Abstract
Study design
Meta-analysis.
Objective
To conduct a meta-analysis investigating the relationship between spinopelvic alignment parameters and development of adjacent level disease (ALD) following lumbar fusion for degenerative disease.
Summary of background data
ALD is a degenerative pathology that develops at mobile segments above or below fused spinal segments. Patient outcomes are worse, and the likelihood of requiring revision surgery is higher in ALD compared to patients without ALD. Spinopelvic sagittal alignment has been found to have a significant effect on outcomes post-fusion; however, studies investigating the relationship between spinopelvic sagittal alignment parameters and ALD in degenerative lumbar disease are limited.
Methods
Six e-databases were searched. Predefined endpoints were extracted and meta-analyzed from the identified studies.
Results
There was a significantly larger pre-operative PT in the ALD cohort versus control (WMD 3.99, CI 1.97–6.00, p = 0.0001), a smaller pre-operative SS (WMD − 2.74; CI − 5.14 to 0.34, p = 0.03), and a smaller pre-operative LL (WMD − 4.76; CI − 7.66 to 1.86, p = 0.001). There was a significantly larger pre-operative PI-LL in the ALD cohort (WMD 8.74; CI 3.12–14.37, p = 0.002). There was a significantly larger postoperative PI in the ALD cohort (WMD 2.08; CI 0.26–3.90, p = 0.03) and a larger postoperative PT (WMD 5.23; CI 3.18–7.27, p < 0.00001).
Conclusion
The sagittal parameters: PT, SS, PI-LL, and LL may predict development of ALD in patients’ post-lumbar fusion for degenerative disease. Decision-making aimed at correcting these parameters may decrease risk of developing ALD in this cohort.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative disease of the spine occurs due to gradual degeneration of intervertebral disk with increasing age and most commonly involves the lumbar levels [1]. Instrumented fusion to stabilize affected vertebral segments has proven to be the treatment of choice for many degenerative spine disorders [2], with effective results reported for various techniques including anterior [3,4,5], posterior [6,7,8], and lateral approaches [9]. National statistics collected by the US Department of Health and Human Services show an increased frequency of spinal fusion surgeries, irrespective of spinal level, during the past 20 years [10]. Between 2002 and 2009, the annual rates for fusion surgery were greatest for the lumbar spine compared to cervical and thoracic levels, increasing from 45 per 100,000 in 2002 to 72 per 100,000 in 2009 [11]. Estimated expenditures for diagnosis and management of back pain can be up to $90 billion each year, and additionally, $10–$20 billion per year in economic loss of productivity each year [12].
Due to the high volume and cost of lumbar fusion surgery, there has been an increasing emphasis on understanding the etiology and risk factors for postoperative complications following lumbar spinal fusion. Adjacent level disease (ALD) is a degenerative pathology that develops at mobile segments above or below a fused spinal segment. Long-term patient functional outcomes are less favorable and the likelihood for revision surgery is higher in those whom develop ALD. Several theories have been proposed regarding the pathogenesis of ALD. The loss of naturally mobile vertebral segments after fusion may result in an increased transmission of forces to adjacent non-fused segments [13]. Cadaveric studies have shown increased adjacent segment motion and intervertebral stress in adjacent motion segments after fusion [14, 15]. Other studies have suggested that damage to stabilizing soft tissue and bony structures during open procedures may distort the distribution of forces on the spine [16,17,18,19]. In both cases, altered biomechanical stresses on the vertebral column lead to acceleration in degenerative disk disease in these adjacent segments.
Although the concept of ALD is widely recognized, there is controversy regarding proper classification. Hilibrand et al. distinguished ALD from “adjacent segment degeneration” by the presence of clinical symptoms in the former but only radiological evidence in the latter [13]. Prior studies have proposed the broader term “adjacent segment pathology” with clinical and radiological subtypes, reflecting a common disease process with varying manifestations [20]. The incidence of radiological ALD may be as high as 100% and clinical ALD as high as 27.5%, suggesting that pathological changes occur commonly but are less frequently symptomatic [21].
