Abstract
Purpose
To determine the incidence of and risk factors for residual back pain in osteoporotic vertebral compression fracture (OVCF) patients after percutaneous kyphoplasty (PKP) treatment, we performed a retrospective analysis of prospective data.
Methods
Patients who underwent bilateral PKP and met this study’s inclusion criteria were retrospectively reviewed. Back pain intensity was assessed using a visual analogue scale (VAS) after surgery. Residual back pain was defined as the presence of postoperative moderate-severe pain (average VAS score ≥ 4), and the variables included patient characteristics, baseline symptoms, radiological parameters and surgical factors. Univariate and multivariate logistic regression analyses were performed to identify risk factors.
Results
A total of 809 patients were included, and residual back pain was identified in 63 (7.8%) patients. Of these patients, 52 patients had complete data for further analysis. Multivariate logistic regression analysis showed that risk factors for back pain included the presence of an intravertebral vacuum cleft (OR 2.93, P = 0.032), posterior fascia oedema (OR 4.11, P = 0.014), facet joint violations (OR 12.19, P < 0.001) and a separated cement distribution (OR 2.23, P = 0.043).
Conclusion
The incidence of postoperative residual back pain was 7.8% among 809 OVCF patients following PKP. The presence of an intravertebral vacuum cleft, posterior fascia oedema, facet joint violations and a separated cement distribution were identified as independent risk factors for residual back pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is the most common metabolic bone disorder, characterized by bone mineral loss and compromised bone strength, and it is the major cause of vertebral compression fractures typically present with sudden onset back pain in the elderly population [1]. Osteoporotic vertebral compression fractures (OVCFs) are becoming a common source of back pain and progressive spinal deformity, reducing quality of life and becoming an increasingly serious health problem worldwide [2,3,4].
Treatment for OVCFs includes conservative and surgical approaches. Most painful OVCFs are successfully managed with conservative treatment, including analgesic medication, stability interventions and physical therapy [5, 6]. Percutaneous kyphoplasty (PKP) is a minimally invasive surgical method that has come to be a well-accepted option for symptomatic OVCFs and OVCFs that fail to respond to conservative treatments [7]. Although PKP provides quick pain relief, improved physical function and good restoration of vertebral height, some patients still experience considerable residual back pain [8,9,10].
Several studies have reported a variety of risk factors for obvious residual back pain after surgery, including infection, cement leakage, a non-healing bone-cement interface and idiopathic pain [11,12,13]. However, some of the factors are still controversial or rarely mentioned, and a few conclusions may be constrained by the small sample population. Therefore, the current study aimed to analyse the incidence and independent risk factors for moderate-to-severe residual back pain following PKP.
Patients and methods
Study design and patients
This study was approved by the ethics committee of our hospital, and all subjects gave informed consent to participate in the study. We retrospectively analysed prospectively collected data from consecutive patients with OVCFs who underwent bilateral PKP from March 2015 to April 2019 in our hospital. The inclusion criteria were an acute (within 2 weeks) or subacute (within 2–8 weeks) vertebral compression fracture with a score of five or more on the visual analogue scale (VAS) for back pain, a hypointense signal on T1-weighted images and a hyperintense signal on T2-weighted images via magnetic resonance imaging (MRI), decreased bone mineral density (T scores less than − 1.5), a fracture level lower than T5 and three or less fracture segments. The exclusion criteria included patients younger than 55 years of age, vertebral body posterior column fractures, the spinal canal having been invaded by retropulsion of bony fragments, spinal infection, pathological fractures and chronic back pain prior to trauma, including a history of previous spinal decompression or fusion surgery, spondylolisthesis and unidentified pain. Patients with severe cardiopulmonary comorbidity, cognitive disorders or cerebral disease who could not communicate independently were also excluded.
