Abstract
Background
Malignant spinal cord compression (MSCC) has been noted in 3–5% of children with primary tumours. MSCC can be associated with permanent neurological deficits and prompt treatment is necessary. Our aim was to perform a systematic review on MSCC in children < 18 years to help formulate national guidelines.
Methods
A systematic review of the English language was undertaken using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Search criteria included ‘MSCC in children, paediatric and metastases’ for papers published between January1999 and December 2022. Isolated case reports/case series with < 10 patients were excluded.
Results
From a total of 17 articles identified, a final 7 were analysed (Level III/IV). Neuroblastoma constituted the most common cause for MSCC in children (62.7%) followed by sarcoma (14.2%). Soft tissue sarcomas were the most frequent cause of MSCC in children > 5 years old, while for neuroblastomas, the mean age of presentation was 20 months. The median age at time of diagnosis for the entire cohort of patients was 50.9 months (14.8–139). The median follow-up duration was 50.7 months (0.5–204).
Motor deficits were the presenting symptom in 95.6% of children followed by pain in 65.4% and sphincter disturbance in 24%. There was a delay of about 26.05 days (7–600) between the onset of symptoms and diagnosis.
A multimodality approach to treatment was utilised depending on the primary tumour. The prognosis for neurological recovery was found to be inversely proportional to the degree of neurological deficits and duration of symptoms in four studies.
Conclusion
Neuroblastoma is the most common cause for MSCC in children (62.7%) followed by sarcoma (14.2%), whilst soft tissue sarcomas constituted the most frequent cause of MSCC in children > 5 years old. The majority of patients presented with motor deficit, followed by pain. In children with neuroblastoma /lymphoma, chemotherapy was the primary treatment. Early surgery should be a consideration with rapid deterioration of neurology despite chemotherapy. A multimodality approach including chemo-radiotherapy and surgery should be the treatment of choice in metastatic sarcomas. It is worth noting that multi-level laminectomy/decompression and asymmetrical radiation to the spine can lead to spinal column deformity in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute malignant spinal cord compression (MSCC) is an uncommon diagnosis in paediatric population with the incidence reported between 3 and 5% among children with cancer [1, 2]. MSCC represents an oncological emergency and can lead to permanent neurological deficits if prompt and effective treatment is not instituted [3]. The pathophysiology of malignant spinal cord compression in the paediatric population is entirely different from that of the adult population [4]. Hence, the management principles used in adult population cannot be extrapolated into the paediatric group. The diagnosis of MSCC can be particularly difficult at an early phase in infants, which in turn increases the short- and long-term morbidity [5]. Chemotherapy, radiotherapy and surgery are the available modalities of treatment. Multimodality treatment approach using combined chemo-radiotherapy and surgery has been associated with the greatest functional improvement in adult population [6]. However, the optimal therapeutic approach for children with MSCC has not been clearly established.
Treatment strategies vary widely depending upon the primary tumour type and also as per the study groups with no definite guidelines for the management of children with MSCC. Furthermore, there are short- and long-term morbidities associated with each of these treatment modalities in a growing child [7]. Our aim was to perform a systematic literature review on MSCC in children < 18 years to assist with the formulation of management guidelines in this population.
Materials and methods
Study design
Systematic review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The Prisma flowchart is illustrated in Fig. 1.
Databases searched
PubMed, Medline, NICE, Google Scholar.
Search criteria/key words
-
Metastatic OR Malignant spinal cord compression AND children
-
Metastatic OR Malignant spinal cord compression AND paediatric
-
Spinal cord compression AND metastases AND paediatric
Inclusion criteria
-
Paediatric population < 18 years
-
Articles published between January 1999 and December 2022
-
Metastatic/Malignant spinal cord compression
Exclusion criteria
-
Case reports and cohort studies with less than 10 patients
-
All other causes of paediatric spinal cord compression
Outcomes
Demographics, clinical presentation, imaging modalities, management principles and therapeutic options, neurological recovery and prognosis.
Analysis
Descriptive Statistics.
Results
The initial search generated 1021 articles, out of which 17 were unique and relevant. After subsequent screening, 7 studies fulfilled the inclusion criteria and were included in the study(1 prospective case series- level III [8]; 6 retrospective case series- level IV [4,5,6,7, 9, 10]).
Demographics (Table 1 )
A total of 303 patients who presented with clinical manifestations of spinal cord compression were identified with average follow-up duration of 50.7 months (0.5-204). The median age of the study population at the time of diagnosis was 50.9 months (14.8-139).
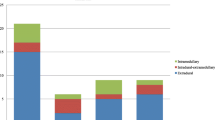
Aetiology of cord compression (Fig. 2)
Overall, neuroblastoma was the most common tumour causing spinal cord compression (62.7%) with the average age of diagnosis being 20 months, followed by soft tissue sarcoma (14.2%) which is more common in patients > 5 years.