Spinopelvic sagittal alignment has been found to have a significant effect on clinical outcomes after fusion surgery [22]. Furthermore, it has been suggested that spinopelvic sagittal alignment may contribute to ALD [23, 24]. The current literature has reported on the relationship between sagittal alignment and ALD in predominantly spinal deformity patients only. Previous studies investigating the relationship between spinopelvic sagittal alignment parameters and ALD in degenerative lumbar disease are limited. Therefore, we aim to evaluate the current literature on the role of spinopelvic alignment parameters in the development of ALD following lumbar fusion surgery for degenerative disk disease.
Methods
Search strategy
The recommended PRISMA statement and guidelines were followed for the present systematic review and meta-analysis [25,26,27]. Electronic searches were performed using Ovid Medline, PubMed, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Database of Systematic Reviews (CDSR), ACP Journal Club and Database of Abstracts of Review of Effectiveness (DARE) from their dates of inception to February 2017. To achieve maximum sensitivity of the search strategy and identify all studies, we combined the terms: “spinopelvic”, “sagittal balance”, “pelvic incidence”, “pelvic tilt”, “sacral slope”, “lumbar lordosis”, “adjacent segment disease”, “lumbar spine”, “fusion”, as either keywords or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All identified articles were systematically assessed using the inclusion and exclusion criteria.
Selection criteria
Eligible comparative studies for the present systematic review and meta-analysis included those where patients underwent fusion surgery for degenerative lumbar spinal diseases, with patients split into groups: those with ALD compared to those without ALD. When institutions published duplicate studies with accumulating numbers of patients or increased lengths of follow-up, only the most complete reports were included for quantitative assessment at each time interval. All publications were limited to those involving human subjects and in the English language. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded. Review articles were omitted because of potential publication bias and duplication of results.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. Two investigators independently reviewed each retrieved article (K.P. and A.N.). Discrepancies between the two reviewers were resolved by discussion and consensus. Because quality scoring is controversial in meta-analyses of observational studies, the reviewers also independently appraised each article included in our analysis according to recommended Cochrane guidelines, including the following points: (1) clear definition of study population; (2) clear definition of outcomes and outcome assessment; (3) independent assessment of outcome parameters; (4) sufficient duration of follow-up; (5) no selective loss during follow-up; and (6) important confounders and prognostic factors identified.
Statistical analysis
The weighted mean difference (WMD) was used as a summary statistic. In the present study, both fixed- and random-effect models were tested. In the fixed-effects model, it was assumed that treatment effect in each study was the same, whereas in a random-effects model, it was assumed that there were variations between studies. χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, owing to heterogeneity rather than chance, with values greater than 50% considered as substantial heterogeneity. I2 can be calculated as: I2 = 100% × (Q − df)/Q, with Q defined as Cochrane’s heterogeneity statistics and df defined as degree of freedom. If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. In the present meta-analysis, the results using the random-effects model were presented to account for possible clinical diversity and methodological variation between studies. Specific analyses considering confounding factors were not possible as corresponding raw data were not available. All p values were two-sided. All statistical analysis was conducted with Review Manager Version 5.3.2. (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Publication bias
Publication bias was assessed using funnel plot asymmetry.
Results
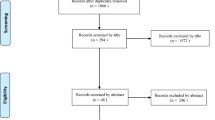
A flow diagram outlining the systematic review process is provided in Fig. 1. The initial database search yielded 1747 citations and 6 citations from additional sources. Following elimination of duplicates, a total of 1735 records were screened and 56 relevant full-text articles were assessed for eligibility. Of those assessed, 48 articles were excluded due to lack of comparative data, no evaluation of lumbar fusion surgery, and comment/editorial articles. A total of 8 articles were included for quantitative synthesis (Table 1). Pelvic parameters were measured in all studies: pre-operative pelvic tilt (PT) (N = 6), sacral slope (SS) (N = 5), lumbar lordosis (LL) (N = 8), pelvic incidence and lumbar lordosis mismatch (PI-LL) (N = 3), and postoperative PI (N = 4) and PT (N = 4). All included articles were single-center studies. They were either prospective cohort (N = 1) or retrospective case control studies (N = 7). The definition used for ALD for each included study is shown in Supplementary Table 1. Assessment of quality of included studies is shown in Supplementary Table 2.
