Abstract
Purpose
Although risk factors for new low back pain (LBP) episodes and acute-to-chronic transition have been identified, risk factors for flares of LBP remain largely unknown. This case-crossover study aimed to identify: (1) risk factors LBP flares and (2) whether risk factors differed when flare is defined by pain increase (pain-defined flare: PDF) or identified by participants according to a broader flare definition that considered emotions and coping (self-reported flare: SRF).
Methods
One hundred and twenty-six participants with LBP for > 3 months were included. Candidate risk factors and flares (PDF/SRF) were assessed daily using a smartphone application for 28 days. Data on exposure to risk factors one, two and three days preceding PDF/SRF were compared to control periods. Conditional logistic regression estimated associations between risk factors and PDF/SRF.
Results
Odds of PDF and SRF were increased by poor sleep quality and morning pain. Good sleep quality reduced odds of flare. Odds for increased pain (PDF), but not SRF, were increased after days with higher afternoon and evening pain, fatigue, fear of physical activity and leisure physical activity.
Conclusion
LBP flare has been largely ignored but is more reflective of the LBP experience than conventional definitions of acute, sub-acute and chronic LBP. This study highlights risk factors for flare and that these differ depending on whether flare is defined by pain alone (PDF) or a broad multidimensional definition (SRF). Potential targets to reduce the intensity/frequency of LBP flares are identified, with strong indication for the potential role of sleep intervention to mitigate LBP flare risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In contrast to the conventional consideration of low back pain (LBP) as acute, sub-acute or chronic, for up to ~ 80% of individuals [1], LBP is an ongoing fluctuating condition, characterised by “flares” interspersed by periods of no or lesser pain. LBP flares have a major impact on quality of life [2] for individuals with persistent and short-term symptoms [3]. Research on risk factors for LBP has almost exclusively considered those associated with new episodes [4] or acute-to-chronic transition [5]. Although informative, these scenarios represent a small fraction of LBP cases. Understanding risk factors for LBP flare is likely to provide foundation for interventions to reduce their frequency and/or intensity.
Risk factors for flare could differ from those for a new episode or transition to chronicity for several reasons. First, most research considers retrospective exposure over long periods (e.g. smoking, typical physical activity [6]) rather than transient exposures relevant for LBP flare. Second, factors that are insufficient to cause a new episode could cause a flare (e.g. psychological factors do not provoke new episodes [7] but a transient change might induce a flare). Third, some factors considered as a consequence of LBP might also induce a flare (e.g. although pain interferes with sleep [8] transient poor sleep might increase pain [9]). People with LBP consider biological (e.g. physical activity and medication), psychological (e.g. stress), behavioural (e.g. sleep) and social (e.g. work characteristics) factors [10] are responsible for flares. Candidate risk factors should be assessed across domains.
Risk factors for LBP flare might depend on how a flare is defined. Although LBP flares [11] have been defined as a period of increased pain (e.g. 2-point increase on an 11-point scale), people with LBP do not consider pain intensity the only determinant of flare [12] as reflected in the consensus definition of flare: “A flare is an increase in symptoms that lasts from hours to weeks, is difficult to tolerate and generally impacts your usual activities and/or emotions” [13].
This study used a case-crossover design to compare periods that do (case) and do not (control) precede a flare to: (1) identify whether transient exposure to candidate risk factors is associated with LBP flare and (2) consider whether risk factors differ depending on how flare is defined (simple pain increase vs. participants’ interpretation of flare according to a multidimensional definition [13]).
Methods
Participants
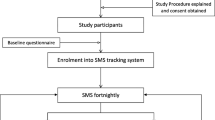
Participants were recruited through advertisements placed on social media and in the local community. Inclusion criteria were: age 18–50 years; LBP for at least three months; expectation of experiencing LBP for days/weeks over the following month; access to a smartphone/Internet; and understanding of English. Exclusion criteria included: spinal infection, fracture, or neoplasm; previous or forthcoming spinal surgery; rheumatoid arthritis; ankylosing spondylitis; and pregnancy in past year. Figure 1 summarises the participant flow. The Institutional Medical Research Ethics Committee approved the study and participants provided written informed consent.