Pollano et al. reported neuroblastoma as the most common cause of spinal cord compression in children below 5 years, while soft tissue sarcoma was the most common cause in children aged above 5 years [10]. Thoracic cord was the most common site of neural compression (45.5%) [10]. The biological behaviour of MSCC in children is entirely different from that in adults. Metastatic spinal cord compression in adults occurs secondary to invasion of epidural space from the adjacent vertebral bodies, whereas in children, it occurs most commonly via direct spread or invasion from paraspinal tumours through the neural foramen (sarcoma, neuroblastoma) [4, 10]. Tumour extension through the neural foramen causes circulatory disturbances due to involvement of local venous plexus, which in turn impairs the spinal cord circulation leading to infarction and paraplegia [10].
Clinical presentation
Overall, 95.6% of children in the study group had motor deficits at the time of presentation, followed by pain in 65.4% and sphincter disturbance in 24%. Pain was an important symptom of MSCC in children aged above 5 years (p = 0.04) [8]. The average duration between the onset of symptoms and diagnosis was 26.05 days (7-600 days).
Imaging
Simple X-ray radiographs were adequate to identify lesions in a number of patients with bony involvement [10]. Myelography was utilised till the 1980’s and has been largely replaced by CT scans and MRI scans subsequently [9]. MRI is currently the diagnostic modality of choice and has been diagnostic in all patients [5, 8, 10]. MRI is highly sensitive in diagnosing intradural and extradural tumours as well as defining the extent of spinal cord compression [10]. Diagnostic sensitivity of CT scan in delineating bony involvement was well beyond that of MRI [10].
First line of treatment (Table 2)
Chemotherapy, radiotherapy and surgery are the most commonly used modalities in the treatment of MSCC [11]. The first line of treatment varied significantly among the different study groups depending on the local management guidelines.
Steroid therapy
Dexamethasone has shown to reduce oedema, inhibit inflammatory response, increase vascular membrane stability and delay the onset of neurological deficits in children with spinal cord compression [12, 13]. Parenteral steroids were administered to all patients at presentation as emergency medical management irrespective of the subsequent treatment modality [9, 10]. Tantawy et al. [8] reported administering steroids to 83.3% of patients at admission with MSCC until pathological diagnosis with 75% of patients demonstrating neurological improvement.
Chemo-radiotherapy
First line chemotherapy has been recommended as the treatment of choice for neuroblastomas irrespective of the grade of neurological deficits [4, 6, 7]. Regression of the tumour and the neurological recovery has been similar in patients treated with chemotherapy compared to surgery or radiotherapy [6]. Surgical decompression for neuroblastoma is recommended only in cases of progressive neurological worsening while on chemotherapy [6, 9] Similarly, lymphomas and Ewing sarcomas are chemo-sensitive, and hence, it should be the first line of treatment, except in patients presenting with progressive neurological deficits [5, 10].
Surgical management
Patients with soft tissue or bone sarcomas with severe cord compression showed improvement with neurological recovery following laminectomy compared to chemo-radiotherapy [14]. However, laminectomy has been associated with spinal instability and subsequent spinal deformities in (29.1%) of patients compared to patients not undergoing laminectomy (2.04%) (p < 0.001) [7]
Neurological recovery
The two factors found to be significantly associated with the neurological outcome were the severity of neurological symptoms at the time of presentation as well as the duration of symptoms prior to presentation. In one study, the likelihood of complete neurological recovery was found to be five times more likely in patients presenting with mild neurological symptoms when compared to those presenting with more severe symptoms (P = 0.017) [7].
Late sequelae
Forty-four per cent of patients presented with significant sequelae secondary to the effects of tumour and/or treatment [9]. Scoliosis was the most common sequel in their study found in 31% of the study population. More frequently, scoliosis was identified in patients treated with laminectomy or radiotherapy compared to chemotherapy (33% and 60% vs 7% respectively) [9]. Treatment-related scoliosis has been diagnosed with X-rays on the follow-up of the patients. The incidence of scoliosis was recognised in 29% of patients who underwent laminectomy when compared to 2% of patients who underwent chemotherapy (P = 0.001) [7]. Multi-level laminectomy, orthovoltage radiation therapy exceeding 3000 rads and asymmetrical radiation therapy were the factors attributed to the development of spinal deformity in patients with neuroblastoma and MSCC who were followed up for more than 60 months [15]. No patients in the current cohort underwent early, upfront instrumentation for prevention or correction of scoliosis. Additionally, no patients presented with scoliosis previous to treatment.