Patient cohort
There were a total of 253 ALD patients and 860 patients without ALD included in the present analysis. The mean age of participants for ALD group (N = 6) was 64.04 years and 61.57 years for the control group (N = 6), which was significantly different (p = 0.01). There was no difference in the proportion of males between the groups: (N = 4) 41% male of ALD group were compared with (N = 4) 40.3% male of control group. There were also no significant differences in terms of other baseline characteristics including BMI (25.9 kg/m2 vs 25.4 kg/m2, p = 0.56), proportion of patients with degenerative spondylolisthesis (55.6 vs 48.3%, p = 0.78), foraminal stenosis (34.7 vs 31.8%, p = 0.19), and disk herniation (15.5 vs 19.2%, p = 0.45) (Table 2).
Pelvic parameter analyses
There was a significantly larger pre-operative PT in the ALD cohort compared to control (WMD 3.99, 95% CI 1.97–6.00, p = 0.0001) associated with moderate heterogeneity (I2 = 65%) (Fig. 2). There was a significantly smaller pre-operative SS compared to control (WMD − 2.74; 95% CI − 5.14, − 0.34, p = 0.03) associated with moderate heterogeneity (I2 = 66%). There was a significantly smaller pre-operative LL in the ALD cohort compared to control (WMD − 4.76; 95% CI − 7.66, − 1.86, p = 0.001) associated with high heterogeneity (I2 = 73%). There was a significantly larger pre-operative PI-LL in the ALD cohort compared to control (WMD 8.74; 95% CI 3.12, 14.37, p = 0.002) with high heterogeneity (I2 = 76%). No significant differences were found for PI (p = 0.25), TK (p = 0.25), angle at fused level (p = 0.13) and SVA (p = 0.18) between cohorts pre-operatively (Table 3).
There was a significantly larger postoperative PI in the ALD cohort compared to control (WMD 2.08; 95% CI 0.26, 3.90, p = 0.03) associated with low heterogeneity (I2 = 15%) (Fig. 3). There was a significantly larger postoperative PT in the ALD cohort compared to control (WMD 5.23; 95% CI 3.18, 7.27, p < 0.00001) associated with moderate heterogeneity (I2 = 62%). No significant differences were found for SS (p = 0.07), LL (p = 0.45), TK (p = 0.28), angle at fused level (p = 0.70), PI-LL (p = 0.16) and SVA (p = 0.71) between cohorts postoperatively (Table 4).
Assessment of publication bias demonstrated no significant funnel plot asymmetry, as shown in Supplementary Figure 1.
Discussion
ALD remains one of the most important long-term complications of lumbar spinal fusion surgery, notwithstanding satisfactory solid fusion at the operated levels. There is evidence which demonstrates that spinal fusion at the index level creates significant compensatory increases in motion/micromotion at adjacent levels subsequent to increased stiffness and higher loads during normal activity [28,29,30]. Our understanding of the risk factors contributing to ALD following surgery for degenerative lumbar spine disease remains limited, with the current evidence reporting a variety of predictors including age [31], smoking status [32], pre-existing degeneration, method of fusion, and length of fusion construct [31, 33]. The importance of spinopelvic sagittal alignment and its relationship with clinical outcomes following lumbar surgery is increasingly emphasized in the recent literature; however, its association with ALD is not well understood [34, 35]. To address limitations in the current evidence, we conducted a systematic review and meta-analysis investigating the relationship between spinopelvic alignment parameters and development of ALD following lumbar fusion surgery for degenerative disease. Our pooled analysis demonstrated that patients whom developed ALD had significantly higher PT, lower SS and PI-LL mismatch prior to fusion and higher PI and postoperative PT compared to control patients without ALD.