Baseline and follow-up assessments
At baseline, participants completed questionnaires regarding LBP duration, average pain intensity over the past week, age, gender and comorbidities. Participants downloaded a smartphone application (RealLife Exp, Life Data, USA) to report data each day, for 28 consecutive days. The app provided brief questionnaires to participants, prompted them to respond, recorded responses and transmitted responses to the server. Participants entered data three times per day: morning (random time between 6–10 am), afternoon (random time between 12–6 pm) and evening (8 pm) (Fig. 2). Table 1 summarises assessed variables.
Identification of case and control periods. Case periods were defined as three days prior to a self-reported flare (SRF) or pain-defined flare (PDF). SRFs were defined by positive response to the question asked each evening regarding the participants’ own interpretation whether they had experienced a flare. PDFs were defined as day with pain of 2 or more points above the pain averaged across all days without a flare. The control period was defined as the three days that preceded a day with no flare
Identification of case and control periods
Two definitions of flare were used, based the participants’ daily responses (Fig. 2). First, self-reported flares (SRF) were identified by positive response to the question asked each evening regarding the participants’ own interpretation whether they had experienced a flare. Second, pain-defined flares (PDF) were identified as a pain increase of 2 or more points on the 11-point numerical rating scale [14] above the pain averaged across all days without a SRF. For analysis, we selected only flares (SRFs/PDFs) preceded by at least 3 days without a flare (pre-flare) to compare exposure to the potential risk factors across pre-flare/pre-no flare periods of that duration. The case period was defined as the three days prior to a SRF/PDF (Fig. 2). Similarly, the control period was defined as the three days that preceded a day with no flare (Fig. 2). Case and control periods were identified using MATLAB 2014b (The MathWorks, Natick, USA).
Statistical analysis
Univariate conditional logistic regression determined whether the variables differed during case and control periods. Only participants with both case and control periods were included in the analysis. Exposure to each variable was calculated for three different windows – one, two and three days preceding a SRF/ PDF. Odds ratios (OR) for the occurrence of a flare and 95% confidence intervals (95%CI) were calculated by comparing case and control windows. Analysis was conducted in Stata version 15 (StataCorp, TX, USA).
Results
Among 126 participants, 86 had both case and control periods. Thirty-one and one participants had no case or control periods that met our criteria, respectively, and eight withdrew. Characteristics of the analysed group are presented in Table 2. A total of 813 flare days were identified, including 465 days with SRF only, 222 with PDF only and 126 with SRF and PDF. Pain was reported every day by 48% of participants. For participants with some days without pain, this ranged from 1 to 20 consecutive days.
Risk factors for PDF
Means (standard deviation) of each variable for case and control days preceding a PDF or non-flare day, respectively, are presented in Table 3 and ORs (95%CI) in Fig. 3 (values are presented in Supplementary Table 1). Morning pain increased odds of experiencing a PDF one (OR [95%CI]—1.67 [1.43, 1.95]), two (1.35 [1.15, 1.58]) and three (1.16 [1.00, 1.34]) days later. Afternoon pain was associated with PDF one (1.74 [1.52, 2.00) and two (1.24 [1.06, 1.44]) days later and narrowly missed significance three days later (1.16 [1.00, 1.34]). Evening pain increased odds of a PDF one (1.33 [1.10, 1.61]) and three (1.22 [1.02, 1.47]) days later.