Surgical procedures and management
Patients were placed in a prone position on the operating table and received local or general anaesthesia. Next, bone puncture trocars were placed bilaterally through the lateral margin of the pedicles at the fractured level and progressively passed through the pedicles into the vertebral body under C-arm guidance. Then, an inflatable bone balloon was used, and polymethylmethacrylate (PMMA) was injected carefully into the vertebral body. The injection was stopped if the cement reached the cortical edge of the vertebral body or if it leaked into extraosseous structures or veins. After the procedure, the patients were maintained in a prone position for 10–15 min. All patients were restricted to bed rest after the procedure and encouraged to ambulate on the first day after PKP. To assist ambulation, patients were required to wear a brace for at least 1 month. Patients also received postoperative antero-posterior and lateral plain radiographs and computed tomography (CT).
Patients were prescribed calcium tablets (600–1200 mg/day), calcitriol (0.25–0.5 μg/day) and alendronate (70 mg/week) after surgery. Regarding pain management, patients who acquired adequate pain relief were not prescribed any analgesic drug, and those who still suffered from inadequate pain relief or pain obviously affecting basic daily life were prescribed non-steroidal anti-inflammatory drugs (NSAIDs); non-opioid analgesic drug was added if the NSAIDs were not effective.
Dependent variable
The VAS scores of the included patients were collected during one month of follow-up at five time points, including 1, 3, 7, 14 and 30 days after surgery. The dependent variable was the presence of moderate-to-severe back pain, and based on the average intensity of back pain assessed postoperatively (1, 3, 7, 14 and 30 days after surgery) using a 10-cm VAS, in which a score of 0 represents no pain and a score of 10 represents the worst conceivable pain, moderate-to-severe residual back pain was defined as a VAS score greater than or equal to 4 [14, 15]. Therefore, patients were divided into a back pain group (VAS score greater than or equal to 4) and a control group (VAS score less than 4) for further analysis.
Independent variables
Basic patient data, including age, sex, body mass index (BMI), bone mineral density (BMD), smoking status, fracture type (acute or subacute fractures) and baseline symptoms, including the preoperative VAS score and Oswestry Disability Index (ODI), were obtained from the medical records.
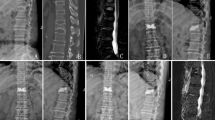
Baseline radiographic parameters included the following: (1) vertebral height loss, i.e. the percentage of anterior vertebral body height relative to the average anterior vertebral body height of the upper and lower adjacent levels (Fig. 1); (2) local kyphotic angle, the angle between the superior and inferior endplate of the fractured level (Fig. 1); (3) thoracic kyphosis angle; (4) intravertebral vacuum cleft; and (5) posterior fascia injury (Fig. 2).
a Vertebral compression fracture without fascia injury shows no remarkable signal changes on T1 WI (A1), T2 WI (A2) and T2-STIR WI (A3) MRI images. b Vertebral compression fracture with fascia injury shows low signal intensity on T1 WI (B1) and high signal intensity on T2 WI (B2) and T2-STIR WI (B3)
Surgical parameters, including anaesthesia, operation time, number of fracture segments and cement volume, were collected from the operative records.
Postoperative radiographic parameters included (1) vertebral height restoration, i.e. the percentage of the anterior vertebral body restoration height relative to the average anterior vertebral body height of the upper and lower adjacent levels (Fig. 1); (2) facet joint violations, defined as needle trajectory contacts with joint surfaces or travel within the facet joint in postoperative CT images (Fig. 3) [9, 16]; (3) cement leakage, including leakage in veins, discs and the spinal canal; and (4) cement distribution, including separated and confluent cement masses [17]. A separated cement distribution was defined when the bilateral cement masses were isolated and rarely connected to each other, and a confluent cement distribution was defined when the bilateral cement masses were integrated into a whole (Fig. 4).
Statistical analysis
The continuous variables are presented as the mean ± standard deviation. Student’s t test was used for comparisons of continuous variables, and the Chi-square test or Fisher’s exact test was applied for analysing categorical variables. Variables associated with significant differences between the two groups were considered potential risk factors for residual back pain in the final multivariate stepwise logistic regression model. Statistical analysis was performed using SPSS 19.0(IBM, Chicago, IL). Differences were considered statistically significant at P < 0.05.