Prognosis
Prognosis for this cohort of patients is extremely heterogeneous as the tumour histology has a significant bearing with regard to the outcome of treatment and survival [8, 10]. Lymphomas had the best outcome in their study population with 100% improvement after definitive treatment followed by soft tissue sarcomas (80% improvement) [8].
Patients who presented within the 4 weeks of the onset of symptoms carried an improved outcome (78.6%) complete recovery of neurology v/s (25%) in patients presenting after 4 weeks (P = 0.008) [6]. The overall five-year survival rate was 72.6% (70.5–79.2%) (6, 7, 8, 10). The incidence of primary malignant osseous spinal tumour is rare in children with Ewing sarcoma and osteosarcoma being the most common causes. The 5-year survival in this population was 41% [16]
Discussion
In this study, we performed a systematic review of the literature on MSCC in the paediatric population and located 7 studies fulfilling our search criteria. Overall, neuroblastoma (67.2%) was identified as the most common tumour causing spinal cord compression in this paediatric population with soft tissue sarcomas being the most common cause above the age of 5 years. Thoracic spine was the most commonly affected site of cord compression (45.5%). Motor deficits were the presenting symptom in 95.6% of children followed by pain in 65.4% and sphincter disturbance in 24%. In the cohort of patients included there was a significant delay of 26 days between the onset of symptoms and final diagnosis by means of histopathology. The above represents a serious issue that should be taken into account in the treatment of the paediatric population as it may affect initiation of treatment and final outcome.
MSCC is s paediatric oncological emergency requiring early recognition and prompt institution of treatment to prevent neurological sequelae. It can cause significant morbidity in children with malignancy and can have profound effect on the quality of life [8]. Clinical presentation can range from minor sensory, motor, and/or autonomic changes to extreme pain, paralysis and bowel/bladder dysfunction [8]. Clinical manifestations of spinal cord compression in young children are difficult to diagnose at an early phase [17, 18].
It was found that first line modality of treatment varied significantly based on the tumour types and also differed among the study groups due to the absence of optimal evidence-based guidelines. The treatment strategies among the various study groups vary and are thus not directly comparable. There remains no standardised guideline to determine the modality of treatment, grading for assessment of neurological deficits or the parameters used to monitor neurological recovery.
However, there is a broad agreement in the literature with low-quality evidence about the management of MSCC in children as follows. Chemotherapy is equally effective as the other therapeutic modalities for the treatment of neuroblastoma/ lymphomas. First line chemotherapy has the advantage that no further specific therapy is needed and has the least risk for the development of long-term sequelae [9]
Sarcomas of the soft tissue or bone causing MSCC responded poorly to chemo-radiotherapy alone and the neurological outcome in patients treated with surgical decompression were significantly better [4]. Combined approach with chemotherapy, radiotherapy and surgery should be the treatment of choice for metastatic spinal sarcoma [4].
The duration of neurological symptoms and their severity are very likely to influence the neurological recovery, irrespective of the modality of treatment [7]—77% of patients presenting with mild neurological symptoms showed complete neurological recovery as against 40% of patients making complete recovery in those presenting with moderate to severe neurological symptoms [7]. Patients who presented within the 4 weeks of the onset of symptoms carried an improved outcome (78.6%) complete recovery of neurology v/s (25%) in patients presenting after 4 weeks (P = 0.008) [6]. This emphasises the importance of early diagnosis of MSCC in children. Positive outcomes are more common in children with MSCC in contrast to the situation in adult population, in terms of neurological recovery and long-term prognosis [4]. With regard to long-term sequelae, post-surgery or post-radiotherapy scoliosis represents a common pathology diagnosed in 31% of paediatric patients undergoing treatment for MSCC. The main mechanism for this seems to be chronic instability due to posterior column insufficiency or integral changes in bone density due to radiotherapy leading to structural changes on the immature paediatric skeleton. Long-term follow-up and observation versus bracing or surgical treatment must be carefully weighted in this population. Overall, for paediatric patients with MSCC, spinal surgery and paediatric oncology teams should be involved in a combined decision-making process regarding the management options for optimal results [19].
Limitations of the study
Malignant spinal cord compression in the paediatric population is a rare pathological entity. As a result, the amount of evidence available in the pertinent literature is limited and derives from Level III and Level IV evidence with heterogeneous data. Given, however, the clinical impact of the topic and the paucity of treatment algorithms, this systematic review, although narrow in terms of patient population and quality of evidence, provides data that may inform current treatment protocols.