Association between changes in participant characteristics and odds of a pain-defined flare (PDF) starting one (black), two (grey) and three (white) days later. Odds ratio and 95% confidence intervals are shown. *—P < 0.05; RMDQ—Roland Morris Disability Questionnaire; PSE—Pain self-efficacy; PA–physical activity; Rumination–rumination about pain; OR could not be calculated when no exposures were recorded (e.g. “treatment” at 1 day before PDF)
Engaging in physical activity during leisure time increased odds of PDF one (1.66 [1.01, 2.74]) and two (1.73 [1.05, 2.85]) days later. Fatigue was associated with a PDF one day later (1.19 [1.06, 1.33]). Analysis of the continuous variable of sleep rate indicated better sleep quality lowered the odds of a PDF the next day (0.88 [0.79, 0.99]). When compared against the reference condition of “very good”, only the category of “very bad” sleep quality increased the odds of a PDF the next day (3.44 [1.11, 10.68]), and the confidence interval was wide.
Fear of physical activity (1.31 [1.04, 1.64]) was associated with greater odds of a PDF three days later, and a tendency was not significant at two days (1.21 [(0.98, 1.49]). There was no strong evidence of associations between PDF and questions that assessed rumination about pain and pain self-efficacy, or measures of disability, work, medication or treatment.
Risk factors for SRF
Means (standard deviation) of each variable for case and control days preceding a SRF or non-flare day, respectively, are presented in Table 4 and ORs (95%CI) in Fig. 4 (values are presented in Supplementary Table 2). Unlike PDF, when flares were identified based on self-report, only morning pain increased odds of a flare the next day (1.26 [1.05, 1.50]). Although not significant, pain in the afternoon tended to increase risk of SRF one, but not two or three, days later. Similar to PDF, higher sleep rate (continuous variable) lowered the odds of SRF on the following day (0.83 [0.72, 0.97]). When compared against the reference condition of “very good sleep”, reduction of sleep quality by just one category (“fairly bad” and “fairly good”) increased the odds of SRF one (fairly bad: 6.28 [2.09, 18.81]; fairly good: 2.98 [1.09, 8.16]) and two (fairly bad: 3.11 [1.04, 9.25]; fairly good: 3.14 [1.25, 7.93]) days later. No other variables increased the risk of SRF.
Association between changes in participant characteristics and odds of a self-reported flare (SRF) starting one (black), two (grey) and three (white) days later. Odds ratio and 95% confidence intervals are shown. *—P < 0.05; RMDQ—Roland Morris Disability Questionnaire; PSE—Pain self-efficacy; PA—physical activity; Rumination–rumination about pain; OR could not be calculated when no exposures were recorded (e.g. “treatment” at 1 day before PDF; “sleep: very bad” at 2 days before PDF)
Discussion
This study identified risk factors for LBP flare. There are three main findings. First, poor sleep quality, fatigue, leisure time physical activity and fear of physical activity increased the risk for a transient pain increase (PDF). Second, risk factors differed when flares were defined by a broader definition (SRF). Third, high sleep quality was protective regardless of the flare definition. These findings highlight potentially modifiable factors to target with interventions.
Risk factors differ between PDF and SRF
Risk factors differed if flare was defined by pain alone or a broader definition. Although the odds of experiencing both PDF and SRF was increased in the day(s) following higher pain, the relationship differed. When defined as PDF, pain in the morning, afternoon and/or evening increased risk for flare, up to three days later. One interpretation is that PDF simply represents the peak of a progressive increase in daily pain over 1–3 days and may simply reflect that pain fluctuates and sometimes exceeds a threshold used to define a flare [3, 14]. Participants did not necessarily consider these events to be a flare. When flare was defined using a broader definition, only pain in the preceding morning increased flare risk. There was no increased odds of a flare 2–3 days later and high afternoon or evening pain did not increase the odds of SRF. This suggests a different mechanism for SRF. For instance, rather than reflecting an overall fluctuation in pain, the association with morning pain might be explained by the immune response [15] related to poor sleep quality [15] that modulates nociception/pain [16].
