Abstract
Purpose
This study aimed at investigating the effects of different body positions and axial loads on spinal stiffness to better understand spinal stabilisation mechanisms.
Methods
The posterior-to-anterior lumbar and thoracic spinal stiffness of 100 young healthy adults (mean age 23 years; 50 females) were measured in three test situations: prone, standing and standing while carrying a load equal to 50% of the subject’s body weight. Each test situation comprised three trials.
Results
Spinal stiffness in all test situations showed good reliability. Repeated measures analysis of covariance showed significantly higher spinal stiffness in standing than in the prone position [F(1/1694) = 433.630, p < 0.001]. However, spinal stiffness was significantly lower when standing while carrying a load of 50% of the body weight than when standing without additional load [F(1/1494) = 754.358, p < 0.001].
Conclusion
This study showed that spinal lumbar and thoracic stiffness increases when body position is changed from prone to standing. Additional axial load of 50% of the subject’s body weight results in reduced spinal stiffness during standing.
Graphic abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Control of the human posture during walking or sit-to-stand transfer is a critical aspect of everyday life. Human motor control coordinates muscle recruitment to provide postural stability [1]. Postural control is maintained by sensory inputs from the vestibular and visual systems as well as proprioception, all of which are processed by the central nervous system [1]. Postural control, which is produced by the passive, active and neurological subsystems, can be assessed by measuring spinal stiffness [2]. Measurements of posterior-to-anterior spinal stiffness have been used for the diagnostics, management and treatment of patients with low back pain [3]. These can provide useful biomechanical information for clinical decision making [4] and help to determine whether a joint is hypo- or hypermobile [5]. It is well known from the literature that postural control is load-dependent [6]. Carrying a load or a change in body position leads to a change in spinal stiffness [7,8,9]. Assessing stiffness in different body positions is important because the orientation of the spine towards gravity or changes in loading directly impact spinal stiffness [8, 9]. So far, spinal stiffness measurements have predominantly been performed in resting prone position. In such a context, stiffness assessment mostly excludes the muscular contribution of the motor control system [7]. Change of position from prone to upright results in an increased spinal muscle activity to stabilise the spine towards gravity [8, 9]. In the prone position, most of this stability is achieved by the inherent tension from passive muscle stiffness, ligaments and joint capsules [9].
To facilitate further research, normative data on spinal stiffness of asymptomatic individuals are required [4]. Moreover, information regarding systematic comparison of spinal stiffness with different body positions and/or axial loadings is not available [10]. A better understanding of spinal stiffness may result in novel insights regarding spinal stabilisation. Therefore, this study aimed to investigate the effects of body positions and axial load on spinal stiffness.
Methods
A total of 100 young healthy subjects of the age group 18–30 years were recruited; written informed consent was obtained from all participants. Subjects were excluded from the study if they had acute back pain (thoracic or lumbar), a history of significant back pain (thoracic or lumbar) or radiating pain down the leg, contraindications to spinal mobilisation/manipulation, spinal fractures or spinal tumours. Also, subjects who previously had a surgical intervention in the thoracic or lumbar spine or experienced a local infection of the spine or the surrounding tissue were excluded. Measurements were conducted at the Balgrist University Hospital, Zurich, Switzerland. The ethics committee of the Canton of Zürich approved this study BASEC-Nr: 2017-01245 and registered ClinicalTrials.gov Identifier: NCT03495843.