Results
Demographics and change in pain intensity
According to the inclusion and exclusion criteria, a total of 952 patients were included, and 809 patients completed the follow-up (follow-up rate: 85.0%). Mean pain intensity (as measured by the VAS score) decreased significantly after surgery (7.2 ± 2.1 vs. 3.2 ± 1.3; P < 0.001). Among the patients completing the follow-up, 63 (7.8%) patients were identified as having moderate-to-severe residual back pain (back pain group), but 11 patients were excluded due to a lack of radiographic information or other medical reasons. Finally, 52 patients were included in the analysis; 34 were females, and 18 were males, with an average age of 76.2 ± 8.9 years. Another 163 patients who were identified as having no or mild pain and who underwent PKP surgery during the same period were included as the control group. As shown in Table 1, although patients in the two groups were presented with gradual pain relief during the postoperative follow-up, the VAS score of the residual pain group was significantly higher than that of the control group at 1, 3, 7 and 14 days after surgery.
Univariate analysis
Comparisons of patient-independent variables between the control and residual back pain groups are summarized in Table 2. The comparison of results for the patients in the control group and the residual back pain group indicated that there were no significant differences between the two groups regarding anaesthesia (P = 0.621), operation time (P = 0.097) and cement volume (P = 0.052). The results also showed that the following factors were significantly different between the control and back pain groups: intravertebral vacuum cleft (P = 0.014), posterior fascia injury (P = 0.002), number of fracture segments (P = 0.043), facet joint violations (P < 0.001) and a separated cement distribution (P = 0.017). Collectively, these data indicated that the above factors were potentially associated with postoperative back pain and were included in the multivariate analysis.
Multivariate analysis
Multivariate logistical regression analysis was performed to detect independent risk factors for back pain. As shown in Table 3, intravertebral vacuum cleft (OR 2.93; 95% CI 1.10–7.81; P = 0.032), posterior fascia oedema (OR 4.11; 95% CI 1.33–12.74; P = 0.014), facet joint violations (OR 12.19; 95% C = 3.55–41.84; P < 0.001) and a separated cement distribution (OR 2.23; 95% CI 1.03–4.84; P = 0.043) were identified as independent risk factors for residual back pain.
Discussion
Although percutaneous kyphoplasty (PKP) is an available and effective therapy for the management of pain resulting from osteoporotic vertebral compression fractures (OVCFs), postoperative adverse events, including pulmonary embolism, infection, and insufficient postoperative pain relief, have negative impacts on clinical outcomes [7, 18, 19]. Of these complications, residual back pain considerably decreases surgical satisfaction and quality of life because the main problem resulting from OVCFs is severe pain [9]. Therefore, in the current study, we measured the incidence of residual back pain and determined potential risk factors by retrospectively reviewing OVCF patients treated with PKP.
Several studies have reported the presence of back pain after surgery. Barr et al. [20] included a total of 38 OVCF patients and reported nearly 5% of patients with no significant pain relief and 12% of patients with moderate pain relief after surgical treatment. Cumulative evidence has demonstrated that a minority of patients still suffer from back pain after kyphoplasty treatment. A randomized trial found 20 of 149 patients was considered to have back pain, which is one of the most common adverse events in this study [10]. In another study, approximately 6.3% and 7.3% of patients were identified with procedural pain and residual back pain, assessed within 7 days postoperatively, after kyphoplasty, respectively [8]. In the present study, the incidence of residual back pain after kyphoplasty is 7.8%, which is similar to that reported in previous studies.
An intravertebral vacuum cleft (IVC) has been described as a sign of spinal instability and a cause of persisting pain. Several studies have shown the disadvantageous effects of the presence of an IVC on clinical outcomes of OVCFs following surgical treatment. Yu et al. [21] demonstrated that the immediate postoperative VAS score for OVCFs with IVCs is remarkably higher than that of OVCFs without IVCs after surgery. Li et al. [22] reported that OVCF patients with IVC have relatively higher VAS scores, which range from 3.8 to 4.0 at the follow-up after PKP treatment. In contrast, a comparative study by Wu et al. [23] reported that the improvement in VAS and ODI scores and the radiographic parameters of the groups with and without IVC were not significantly different after PKP. This contradiction may be due to patients who had a coexisting vertebral fracture with or without an IVC. In line with these studies, our results revealed that IVCs contribute to residual back pain after kyphoplasty.