One of the earlier studies to investigate the association between spinopelvic sagittal alignment and ALD was conducted by Kumar et al., who analyzed 83 patients with degenerative disk disease. The authors demonstrated a significant association between abnormal C7-plumb line and SS with higher rates of postoperative ALD [35], highlighting the importance of appropriate sagittal alignment correction during fusion procedures for this population. In our pooled analysis of comparative studies, we found that pre-operative increased PT was significantly associated with the development of ALD. Patients with fixed sagittal malalignment have an increased PT or pelvic retroversion during standing as a compensatory mechanism for their spinal deformity [34]. PT also remained significantly higher in patients that developed ALD after lumbar fusion, suggesting that sagittal alignment was not optimally corrected in these patients and therefore predisposed them to ALD. Our observations suggest that additional attention should be paid to sagittal malalignment and potential correction during every fusion surgery to reduce the incidence of ALD.
PI is a fixed value for any given individual; however, it may vary from person to person. Our results show that after lumbar fusion, PI values are significantly higher in those patients that subsequently develop ALD. Higher PI values were also found before fusion surgery for the ALD cohort compared to control, but did not reach significance. Postoperative hypolordosis is common following fusion and may increase biomechanical loads at adjacent segments [36, 37]. Therefore, patients undergoing lumbar fusion with a higher PI may be more likely to develop ALD because of the increased PI-LL mismatch following failure to increase LL. Similarly, patients whom developed ALD had a significantly higher pre-operative PI-LL compared to controls before lumbar fusion. These results support the notion that in some lumbar spinal fusion cases, patients were present with high PI and there was failure to increase LL in order to match their high PI [38, 39]. Senteler et al. in a biomechanical study found that a PI-LL mismatch greater than 15° was predictive of revision surgery for ALD after lumbar fusion [40]. The relevance of spinopelvic sagittal alignment in the outcomes of adult deformity surgery has only been recently realized. In a prospective study, Schwab et al. found PI-LL mismatch to have the strongest correlation with disability and lower quality of life scores in spinal deformity patients [22]. Authors concluded that PI-LL mismatch should be restored in adult spinal deformity patients, with the authors defining a PI-LL mismatch of ≥ 11 degrees as being unbalanced or compensating. Additionally, they found a higher SVA to be correlated with need for corrective surgery. Our results showed SVA to not be significantly different between ALD and control cohorts, presumably because these patients did not have spinal deformity and sagittal malalignment as their primary concern. Although PI-LL has traditionally been considered the index of appropriate surgical correction for adult deformity, our results implicate that this parameter may be a useful predictor of ALD following lumbar fusion surgery for degenerative lumbar disease.
Previous studies have reported that failure to restore LL in fused lumbar levels is a risk factor for ALD [36, 41, 42]. Djurasovic et al. reported that patients whom developed ALD had significantly less lordosis both at the index fusion level and regionally compared to matched controls [23]. Kim et al. found that maintaining L4-L5 lordosis angle greater than 20 degrees was important for prevention of clinical ALD [42]. Our results showed that patients with ALD had significantly less LL pre-operatively, but similar LL postoperatively compared to controls. These findings are not surprising, as the LL can be considered correspondent to PI. Our results support the paradigm that preoperative sagittal malalignment has a significant association with ALD following lumbar fusion surgery.