Data collection procedures
The first step was to obtain demographic data such as weight, sex, age and height of each participant. This and the spinal stiffness measurement were performed by two medical students, who were thoroughly trained in the use of the mechanical indenter and ultrasound device. All stiffness measurements were taken by the same examiner. To prevent bias, the exact location of the spinous process of L5 was determined with the help of ultrasound. For this purpose, a portable ultrasound device, the Aloka SSD-500 (Aloka Co, Tokyo, Japan) with an Aloka UST-934N-3.5 Electronic Convex Probe, was used. The other spinous processes were manually identified and marked with ink to label the position for indenter placement. To increase accuracy, the marking was verified by both examiners. Before the measurements were initiated, a familiarisation indentation trial in the prone position was conducted to minimise the subject’s anxiety. Because spinal stiffness is influenced by various factors, such as pain [11], increased abdominal pressure [12] and the respiratory cycle [13], subjects were instructed to inhale and exhale comfortably and then hold their breath at the end of a normal exhalation [13]. For the comfort of the participants, a short break was allowed after thoracic and before the lumbar measurements for one breathing cycle.
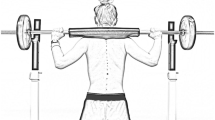
The thoracic and lumbar spinal stiffness of the participants was assessed in three test conditions: prone, neutral standing upright and standing with an additional axial load. The axial loading was accomplished with the help of a long weight bar which the participants were carrying while standing. The weight on the bar was adjusted to equal 50% of the participant’s body weight (standing + 50%). The first test measurement was performed in the prone position, the second in the standing position, and the third with the additional axial loading. Each test comprised three trials. Between each measurement, there was a 2-min break to ensure viscoelastic recovery before the next trial [14]. In the prone position, the participants were laying on a medical couch. For safety reasons, the measurements obtained with additional axial load (standing + 50%) were performed with a squat rack. The long weight bar was placed in the squat rack, slightly below shoulder height of the subject, and 50% of the participant’s body weight (± 0.5%) was put on it. The position of the feet was directly under the middle of the bar, and the hands were evenly spaced. Shortly before taking the measurement, the subject was instructed to lift the bar and remain in the standing position. When the subject was in a stable standing position, the stiffness measurements were taken (see Fig. 1).
Assessments
The spinal stiffness was assessed using a device which measures tissue compliance by employing the concept of impulse response [10, 15]. An impulse is generated by the device and applied to the spine. A force transducer of the device measures the response, the impulse response. The impulse response is the compliance of the muscles, joints and connected structures to the energy generated by the impulse or stiffness [15]. The compliance of the involved tissues by approximation corresponds to a linear time-invariant system and the impulse to a very brief (< 1 ms) input signal. Therefore, the impulse response completely characterises this compliance [16, S. 147ff]. It can be thought of as force with no change in time. Thus, the units of output are Newton (force). This method has the advantage that it can measure spinal stiffness in different body positions. To measure posterior-to-anterior spinal stiffness, a computer-assisted analytic device (PulStar Function Recording and Analysis System, PulStarFRAS, Sense Technology, Inc, Pittsburgh, PA, USA) was used [15]. A force of 80 N was applied from the device to the spinous process. To trigger the measurement, a preload of 18 N was applied to overcome possible confounders caused by the soft tissue components between the device and spinous process. For this study, an impulse head with a single contact probe was used. The participants were asked to report if they experienced any pain during measurements.
Statistical analysis
Descriptive statistics were used to summarise baseline characteristics of the participants, and the mean of the three trials of each test situation was used for further calculations. A graph of mean spinal stiffness and 95% confidence intervals (CI) of each thoracic and lumbar vertebra in all three testing situations was plotted. Measurements of Th1 and Th2 during the test situation involving standing with additional axial load were not possible because the spinous processes were covered by the weight bar.
The test–retest reliability of all three test situations was assessed with an intraclass correlation coefficient (ICC) with 95% CI. Cronbach’s alpha was assessed to evaluate internal consistency. The standard error of measurement (SEM) and smallest detectable change were calculated to determine absolute reliability. Limits of agreement (LoA) and systematic bias were assessed using Bland–Altman plots.