Poor sleep quality was a risk factor for flares defined by both criteria, but SRF was more sensitive. Only the category of “very poor” sleep preceded a PDF, whereas subtle sleep deviations increased the odds of SRF up to 2 days later. This distinction between PDF and SRF may relate to the negative impact of poor sleep on features considered in broader dimensions of SRF – sleep quality impacts mood [17], affective anticipatory brain mechanisms (i.e. responses to threat/danger) [18] and emotional brain regulation [19]. Failure of “very bad” sleep to predict SRF relates to its few occasions. Validity of the association between sleep and PDF/SRF is supported by the contrasting observation that good sleep rate reduced the risk for both flare types. The protective nature of good sleep reinforces sleep interventions to reduce LBP flare.
The increased odds of PDF with fatigue and leisure-time physical activity differs from a recent case-crossover study of acute LBP (< 3 months) at 3- or 7-day intervals for six weeks. In that study, prolonged sitting (inactivity) increased risk of greater pain, whereas engagement in specific physical activities did not [11]. The different outcome between studies is best explained by difference in measure; this study focused on overall activity whereas the previous study assessed exposure to specific tasks (e.g. heavy lifting).
The relationship between physical activity and increased odds for PDF, but not SRF, strengthens the difference between flare definitions. The findings imply that to be identified as a SRF, increased pain is not sufficient and greater importance may be placed on other dimensions such as emotions and function [12]. Several issues might explain why leisure time physical activity did not increase the odds of SRF. First, benefits of leisure time physical activity extend beyond the physical domain [20], and although leisure time physical activity preceded increased pain, it might not be considered a SRF because of its positive effects on mood [21]. Second, acceptance (adapting behaviour to engage in activity despite symptoms [22]) might underlie the difference. This concurs with evidence that acceptance is associated with lower disability and negative emotions [23]. Likewise, increased pain after fatigue might not be considered a SRF because it is expected and accepted.
Of the psychological features, only fear of physical activity increased risk of PDF, significantly three days later, and with a tendency two days later. This might be a chance finding and requires replication before further consideration. The previous study of acute LBP found an association with stress and depression [11], which were not studied here.
Contextualizing findings
This is the first study to investigate flare defined by both increased pain and patients’ perspective, the first to do this continuously for 28 days, and the first to evaluate sleep quality, which was identified as the strongest risk factor. The only other evaluation of risk factors for flare evaluated people at 3- or 7-day intervals for six weeks, and analysis methods made it unclear whether features preceded or followed flare onset [11]. Our findings revealed some similarities between risk factors for PDF flares and those for a LBP episode [4]–physical activity increased risk for both. That study did not consider sleep or psychosocial aspects other than fatigue.
Study strengths and limitations
A strength of this study was the relatively large sample of participants who recorded data for 28 days. The case-crossover study design compares participants to themselves at different times, which controls for known and unknown confounding factors [24]. Thrice daily data collection allowed precise identification of timing of exposure, which limits temporal inaccuracy [11]. The use of smartphones for frequent data collection has acceptable reliability [25].
There are some limitations. First, similar to previous studies [26], fear of physical activity and rumination about pain were assessed using single questions rather than complete questionnaires. The questions cannot be considered to be equivalent or have the same psychometric properties as the questionnaire. Complete questionnaires were unfeasible for 28 days, and thus, selected questions focused on relationships between physical activity and flare. Second, our participant group was broad, and we cannot determine whether risk factors differ between individuals with specific LBP diagnoses or specific occupational groups. Third, risk factor exposures were identified through self-report. Further research should consider objective measures (e.g. wearable sensors). Fourth, the flare definition depends an individual’s interpretation. This could be considered a strength as it considers the patient’s personal experience. Fifth, we asked participants whether they had experienced a flare “today”, which may have anchored participants interpretation of a flare to a brief event rather than one that could last for days/weeks [13]. Finally, for morning pain, it is unknown whether participants woke with pain or whether it started during the morning.