Conclusion
Malignant cord compression in the paediatric population is a rare with devastating clinical sequelae. The main primary diagnoses include neuroblastoma and sarcomas with prognostication heavily depending on the histopathological and molecular background. Chemotherapy is the mainstay treatment in neuroblastoma, Ewing’s sarcoma and lymphoma. Overall, early surgery should be a consideration in patients presenting with neurological deficits and those with rapid deterioration of neurology despite oncological therapy. Multimodality approach including chemo-radiotherapy and surgery after meticulous multidisciplinary discussion should be the treatment of choice in paediatric malignancy leading to spinal cord compression.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Code availability
Not applicable.
References
Lewis DW et al (1986) Incidence, presentation, and outcome of spinal cord disease in children with systemic cancer. Pediatrics 78(3):438–443
Klein SL, Sanford RA, Muhlbauer MS (1991) Pediatric spinal epidural metastases. J Neurosurg 74(1):70–75
Lucia DM, Piero S, Simona V et al (2019) Symptomatic malignant spinal cord compression in children: a single-center experience. Ital J Pediatr 45:80
Tasdemiroglu E, Patchell RA (2001) Spinal cord compression caused by solid malignant tumours in children. Turk Neurosurg 11:101–107
Martino LD, Spennato P, Vetrella S et al (2019) Symptomatic malignant spinal cord compression in children: a single–center experience. Ital J Pediatr 45:80
Fawzy M, El-Beltagy M, El-Shafei M et al (2015) Intraspinal neuroblastoma: treatment options and neurological outcome of spinal cord compression. Oncol Lett 9:907–911
Katzenstein HM, Kent PM, London WB, Cohn SL (2001) Treatment and Outcome of 83 children with intraspinal neuroblastoma: the pediatric oncology group experience. J Clin Oncol 19(4):1047–1055. https://doi.org/10.1200/JCO.2001.19.4.1047
Tantawy A, Fatma SE, Mahmoud MA, Shepl OE (2013) Spinal cord compression in childhood pediatric malignancies: multicentric Egyptian study. J Pediatr Hematol Oncol 35:232–236
De Bernardi B, Pianca C, Pistamiglio P et al (2001) Neuroblastoma with symptomatic spinal cord compression at diagnosis: treatment and results with 76 cases. J Clin Oncol 19(1):183–190. https://doi.org/10.1200/JCO.2001.19.1.183
Pollono D, Tomarchia S, Drut R et al (2013) Spinal cord compression: a review of 70 pediatric patients. Pediatr Hematol Oncol 20(6):457–466
Abrahm JL (2004) Assessment and treatment of patients with malignant spinal cord compression. J Support Oncol 2:377–401
De Bernardi B, Balwierz W, Bejent J et al (2005) Epidural compression in neuroblastoma: diagnostic and therapeutic aspects. Cancer Lett 228:283–299
Siegal T (1995) Spinal cord compression: from laboratory to clinic. Eur J Cancer 31A:1748–1753
Raffel C, Neave VC, Lavine S, McComb JG (1991) Treatment of spinal cord compression by epidural malignancy in childhood. Neurosurgery 28:349–352
Mayfield JK, Riseborough EJ, Jaffe N et al (1981) Spinal deformity in children treated for neuroblastoma. J Bone Joint Surg 63:183–193
Vijay MR, Ilyas M, Meic H, Douglas L (2016) Primary osseous tumors of the pediatric spinal column: review of pathology and surgical decision making. Neurosurg Focus 41(2):E3
Punt J, Pritchard J, Pincott JR et al (1980) Neuroblastoma: a review of 21 cases presenting with intraspinal cord compression. Cancer 45:3095–3101
Hesketh E, Eden OB, Gattamaneni HR et al (1998) Spinal cord compression: do we miss it? Acta Paediatr 87:452–454
Sun H, Nemecek AN (2010) Optimal management of malignant epidural spinal cord compression. Hematol Oncol Clin North Am 24:537–551
Loblaw DA, Laperriere NJ (1998) Emergency treatment of malignant extradural spinal cord compression: an evidence-based guideline. J Clin Oncol 16:1613–1624
Hell AK, Kühnle I, Lorenz HM, Braunschweig L, Lüders KA, Bock HC, Kramm CM, Ludwig HC, Tsaknakis K (2020) Spinal deformities after childhood tumors. Cancers 12(12):3555
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MAH, NP and NQ contributed to the study conception and design. Data collection and analysis were performed by MAH and NP. The first draft of the manuscript was written by MAH and NP, and all authors commented on previous versions of the manuscript. Final version of the article reviewed by NQ and MAH.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Consent for publication
Not applicable
Consent to participate
Not applicable
Ethical approval
Not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quraishi, N.A., Palliyil, N., Hassanin, M.A. et al. Malignant spinal cord compression in the paediatric population—a systematic review, meta-analysis. Eur Spine J 32, 4306–4313 (2023). https://doi.org/10.1007/s00586-023-07820-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07820-3