Moreover, posterior fascial traumatic oedema identified by MRI was another risk factor for postoperative pain in the current study. Although most of the OVCFs resulted from low energy impacts or were even caused by hidden trauma, nearly 7.4% (16/215) of the patients still had a fascial injury and high VAS score. A previous study showed that OVCF patients with a concomitant thoracolumbar fascia injury did not respond well to their pain relief after treatment, indicating that there may be a relationship between thoracolumbar fascia injury and residual back pain [24]. One plausible explanation for this result is that the thoracolumbar fascia is rich in nerve endings.
The finding that facet joint violation is a risk factor for residual pain has rarely been mentioned in previous studies. Facet joint violation has mainly been reported in studies about pedicle screw instrumentation fixation for burst fractures and degeneration disease, where it is one of the major complications and closely related to postoperative pain. Levin et al. [25] reported that patients who have facet joint violations have significantly worse quality of life improvements when compared with control patients. With regard to the relationship between facet joint violations and kyphoplasty, our previous study revealed that approximately 9.6% of facet joints were invaded by puncture needles after kyphoplasty for OVCFs [26]. Yan et al. [27] demonstrated that seven patients had remarkable pain at the puncture sites 1 month after surgery and speculated that the pain was related to facet joint violations because of the pain disappearance with a local block treatment. Li et al. [9] reported that facet joint violations have a detrimental effect on clinical outcomes, as they are associated with high VAS scores and low surgical satisfaction. In our study, facet joint violations have been identified as a risk factor for residual pain. The reason for this may be that the facet joint and capsule ligament are full of mechanoreceptors and pain-detecting nociceptors, which are susceptible to direct mechanical impairment. Another explanation for this result is related to the deleterious effects of facet joint violations on secondary inflammatory reactions and the presence of osteoarthritis.
The relationship between cement injection and clinical outcomes in the treatment of OVCFs was noticed in several studies. Previous studies indicated that cement injection volume is the key factor for enhancing mechanical stability by filling the cavity of bone trabecula, and the ideal value of cement notably influences pain relief and the prevention of cement-related complications [28]. However, no difference was found between the two groups regarding cement injection volume in the current study. On the other hand, cement distribution also played a critical role in postoperative outcomes. He et al. [29] found that a small cement volume with an extensive distribution provides positive restoration as does a large cement volume with a confined distribution. Additionally, Liu et al. [17] reported that their confluent cement distribution group obtained better short-term pain relief than those in their separated cement distribution group, and the authors suggested that a separated cement distribution results in operative back pain owing to the exposed damaged part still suffering from nerve ending stimulation, unstable vertebral micro-fracture and micro-motion under a single side load. Consistent with these studies, we found that the rate of separated cement distributions in the back pain group was higher than that in the control group, and multivariate logistic regression analysis showed that a separated cement distribution was an independent risk predictor for residual back pain after PKP treatment.
There are certainly limitations to determining the risk factors for residual back pain after kyphoplasty in OVCFs, and further study and investigation is still required. For example, because the number of patients was relatively small and the sample was from a single institution, some of the potential factors mentioned by a previous study, including rib fracture, infection, new symptomatic compression fracture and serious cement leakage, did not occur [12]. Therefore, a relatively large sample size and multicentre design would allow for a better understanding. In addition, smoking has been regarded as having a negative role in the clinical outcomes of surgical treatment for spinal diseases [14]. However, this situation was not found in the current study, and the discrepancy may be the result of some patients hiding their smoking history or the lack of a dose-dependent analysis between the amount of lifetime smoking and the pain score. We also noticed that some residual back pain may be partly affected by other spinal pathologies because elderly patients often have accompanying spinal degenerative diseases. Moreover, the patients were divided into two groups based on their average pain score within 1 month of surgery, and a long-term, multiple-time-point follow-up would be more persuasive. Nevertheless, several risk factors for residual back pain were confirmed, at least in part, and they will be important for improving surgical efficiency.