There are multiple implications from the presented results above. Given the association between spinal alignment and the development of ALD, this suggests that spine surgeons should routinely pay attention to spinal alignment in patients undergoing surgery for lumbar degenerative disease, even without overt spinal deformity. Appropriate correction of sagittal alignment parameters during the operation will likely reduce the incidence of postoperative ALD complications. The other implication of our results is that surgeons should employ surgical techniques which appropriately restore LL to minimize ALD. There is evidence which demonstrates that anterior lumbar interbody fusion (ALIF) is effective in restoring LL [43,44,45,46,47]. In a retrospective analysis of 32 ALIF patients and 25 transforaminal lumbar interbody fusion (TLIF) patients, ALIF was able to increase foraminal height by 18.5% and increase local and regional lumbar lordosis compared to TLIF43. The advantages of ALIF are that the anterior approach can provide maximum area of endplate interface, allowing for a larger intervertebral spacer or graft to maximally correct LL. ALIF also showed greater segmental and LL correction compared to lateral lumbar interbody fusion (LLIF); however, LLIF was also able to achieve acceptable LL restoration [47, 48]. A recent randomized study demonstrated no significant differences in correction of LL using either TLIF or a posterolateral fusion (PLF) approach [49]. The current literature suggests that the ALIF approach may be most effective for adequate LL restoration in patients with sagittal malalignment, to reduce the development of ALD. There is evidence to suggest that ALD may be mitigated when an interspinous device or pedicle screw-based dynamic fixator is employed, with the latter imparting somewhat higher stress. However, larger studies are needed [50].
The present study is constrained by several limitations. It is possible that unbalanced cohort sizes, heterogeneity in patient population and the wide range of procedures done at the lumbar spine due to the nature of this systematic review may have limited its power to detect differences between cohorts. In terms of baseline characteristics, parameters such as age were unbalanced, with the ALD group being older by 3 years. We were unable to perform an adjusted analysis to account for age differences as baseline, and as such, this is a confounding factor that undermines the validity of the presented results. In addition, the follow-up of included studies varied from 14 months to 11 years. For longer-term reported outcomes, this could be affected by the natural course and history after surgery and could not be accounted for in our analysis. Additionally, included studies were published in different countries and continents, with different ethnicities. This confounder could not be adjusted for in the present meta-analysis. ALD measurement and definition is not standardized and thus varied between studies. Further investigation is required to establish criteria to differentiate ALD from normal age-related degeneration as adjacent segments to fusion levels may have some pre-existing degenerative changes. Raw data were unavailable for analyzed studies, and therefore, it was not possible to determine normal “cut-off” values for spinopelvic parameters in this population. Future studies aimed at investigating the correlation between spinopelvic parameters and clinical outcomes in patients whom develop ALD are warranted; these were not examined in this study. In addition, studies do not necessarily report the incidence of superior segment facet joint violation which is associated with increased morbidity and reoperation one the basis of ALD [51]. Lastly, importantly, the literature over the past several years suggests that PI may not always be as fixed as has been traditionally assumed. In the context of long instrumentation/fusion such as proximal thoracic to sacral appear to be associated with significant sacroiliac mobility [52, 53].
In conclusion, we have identified a subset of sagittal parameters (PT, SS, PI-LL) that may predict the development of ALD in patients with degenerative disease undergoing lumbar fusion. Surgical decision-making aimed at correcting these parameters intra-operatively may decrease risk of developing ALD in this patient population.