Implications and conclusions
LBP fluctuates over time and it is important to differentiate between types of fluctuation across LBP trajectories. Risk factors for LBP flare depend on how it is defined. As risk factors for PDF and SRF differ, it is plausible that outcomes of trials of treatment efficacy and prognosis might be influenced by how flare is defined. We argue that SRF is likely to provide a measure that is more meaningful for a patient.
This study highlights the potential role of assessment and treatment of sleep in LBP management. Extending previous research that showed a reciprocal relationship between sleep and LBP [9], we revealed a strong relationship between sleep quality and subsequent LBP flare. As higher sleep rate was protective for LBP flares, strategies to improve sleep quality could potentially mitigate LBP flares.
Code availability
Not applicable.
References
Kongsted A, Kent P, Hestbaek L, Vach W (2015) Patients with low back pain had distinct clinical course patterns that were typically neither complete recovery nor constant pain. A latent class analysis of longitudinal data. Spine J 15:885–894
Young AE, Wasiak R, Phillips L, Gross DP (2011) Workers’ perspectives on low back pain recurrence: “it comes and goes and comes and goes, but it’s always there.” Pain 152:204–211. https://doi.org/10.1016/j.pain.2010.10.033
Suri P, Rainville J, Fitzmaurice GM, Katz JN, Jamison RN, Martha J, Hartigan C, Limke J, Jouve C, Hunter DJ (2011) Acute low back pain is marked by variability: An internet-based pilot study. BMC Musculoskelet Disord 12:220. https://doi.org/10.1186/1471-2474-12-220
Steffens D, Ferreira ML, Latimer J, Ferreira PH, Koes BW, Blyth F, Li Q, Maher CG (2015) What triggers an episode of acute low back pain? A case-crossover study. Arthritis Care Res 67:403–410. https://doi.org/10.1002/acr.22533
Campbell P, Foster NE, Thomas E, Dunn KM (2013) Prognostic indicators of low back pain in primary care: five-year prospective study. J Pain 14:873–883. https://doi.org/10.1016/j.jpain.2013.03.013
Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E (2010) The association between smoking and low back pain: a meta-analysis. Am J Med 123(87):e87–e35. https://doi.org/10.1016/j.amjmed.2009.05.028
Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM (2000) Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine 25:2114–2125
Tang NK, Wright KJ, Salkovskis PM (2007) Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res 16:85–95. https://doi.org/10.1111/j.1365-2869.2007.00571.x
Alsaadi SM, McAuley JH, Hush JM, Lo S, Lin CW, Williams CM, Maher CG (2014) Poor sleep quality is strongly associated with subsequent pain intensity in patients with acute low back pain. Arthritis Rheumatol 66:1388–1394. https://doi.org/10.1002/art.38329
Costa N, Hodges PW, Ferreira ML, Makovey J, Setchell J (2019) What Triggers an LBP Flare? A Content Analysis of Individuals’ Perspectives Pain Med. https://doi.org/10.1093/pm/pnz021
Suri P, Rainville J, de Schepper E, Martha J, Hartigan C, Hunter DJ (2018) Do Physical Activities Trigger Flare-ups During an Acute Low Back Pain Episode?: A Longitudinal Case-Crossover Feasibility Study. Spine 43:427–433. https://doi.org/10.1097/BRS.0000000000002326
Setchell J, Costa N, Ferreira M, Makovey J, Nielsen M, Hodges PW (2017) What constitutes back pain flare? A cross sectional survey of individuals with low back pain. Scand J Pain 17:294–301. https://doi.org/10.1016/j.sjpain.2017.08.003