In conclusion, the incidence of residual back pain after kyphoplasty in OVCFs was 7.8%. Through a matched case–control study evaluating various parameters, the presence of an intravertebral vacuum cleft, posterior fascia oedema, facet joint violations and a separated cement distribution were identified as independent risk factors for residual back pain. Patients with these factors also had remarkably lower functional outcomes following surgery for OVCFs than other patients. Surgeons should pay attention to these risk factors and adequately manage them to improve surgical effectiveness and satisfaction.
References
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733. https://doi.org/10.1007/s00198-006-0172-4
Lau E, Ong K, Kurtz S, Schmier J, Edidin A (2008) Mortality following the diagnosis of a vertebral compression fracture in the medicare population. J Bone Joint Surg Am 90:1479–1486. https://doi.org/10.2106/JBJS.G.00675
Lindsay R, Burge RT, Strauss DM (2005) One year outcomes and costs following a vertebral fracture. Osteoporos Int 16:78–85. https://doi.org/10.1007/s00198-004-1646-x
Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA (2018) Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty “sham” trials? Osteoporos Int 29:375–383. https://doi.org/10.1007/s00198-017-4281-z
Expert Panels on Neurological Imaging IR, Musculoskeletal I, Shah LM, Jennings JW, Kirsch CFE, Hohenwalter EJ, Beaman FD, Cassidy RC, Johnson MM, Kendi AT, Lo SS, Reitman C, Sahgal A, Scheidt MJ, Schramm K, Wessell DE, Kransdorf MJ, Lorenz JM, Bykowski J (2018) ACR appropriateness criteria((R)) management of vertebral compression fractures. J Am Coll Radiol JACR 15:S347–S364. https://doi.org/10.1016/j.jacr.2018.09.019
Mattie R, Laimi K, Yu S, Saltychev M (2016) Comparing percutaneous vertebroplasty and conservative therapy for treating osteoporotic compression fractures in the thoracic and lumbar spine: a systematic review and meta-analysis. J Bone Joint Surg Am 98:1041–1051. https://doi.org/10.2106/JBJS.15.00425
Garfin SR, Yuan HA, Reiley MA (2001) New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 26:1511–1515
Dohm M, Black CM, Dacre A, Tillman JB, Fueredi G, investigators K (2014) A randomized trial comparing balloon kyphoplasty and vertebroplasty for vertebral compression fractures due to osteoporosis. AJNR Am J Neuroradiol 35:2227–2236. https://doi.org/10.3174/ajnr.A4127
Li Y, Huang M, Chen J, Wu Y, Wang X (2018) The impact of facet joint violation on clinical outcomes after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. World Neurosurg 119:e383–e388. https://doi.org/10.1016/j.wneu.2018.07.170
Rodriguez AJ, Fink HA, Mirigian L, Guanabens N, Eastell R, Akesson K, Bauer DC, Ebeling PR (2017) Pain, quality of life, and safety outcomes of kyphoplasty for vertebral compression fractures: report of a task force of the American society for Bone and Mineral Research. J Bone Miner Res 32:1935–1944. https://doi.org/10.1002/jbmr.3170
Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P (2000) Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology 39:1410–1414. https://doi.org/10.1093/rheumatology/39.12.1410
Lin CC, Shen WC, Lo YC, Liu YJ, Yu TC, Chen IH, Chung HW (2010) Recurrent pain after percutaneous vertebroplasty. AJR Am J Roentgenol 194:1323–1329. https://doi.org/10.2214/AJR.09.3287
Lin CC, Wen SH, Chiu CH, Chen IH, Yu TC (2009) The clinical influence of fluid sign in treated vertebral bodies after percutaneous vertebroplasty. Radiology 251:866–872. https://doi.org/10.1148/radiol.2513080914
Kimura A, Shiraishi Y, Inoue H, Endo T, Takeshita K (2018) Predictors of persistent axial neck pain after cervical laminoplasty. Spine 43:10–15. https://doi.org/10.1097/BRS.0000000000002267
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17:45–56