Change history
13 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00586-021-07000-1
References
Lotz JC, Haughton V, Boden SD et al (2012) New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology 264:6–19
Mobbs RJ, Phan K, Malham G et al (2015) Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg (Hong Kong) 1:2–18
Mobbs RJ, Phan K, Daly D et al (2016) Approach-related complications of anterior lumbar interbody fusion: results of a combined spine and vascular surgical team. Glob Spine J 6:147–154
Phan K, Lee NJ, Kothari P et al (2016) Risk factors for readmissions following anterior lumbar interbody fusion. Spine 43:364–369
Rao PJ, Ghent F, Phan K et al (2015) Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci 22:1619–1624
Phan K, Thayaparan GK, Mobbs RJ (2015) Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion–systematic review and meta-analysis. Br J Neurosurg 29:705–711
Di Capua J, Somani S, Kim JS et al (2017) Analysis of risk factors for major complications following elective posterior lumbar fusion. Spine 42:1347–1354
Lee NJ, Kothari P, Phan K et al (2016) The incidence and risk factors for 30-day unplanned readmissions after elective posterior lumbar fusion. Spine 43:41–48
Phan K, Rao PJ, Scherman DB et al (2015) Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci 22:1714–1721
Rajaee SS, Bae HW, Kanim LE et al (2012) Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine 37:67–76
Yelin E, Weinstein S, King T (2016) The burden of musculoskeletal diseases in the United States. Semin Arthritis Rheum 46:259–260
Davies M (2013) Where the United States Spends its Spine Dollars: expenditures on different ambulatory services for the management of back and neck conditions. Spine 37(19):1693–1701
Hilibrand AS, Robbins M (2004) Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J 4:190S–194S
Yang SW, Langrana NA, Lee CK (1986) Biomechanics of lumbosacral spinal fusion in combined compression-torsion loads. Spine 11:937–941
Lee CK (1988) Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine 13:375–377
Bresnahan L, Ogden AT, Natarajan RN et al (2009) A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine 34:17–23
Regev GJ, Lee YP, Taylor WR et al (2009) Nerve injury to the posterior rami medial branch during the insertion of pedicle screws: comparison of mini-open versus percutaneous pedicle screw insertion techniques. Spine 34:1239–1242
Battie MC, Videman T, Kaprio J et al (2009) The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J 9:47–59
Kim DY, Lee SH, Chung SK et al (2005) Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine 30:123–129
Lee JC, Choi S-W (2015) Adjacent segment pathology after lumbar spinal fusion. Asian Spine J 9:807–817
Harrop JS, Youssef JA, Maltenfort M et al (2008) Lumbar adjacent segment degeneration and disease after arthrodesis and total disc arthroplasty. Spine 33:1701–1707
Schwab FJ, Blondel B, Bess S et al (2013) Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine 38:E803–E812
Djurasovic MO, Carreon LY, Glassman SD et al (2008) Sagittal alignment as a risk factor for adjacent level degeneration: a case-control study. Orthopedics 31:546
Radcliff KE, Kepler CK, Jakoi A et al (2013) Adjacent segment disease in the lumbar spine following different treatment interventions. Spine J 13:1339–1349
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Phan K, Tian DH, Cao C et al (2015) Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Anna Cardiothorac Surg 4:112–122
Phan K, Mobbs RJ (2015) Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg 1:19–27
Cole T-C, Ghosh P, Hannan NJ et al (1987) The response of the canine intervertebral disc to immobilization produced by spinal arthrodesis is dependent on constitutional factors. J Orthop Res 5:337–347
Lee CK, Langrana NA (1984) Lumbosacral spinal fusion. A biomechanical study. Spine 9:574–581
Cole T-C, Burkhardt D, Ghosh P et al (1985) Effects of spinal fusion on the proteoglycans of the canine intervertebral disc. J Orthop Res 3:277–291
Cheh G, Bridwell KH, Lenke LG et al (2007) Adjacent segment disease following lumbar/thoracolumbar fusion with pedicle screw instrumentation. Spine 32:2253–2257
Mok JM, Cloyd JM, Bradford DS et al (2009) Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine 34:832–839
Sears WR, Sergides IG, Kazemi N et al (2011) Incidence and prevalence of surgery at segments adjacent to a previous posterior lumbar arthrodesis. Spine J 11:11–20
