,183 consecutive degenerative lumbar cases. Spine (Phila Pa 1976) 31(22):2609–2613
Ahn Y, Lee HY, Lee S-H et al (2011) Dural tears in percutaneous endoscopic lumbar discectomy. Eur Spine J 20(1):58–64. https://doi.org/10.1007/s00586-010-1493-8
Dafford EE, Anderson PA (2015) Comparison of dural repair techniques. Spine J 15(5):1099–1105. https://doi.org/10.1016/j.spinee.2013.06.044
Epstein NE (2010) Dural repair with four spinal sealants: focused review of the manufacturers’ inserts and the current literature. Spine J 10(12):1065–1068. https://doi.org/10.1016/j.spinee.2010.09.017
Park H-J, Kim S-K, Lee S-C et al (2020) Dural tears in Percutaneous Biportal endoscopic spine surgery: anatomical location and management. World Neurosurg 136:e578–e85. https://doi.org/10.1016/j.wneu.2020.01.080
Shin JK, Youn MS, Seong YJ et al (2018) Iatrogenic dural tear in endoscopic lumbar spinal surgery: full endoscopic dural suture repair (Youn’s technique). Eur Spine J 27(Suppl 3):544–548. https://doi.org/10.1007/s00586-018-5637-6
Bergamaschi JPM, de Araújo FF, Soares TQ et al (2022) Dural Injury Treatment with a full-endoscopic Transforaminal Approach: a Case Report and description of Surgical technique. Case Rep Orthop 2022:6570589. https://doi.org/10.1155/2022/6570589
Pan M, Li Q, Li S et al (2020) Percutaneous endoscopic lumbar discectomy: indications and complications. Pain Physician 23(1):49–56
Tsutsumimoto T, Yui M, Uehara M et al (2014) A prospective study of the incidence and outcomes of incidental dural tears in microendoscopic lumbar decompressive surgery. Bone Joint J 96–B(5):641–645. https://doi.org/10.1302/0301-620X.96B5.32957
Durand WM, DePasse JM, Kuris EO et al (2018) Late-presenting dural tear: incidence, risk factors, and associated complications. Spine J 18(11):2043–2050. https://doi.org/10.1016/j.spinee.2018.04.004
Radcliff KE, Sidhu GDS, Kepler CK et al (2016) Complications of flat Bed Rest after Incidental Durotomy. Clin Spine Surg 29(7):281–284. https://doi.org/10.1097/BSD.0b013e31827d7ad8
Alluri R, Kang HP, Bouz G et al (2020) The true effect of a lumbar Dural tear on complications and cost. Spine (Phila Pa 1976) 45(3):E155–E62. https://doi.org/10.1097/BRS.0000000000003213
Guerin P, El Fegoun AB, Obeid I et al (2012) Incidental durotomy during spine surgery: incidence, management and complications. A retrospective review. Injury 43(4):397–401. https://doi.org/10.1016/j.injury.2010.12.014
Epstein NE (2013) A review article on the diagnosis and treatment of cerebrospinal fluid fistulas and dural tears occurring during spinal surgery. Surg Neurol Int 4(Suppl 5):S301–S17. https://doi.org/10.4103/2152-7806.111427
Papavero L, Engler N, Kothe R (2015) Incidental durotomy in spine surgery: first aid in ten steps. Eur Spine J 24(9):2077–2084. https://doi.org/10.1007/s00586-015-3837-x
Tafazal SI, Sell PJ (2005) Incidental durotomy in lumbar spine surgery: incidence and management. Eur Spine J 14(3):287–290
Locke MC, Davis JC, Brothers RJ et al (2018) Assessing the outcomes, risks, and costs of local versus general anesthesia: a review with implications for cutaneous surgery. J Am Acad Dermatol 78(5). https://doi.org/10.1016/j.jaad.2018.01.009
Grannum S, Patel MS, Attar F et al (2014) Dural tears in primary decompressive lumbar surgery. Is primary repair necessary for a good outcome? Eur Spine J 23(4):904–908. https://doi.org/10.1007/s00586-013-3159-9
Narotam PK, José S, Nathoo N et al (2004) Collagen matrix (DuraGen) in dural repair: analysis of a new modified technique. Spine (Phila Pa 1976). 29(24)
Cammisa FP, Girardi FP, Sangani PK et al (2000) Incidental durotomy in spine surgery. Spine (Phila Pa 1976) 25(20):2663–2667
Jankowitz BT, Atteberry DS, Gerszten PC et al (2009) Effect of fibrin glue on the prevention of persistent cerebral spinal fluid leakage after incidental durotomy during lumbar spinal surgery. Eur Spine J 18(8):1169–1174. https://doi.org/10.1007/s00586-009-0928-6
Funding
This research was supported by Development and Transformation of Digital Intelligent Orthopedic Endoscopic Surgery System (Grant No. cyyy-xkdfjhcgzh-202303).
Author information
Authors and Affiliations
Contributions
All authors have contributed to this study. The manuscript was written by R.Z, N.L, and J.Z; case data was organized by R.Z, N.L, and J.Z; case follow-up was handled by R.Z, N.L, and J.Z; the endoscopic double line suture repair technique was designed by X.L and X.Z; the manuscript was reviewed by X.L and X.Z. All authors commented on previous versions of the manuscript, read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by Clinical Research Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (ID: K2024-053-01).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, R., Li, N., Zhang, J. et al. Endoscopic double line suture repair technique for repairing Iatrogenic dural tear: a technical case report. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08383-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08383-7