Babu R, Park JG, Mehta AI, Shan T, Grossi PM, Brown CR, Richardson WJ, Isaacs RE, Bagley CA, Kuchibhatla M, Gottfried ON (2012) Comparison of superior-level facet joint violations during open and percutaneous pedicle screw placement. Neurosurgery 71:962–970. https://doi.org/10.1227/NEU.0b013e31826a88c8
Liu H, Zhang J, Liang X, Qian Z, Zhou Z, Lu H, Bou EH, Meng B, Mao H, Yang H, Liu T (2019) Distribution pattern making sense: patients achieve rapider pain relief with confluent rather than separated bilateral cement in percutaneous kyphoplasty for osteoporotic vertebral compression fractures. World Neurosurg. https://doi.org/10.1016/j.wneu.2019.03.063
Chen YJ, Tan TS, Chen WH, Chen CC, Lee TS (2006) Intradural cement leakage: a devastatingly rare complication of vertebroplasty. Spine 31:E379–E382. https://doi.org/10.1097/01.brs.0000219495.57470.67
Wang LJ, Yang HL, Shi YX, Jiang WM, Chen L (2012) Pulmonary cement embolism associated with percutaneous vertebroplasty or kyphoplasty: a systematic review. Orthopaed Surg 4:182–189. https://doi.org/10.1111/j.1757-7861.2012.00193.x
Barr JD, Barr MS, Lemley TJ, McCann RM (2000) Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine 25:923–928
Yu W, Liang Yao Z, Qiu T, Ye L, Jiang X (2017) The therapeutic effect of intravertebral vacuum cleft with osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Int J Surg 40:17–23. https://doi.org/10.1016/j.ijsu.2017.02.019
Li Z, Liu T, Yin P, Wang Y, Liao S, Zhang S, Su Q, Hai Y (2019) The therapeutic effects of percutaneous kyphoplasty on osteoporotic vertebral compression fractures with or without intravertebral cleft. Int Orthop 43:359–365. https://doi.org/10.1007/s00264-018-4007-7
Wu AM, Lin ZK, Ni WF, Chi YL, Xu HZ, Wang XY, Huang QS (2014) The existence of intravertebral cleft impact on outcomes of nonacute osteoporotic vertebral compression fractures patients treated by percutaneous kyphoplasty: a comparative study. J Spinal Disorders Tech 27:E88–E93. https://doi.org/10.1097/BSD.0b013e31829142bf
Yan Y, Xu R, Zou T (2015) Is thoracolumbar fascia injury the cause of residual back pain after percutaneous vertebroplasty? A prospective cohort study. Osteoporos Int 26:1119–1124. https://doi.org/10.1007/s00198-014-2972-2
Levin JM, Alentado VJ, Healy AT, Steinmetz MP, Benzel EC, Mroz TE (2018) Superior segment facet joint violation during instrumented lumbar fusion is associated with higher reoperation rates and diminished improvement in quality of life. Clin Spine Surg 31:E36–E41. https://doi.org/10.1097/BSD.0000000000000566
Li Y, Wang X, Jiang K, Chen J, Lin Y, Wu Y (2019) Incidence and risk factors of facet joint violation following percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Radiol 60:755–761. https://doi.org/10.1177/0284185118799515
Yan L, He B, Guo H, Liu T, Hao D (2016) The prospective self-controlled study of unilateral transverse process-pedicle and bilateral puncture techniques in percutaneous kyphoplasty. Osteoporos Int 27:1849–1855. https://doi.org/10.1007/s00198-015-3430-5
Fu Z, Hu X, Wu Y, Zhou Z (2016) Is there a dose-response relationship of cement volume with cement leakage and pain relief after vertebroplasty? Dose Response 14:1559325816682867. https://doi.org/10.1177/1559325816682867
He X, Li H, Meng Y, Huang Y, Hao DJ, Wu Q, Liu J (2016) Percutaneous kyphoplasty evaluated by cement volume and distribution: an analysis of clinical data. Pain Phys 19:495–506
Acknowledgements
This work was supported by grants from the Zhejiang Public Service Technology Research Program and Social Development (LGF18H060008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Y., Yue, J., Huang, M. et al. Risk factors for postoperative residual back pain after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Eur Spine J 29, 2568–2575 (2020). https://doi.org/10.1007/s00586-020-06493-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-020-06493-6