Costa N, Ferreira ML, Setchell J, Makovey J, Dekroo T, Downie A, Diwan A, Koes B, Natvig B, Vicenzino B, Hunter D, Roseen EJ, Rasmussen-Barr E, Guillemin F, Hartvigsen J, Bennell K, Costa L, Macedo L, Pinheiro M, Underwood M, Van Tulder M, Johansson M, Enthoven P, Kent P, O’Sullivan P, Suri P, Genevay S, Hodges PW (2019) A Definition of “Flare” in Low Back Pain: A Multiphase Process Involving Perspectives of Individuals With Low Back Pain and Expert Consensus. J Pain 20:1267–1275. https://doi.org/10.1016/j.jpain.2019.03.009
Makovey J, Metcalf B, Zhang Y, Chen JS, Bennell K, March L, Hunter DJ (2015) Web-Based Study of Risk Factors for Pain Exacerbation in Osteoarthritis of the Knee (SPARK-Web): Design and Rationale. JMIR Res Protoc 4:e80. https://doi.org/10.2196/resprot.4406
Heffner KL, France CR, Trost Z, Ng HM, Pigeon WR (2011) Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain 27:35–41. https://doi.org/10.1097/ajp.0b013e3181eef761
Abbadie C (2005) Chemokines, chemokine receptors and pain. Trends Immunol 26:529–534. https://doi.org/10.1016/j.it.2005.08.001
Harvey AG, Murray G, Chandler RA, Soehner A (2011) Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev 31:225–235. https://doi.org/10.1016/j.cpr.2010.04.003
Goldstein AN, Greer SM, Saletin JM, Harvey AG, Nitschke JB, Walker MP (2013) Tired and apprehensive: anxiety amplifies the impact of sleep loss on aversive brain anticipation. J Neurosci 33:10607–10615. https://doi.org/10.1523/JNEUROSCI.5578-12.2013
Gujar N, McDonald SA, Nishida M, Walker MP (2011) A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex 21:115–123. https://doi.org/10.1093/cercor/bhq064
Sanchez-Villegas A, Ara I, Dierssen T, de la Fuente C, Ruano C, Martinez-Gonzalez MA (2012) Physical activity during leisure time and quality of life in a Spanish cohort: SUN (Seguimiento Universidad de Navarra) project. Br J Sports Med 46:443–448. https://doi.org/10.1136/bjsm.2010.081836
Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, McMurdo M, Mead GE (2013) Exercise for depression. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004366.pub6
McCracken LM, Vowles KE (2006) Acceptance of chronic pain. Curr Pain Headache Rep 10:90–94. https://doi.org/10.1007/s11916-006-0018-y
Serbic D, Pincus T (2017) The relationship between pain, disability, guilt and acceptance in low back pain: a mediation analysis. J Behav Med 40:651–658. https://doi.org/10.1007/s10865-017-9826-2
Maclure M, Mittleman MA (2000) Should we use a case-crossover design? Annu Rev Public Health 21:193–221. https://doi.org/10.1146/annurev.publhealth.21.1.193
Johansen B, Wedderkopp N (2010) Comparison between data obtained through real-time data capture by SMS and a retrospective telephone interview. Chiropr Osteopat 18:10. https://doi.org/10.1186/1746-1340-18-10
Gerhart JI, Burns JW, Post KM, Smith DA, Porter LS, Burgess HJ, Schuster E, Buvanendran A, Fras AM, Keefe FJ (2017) Relationships Between Sleep Quality and Pain-Related Factors for People with Chronic Low Back Pain: Tests of Reciprocal and Time of Day Effects. Ann Behav Med 51:365–375. https://doi.org/10.1007/s12160-016-9860-2
Funding
This study is supported by a Program grant (APP1091302); Centre of Research Excellence grant (APP1079078) and Fellowships (PH—APP1102905; MF—APP1143593) from the National Health and Medical Research Council (NHMRC) of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to declare.
Availability of data and material
Available on request.
Consent to participate
Participants provided written informed consent.
Ethics approval
The University of Queensland Approval #: 2,010,000,045.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Costa, N., Smits, E., Kasza, J. et al. ISSLS PRIZE IN CLINICAL SCIENCE 2021: What are the risk factors for low back pain flares and does this depend on how flare is defined?. Eur Spine J 30, 1089–1097 (2021). https://doi.org/10.1007/s00586-021-06730-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-06730-6