The differences in spinal stiffness between body positions (factors prone and standing) and additional axial loading (factors standing and standing + 50%) were tested with a two-factor repeated measures analysis of covariance (ANCOVA) with body mass index (BMI) and sex as the between-subjects factors. Three BMI categories were defined (< 20, 20–24, > 25 kg/m2). For post hoc analysis, a one-way analysis of variance for each vertebra was used (Bonferroni correction p < 0.003). All statistical analyses were performed using SPSS 23 (IBM, PASW Statistics, Chicago, IL). The REDCap (8.2.0, Vanderbilt University) was used to collect and store data.
Results
Participants
One hundred participants were recruited and spinal stiffness was measured; none of them had to be excluded.
The characteristics of participants are summarised in Table 1; none of them experienced pain during the measurements. The mean spinal stiffness with 95% CI in all three test situations is presented in Fig. 2.
Reliability
Spinal stiffness in all test situations showed good reliability, with the ICCs ≥ 0.83, Cronbach’s alpha between 0.83 and 0.88 and SEM ≤ 2.02. All outcomes are shown in Table 2. The Bland–Altman plot indicated that most points were located within the 95% LoA for test–retest reliability. No systematic error was observed. The results of each trial and Bland–Altman plots can be found in supplementary file S1.
Influence of body position
Repeated measures ANCOVA main effect with sphericity assumed showed mean spinal stiffness significantly higher when standing than when in the prone position [F(1/1694) = 433.630, p < 0.001]. Results of each vertebra are shown in Table 3. We also found that there was an interaction between BMI and body position [F(2/1694) = 29.358, p < 0.001]; however, no relation between sex and body position was observed [F(1/1694) = 0.828, p = 0.363]. Testing all three BMI groups (BMI < 20, 20–24, > 25) separately showed significant differences between the prone and standing positions in all three groups: BMI < 20, F(1/220) = 128.001, p < 0.001; BMI 20–24, F(1/1070) = 710.029, p < 0.001; BMI > 25, F(1/407) = 65.482, p < 0.001. The effects of different positions on spinal stiffness were similar in all three BMI categories.
Influence of additional axial loading
Repeated measures ANCOVA main effect with sphericity assumed showed that mean spinal stiffness was significantly lower in the configuration of standing + 50% than that in normal standing [F(1/1494) = 754.358, p < 0.001] (Table 3). Investigating the frequencies of the direction of change across vertebras and across subjects, 82% of the vertebras showed a decrease (mean decrease 8.3% ± 7.3SD), 2% showed no change, and 16% presented an increase (mean increase 2.8% ± 2.7SD) in spinal stiffness. There was an interaction between BMI and the loading [F(2/1494) = 7.041, p = 0.001]; however, there was no relation between sex and axial loading [F(1/1494) = 0.002, p = 0.965]. Testing all three BMI categories separately showed significant differences between standing and standing with additional load in all three groups: BMI < 20, F(1/194) = 175.219, p < 0.001; BMI 20–24, F(1/944) = 699.787, p < 0.001; BMI > 25, F(1/359) = 199.694, p < 0.001. Additionally, the effects of different positions on spinal stiffness were similar in all 3 BMI categories.
Discussion
Here, we present spinal stiffness data in different body positions and/or with different axial loadings. The data were found to be reliable, thereby providing normative data on spinal stiffness in asymptomatic individuals. In contrast to our expectations, this study showed no significant difference in spinal stiffness between males and females in all body positions. While this is in line with results by Stanton and Kawchuk [17], two previous studies showed higher spinal stiffness values in males than in females [11, 18]. But one study found this difference only for the vertebra Th7 [11] and in the other study, the males were 14 years older than the females [18], which might have influenced the results [19]. In the present study, spinal stiffness did demonstrate a dependency on the BMI of the participants. Our finding of decreasing stiffness with higher BMI is supported by the literature [18, 19]. Despite the influence of BMI on spinal stiffness, the different BMI groups showed the same effects in different body positions and with different axial loads.