Di Martino A, Quattrocchi CC, Scarciolla L et al (2014) Estimating the risk for symptomatic adjacent segment degeneration after lumbar fusion: analysis from a cohort of patients undergoing revision surgery. Eur Spine J 23(Suppl 6):693–698
Kumar MN, Baklanov A, Chopin D (2001) Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J 10:314–319
Umehara S, Zindrick MR, Patwardhan AG et al (2000) The biomechanical effect of postoperative hypolordosis in instrumented lumbar fusion on instrumented and adjacent spinal segments. Spine 25:1617–1624
Oda I, Cunningham BW, Buckley RA et al (1999) Does spinal kyphotic deformity influence the biomechanical characteristics of the adjacent motion segments? An in vivo animal model. Spine 24:2139–2146
Barrey C, Roussouly P, Perrin G et al (2011) Sagittal balance disorders in severe degenerative spine. Can we identify the compensatory mechanisms? Eur Spine J 20(Suppl 5):626–633
Roussouly P, Pinheiro-Franco JL (2011) Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J 20(Suppl 5):609–618
Senteler M, Weisse B, Snedeker JG et al (2014) Pelvic incidence-lumbar lordosis mismatch results in increased segmental joint loads in the unfused and fused lumbar spine. Eur Spine J 23:1384–1393
Akamaru T, Kawahara N, Tim Yoon S et al (2003) Adjacent segment motion after a simulated lumbar fusion in different sagittal alignments: a biomechanical analysis. Spine 28:1560–1566
Kim KH, Lee SH, Shim CS et al (2010) Adjacent segment disease after interbody fusion and pedicle screw fixations for isolated L4-L5 spondylolisthesis: a minimum five-year follow-up. Spine 35:625–634
Hsieh PC, Koski TR, O’Shaughnessy BA et al (2007) Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 7:379–386
Pavlov PW, Meijers H, van Limbeek J et al (2004) Good outcome and restoration of lordosis after anterior lumbar interbody fusion with additional posterior fixation. Spine 29:1893–1899 (discussion 900)
Rao PJ, Maharaj MM, Phan K et al (2015) Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J 15:817–824
Lee N, Kim KN, Yi S et al (2017) Comparison of outcomes of anterior-, posterior- and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 101:216–226
Sembrano JN, Yson SC, Horazdovsky RD et al (2015) Radiographic comparison of lateral lumbar interbody fusion versus traditional fusion approaches: analysis of sagittal contour change. Int J Spine Surg 9:16
Malham GM, Parker RM, Blecher CM et al (2016) Choice of approach does not affect clinical and radiologic outcomes: a comparative cohort of patients having anterior lumbar interbody fusion and patients having lateral lumbar interbody fusion at 24 months. Glob Spine J 6:472–481
Challier V, Boissiere L, Obeid I et al (2016) One-level lumbar degenerative spondylolisthesis and posterior approach. is transforaminal lateral interbody fusion mandatory? A randomized controlled trial with two year follow-up. Spine 42(8):531–539
Levin JM, Alentado VJ, Healy AT et al (2017) Superior segment facet joint violation during instrumented lumbar fusion is associated with higher reoperation rates and diminished improvement in quality of life. Clin Spine Surg. https://doi.org/10.1097/BSD.0000000000000566
Lee CH, Kim YE, Lee HJ et al (2017) Biomechanical effects of hybrid stabilization on the risk of proximal adjacent-segment degeneration following lumbar spinal fusion using an interspinous device or a pedicle screw-based dynamic fixator. J Neurosurg Spine 22:1–7. https://doi.org/10.3171/2017.3.SPINE161169
Cecchinato R, Redaelli A, Martini C et al (2017) Long fusions to S1 with or without pelvic fixation can induce relevant acute variations in pelvic incidence: a retrospective cohort study of adult spine deformity surgery. Eur Spine J. https://doi.org/10.1007/s00586-017-5154-z
Lee JH, Na KH, Kim JH et al (2016) Is pelvic incidence a constant, as everyone knows? Changes of pelvic incidence in surgically corrected adult sagittal deformity. Eur Spine J 25(11):3707–3714
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Phan, K., Nazareth, A., Hussain, A.K. et al. Relationship between sagittal balance and adjacent segment disease in surgical treatment of degenerative lumbar spine disease: meta-analysis and implications for choice of fusion technique. Eur Spine J 27, 1981–1991 (2018). https://doi.org/10.1007/s00586-018-5629-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5629-6