Influence of body position
There was higher spinal stiffness in the upright than that in the prone position in most thoracic and lumbar vertebrae. This result supports the concept that increased activation of the back extensor muscles in an upright neutral position results in higher stiffness values than in prone position [8]. Only Th1–Th4 showed lower spinal stiffness values in the upright position. A possible explanation could be that the sternum stabilised the upper thoracic spine while the subject laid prone on the table [20]. According to our knowledge, there is only one study which has measured both thoracic and lumbar spinal stiffness [15], and it involved assessing 18 healthy young adults in the prone position. Similar to our results, higher stiffness values were found for Th1–Th4 with lower values for the lumbar spine in the prone position [15]. In contrast, another study has reported lower spinal stiffness in the upper than in the lower thoracic spine in healthy participants aged 18–45 years [21]. However, the thoracic spine was not measured entirely in this study (only the four vertebrae adjacent to the stiffest vertebra).
Influence of additional axial loading
Spinal stiffness in most vertebrae decreased while the subjects were carrying an additional axial load compared to standing upright neutrally. This is similar to a study that investigated spinal stiffness of the L3 vertebra in prone and upright positions during parabolic flight, where decreased spinal stiffness was observed during hypergravity (1.8 g) conditions [22]. These results are contrary to what has been found using in vitro samples [9] or in vitro porcine models [23]. Such in vitro experiments test the stiffness of passive structures, including bones and ligaments, but obviously do not include the assessment of muscle activity or spinal motor control. One explanation could be where/how the load in this study was applied. In our study, the load was placed on the shoulders. This produces a similar axial load as carrying a backpack with a minimum of moment arms, which has been shown to result in particularly low spine loads [24]. In line with this notion, earlier studies found no change or even a decreased lumbar erector spinae EMG activity while carrying a backpack compared to the unloaded spine [25]. Accordingly, a reduction of the erector spinae activity leads to a forward trunk lean to counterbalance the weight [25]. In our study, the axial load placed on the participant’s shoulders creates an extension moment in the same way as a backpack. Because the stability provided by the passive structures is small [7], active structures and motor control of the spine likely contribute to the decrease in stiffness found in the present study.
Limitations
Due to the squat rack lying over the participants’ shoulders, we could not measure the stiffness of Th1 and Th2 in the upright position with an additional load. Furthermore, several factors which influence spinal stiffness were not assessed, e.g. trunk muscle activity and abdominal pressure.
Conclusion
This study provides new insights regarding spinal motor control. We confirmed the increase in spinal lumbar and thoracic stiffness when body position is changed from prone to standing upright. Additional axial load of 50% of the body weight during standing leads to reduced spinal stiffness.
References
Shumway-Cook A, Woollacott MH (2001) Motor control: theory and practical applications. Lippincott Williams & Wilkins, Philadelphia
Panjabi MM (1992) The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J Spinal Disord 5:390–396 (discussion 397)
Henderson CNR (2012) The basis for spinal manipulation: chiropractic perspective of indications and theory. J Electromyogr Kinesiol 22:632–642
Wong AYL, Kawchuk GN (2017) The clinical value of assessing lumbar posteroanterior segmental stiffness: a narrative review of manual and instrumented methods. J Phys Med Rehabil 9:816–830
Fritz JM, Whitman JM, Childs JD (2005) Lumbar spine segmental mobility assessment: an examination of validity for determining intervention strategies in patients with low back pain. Arch Phys Med Rehabil 86:1745–1752
Mergner T, Rosemeier T (1998) Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions—a conceptual model. Brain Res Brain Res Rev 28:118–135
Hodges PW, Cholewicki J, van Dieen JH (2013) Spinal control: the rehabilitation of back pain e-book: state of the art and science. Elsevier Health Sciences, Philadelphia
Chan ST, Fung PK, Ng NY, Ngan TL, Chong MY, Tang CN, He JF, Zheng YP (2012) Dynamic changes of elasticity, cross-sectional area, and fat infiltration of multifidus at different postures in men with chronic low back pain. Spine J 12:381–388. https://doi.org/10.1016/j.spinee.2011.12.004
Stokes IA, Gardner-Morse M (2003) Spinal stiffness increases with axial load: another stabilizing consequence of muscle action. J Electromyogr Kinesiol 13:397–402
Hofstetter L, Hausler M, Wirth B, Swanenburg J (2019) Instrumented measurement of spinal stiffness: a systematic literature review of reliability. J Manip Physiol Ther. https://doi.org/10.1016/j.jmpt.2018.03.002
Brodeur R, DelRe L (1999) Stiffness of the thoraco-lumbar spine for subjects with and without low back pain. J Neuromuscul Syst 7:127–133
Hodges PW, Eriksson AE, Shirley D, Gandevia SC (2005) Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech 38:1873–1880. https://doi.org/10.1016/j.jbiomech.2004.08.016
Shirley D, Hodges PW, Eriksson AE, Gandevia SC (2003) Spinal stiffness changes throughout the respiratory cycle. J Appl Physiol 95:1467–1475. https://doi.org/10.1152/japplphysiol.00939.2002
Stanton TR, Kawchuk GN (2009) Reliability of assisted indentation in measuring lumbar spinal stiffness. Man Ther 14:197–205. https://doi.org/10.1016/j.math.2008.01.011
Leach RA, Parker PL, Veal PS (2003) PulStar differential compliance spinal instrument: a randomized interexaminer and intraexaminer reliability study. J Manip Physiol Ther 26:493–501. https://doi.org/10.1016/S0161-4754(03)00106-4
Girod B, Rabenstein R, Stenger A (2003) Einführung in die Systemtheorie. Vieweg+Teubner Verlag, Wiesbaden
Stanton T, Kawchuk G (2008) The effect of abdominal stabilization contractions on posteroanterior spinal stiffness. Spine 33:694–701
Lee M, Steven GP, Crosbie J, Higgs R (1998) Variations in posteroanterior stiffness in the thoracolumbar spine: preliminary observations and proposed mechanisms. Phys Ther 78:1277–1287
Owens EF, DeVocht JW, Gudavalli MR, Wilder DG, Meeker WC (2007) Comparison of posteroanterior spinal stiffness measures to clinical and demographic findings at baseline in patients enrolled in a clinical study of spinal manipulation for low back pain. J Manip Physiol Ther 30:493–500
Holdsworth F (1970) Fractures, dislocations, and fracture-dislocations of the spine. J Bone Jt Surg Am 52:1534–1551
Campbell BD, Snodgrass SJ (2010) The effects of thoracic manipulation on posteroanterior spinal stiffness. J Orthop Sports Phys Ther 40:685–693
Swanenburg J, Meier ML, Langenfeld A, Schweinhardt P, Humphreys K (2018) Spinal stiffness in prone and upright postures during 0–1.8 g induced by parabolic flight. Aerosp Med Hum Perform 89:563–567
Gardner-Morse MG, Stokes IA (2003) Physiological axial compressive preloads increase motion segment stiffness, linearity and hysteresis in all six degrees of freedom for small displacements about the neutral posture. J Orthop Res 21:547–552
Rose JD, Mendel E, Marras WS (2013) Carrying and spine loading. Ergonomics 56:1722–1732
Bobet J, Norman RW (1984) Effects of load placement on back muscle activity in load carriage. Eur J Appl Physiol 53:71–75
Funding
No Funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The ethics committee of the Canton of Zurich approved this study (BASEC-Nr: 2017–01245). It was registered at ClinicalTrials.gov (Identifier: NCT03495843).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Häusler, M., Hofstetter, L., Schweinhardt, P. et al. Influence of body position and axial load on spinal stiffness in healthy young adults. Eur Spine J 29, 455–461 (2020). https://doi.org/10.1007/s00586-019-06254-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06254-0