Abstract
Study design
A prospective cross-sectional case series study.
Objective
To investigate the prevalence of low virulence disc infection and its associations with characteristics of patients or discs in the cervical spine.
Background
Low virulence bacterial infections could be a possible cause of intervertebral disc degeneration and/or back pain. Controversies are continuing over whether these bacteria, predominantly Propionibacterium acnes (P. acnes), represent infection or contamination. However, the current studies mainly focus on the lumbar spine, with very limited data on the cervical spine.
Methods
Thirty-two patients (20 men and 12 women) who underwent anterior cervical fusion for degenerative cervical spondylosis or traumatic cervical cord injury were enrolled. Radiological assessments included X-ray, CT, and MRI of the cervical spine. Endplate Modic changes, intervertebral range of motion, and disc herniation type were evaluated. Disc and muscle tissues were collected under strict sterile conditions. Samples were enriched in tryptone soy broth and subcultured under anaerobic conditions, followed by identification of the resulting colonies by the PCR method.
Results
Sixty-six intervertebral discs were excised from thirty-two patients. Positive disc cultures were noted in eight patients (25%) and in nine discs (13.6%). The muscle biopsy (control) cultures were negative in 28 patients and positive in 4 patients (12.5%); three of whom had a negative disc culture. Seven discs (10.6%) were positive for coagulase-negative Staphylococci (CNS) and two discs were positive for P. acnes (3.0%). A younger patient age and the extrusion or sequestration type of disc herniation, which represented a complete annulus fibrous failure, were associated with positive disc culture.
Conclusions
Our data show that CNS is more prevalent than P. acnes in degenerative cervical discs. The infection route in cervical discs may be predominantly through an annulus fissure. Correlation between these infections and clinical symptoms is uncertain; therefore, their clinical significance needs to be investigated in the future.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Stirling et al. [1] first reported the presence of low virulent Gram-positive anaerobic bacteria in the disc tissue of patients with severe sciatica, the hypothesis that infection might be one of the causes of intervertebral disc degeneration [2,3,4,5,6] and back pain [1, 5, 7, 8] has gained increasing attention as well as ongoing controversy [8,9,10,11,12]. The detection of these bacteria in disc tissues was very common, though the prevalence varied from 1.6 to 82% [1,2,3,4,5,6, 9,10,11, 13,14,15,16,17,18]. Among the pathogens isolated from the disc tissues, Propionibacterium acnes (P. acnes), a slow-growing anaerobic bacteria associated with the skin condition of acne, was the most common, followed by coagulase-negative Staphylococci (CNS) [1,2,3,4,5,6, 13,14,15,16,17,18].
Despite controversies over whether these positive cultures represent true infection or contamination [9, 11, 12], many researchers found that their presence was correlated to disc-related factors such as low back pain or sciatica [1, 5, 7, 8, 18], annulus fibrous (AF) tears [15], and Modic changes (MCs) [3, 16], indicating that there might be some unknown link between these pathogens and disc degeneration. However, most of these studies were conducted on the lumbar spine only. Whether this link exists in the cervical spine and whether the pathogen spectrum in the cervical spine is different from the lumbar spine is uncertain.
Studies reporting the prevalence of low virulence disc infection in the cervical spine are very rare. Only two studies reported it in their subgroup of cervical spine degeneration [5, 14], and neither of them investigated associations between low virulence cervical disc infection and characteristics of patients or discs. For this purpose, we selected patients who underwent anterior cervical fusion for degenerative cervical spondylosis or traumatic cervical cord injury in the present study, which was the largest case series to date concerning cervical disc infections.
Methods
Patients
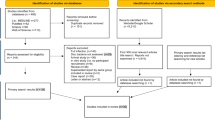
Between October 2016 and June 2017, 35 patients (22 men and 13 women) underwent anterior cervical discectomy and fusion (ACDF) or anterior cervical corpectomy and fusion (ACCF) in our institution due to degenerative cervical spondylosis or traumatic cervical cord injury. Patients without trauma presented with neck and shoulder pain, radiculopathy and/or myelopathy for 2–6 months, and failed to respond to conservative treatment for at least 2 months. Other patients with trauma presented with myelopathy or spinal cord injury. Exclusion criteria were: tumor, global cervical spine deformity, clinical infection, revision surgery, and receiving any antibiotics up to 1 month before surgery. 32 patients (20 men and 12 women) were finally included, with an average age of 57.8 ± 12.3 years (range 35–77 years).
Radiological assessment
A standard cervical X-ray series was obtained for all patients before surgery, including anterior–posterior, lateral, and flexion and extension views. Also obtained were computerized tomography (CT) and magnetic resonance imaging (MRI). MCs were evaluated on sagittal T1-weighted and T2-weighted images on MRI. Two observers (Z.F.D., a senior spine surgeon, and R.H., a senior radiologist) classified the MCs independently on sagittal MR images according to the definitions of Modic et al. [19, 20].
Intervertebral range of motion (ROM) was measured from C2–3 to C6–7. It was defined as the difference of angle between two endplates of each intervertebral disc from flexion to extension on X-ray.
Disc herniation was classified as one of the four stages: disc bulge which consisted of a diffuse or broad-based disc protrusion; disc protrusion which was identified as a focal herniation with the outer AF intact; disc extrusion which was a focal herniation with the outer AF torn; and disc sequestration which was identified as an intra-canal herniation of nucleus pulposus totally separated from nucleus in situ.
Tissue collection
Patients were placed in a supine position with the head slightly elevated. Strict sterile protocols were followed to avoid skin contamination. Skin overlying the surgical area was sterilized three times with povidone iodine, dried with sterile gauze, and covered with a plastic adhesive drape (3M Health Care, St. Paul, MN, USA). Surgery was performed via a right-sided anterior approach. Either a transverse or a longitudinal incision was made, depending on the number of motion segments to be fused. Dissection was carried out down to the anterior aspect of the cervical spine between the carotid sheath and the visceral sheath. The intervertebral disc tissues were removed, and a small piece of sternocleidomastoid muscle (which served to identify contamination during surgery) was also collected. Disc and muscle samples were cut into small pieces for culture.
Bacteria culture of surgical specimens
Each surgical specimen was added into a tube with 10 ml tryptone soy broth and incubated in a sealed anaerobic bag at 37 °C for 14 days. The pure culture medium without tissue was incubated in the same conditions as a negative control. The broth was then subcultured onto blood agar plates, incubated at 37 °C under anaerobic conditions for 7 days, and inspected for microbial growth. A single colony of samples with positive culture result was selected and investigated by Gram staining and identified by polymerase chain reaction (PCR) method. DNA was extracted from single presumptive colonies with ZymoBead Genomic DNA Kit (Zymo Research). Broad range universal bacterial primers were used to amplify bacterial 16S rDNA: forward primer 27F AGAGTTTGATCCTGGCTCAG and reverse primer 1492R GGTTACCTTGTTACGACTT. A negative control using sterile water as template was included. The PCR protocol was used as follows: initial 94 °C denature for 2 min; 35 cycles of 94 °C denature for 30 s, 55 °C annealing for 30 s, and 72 °C extension for 30 s; 72 °C extension for 2 min; 4 °C hold.
Statistical analysis
Raw data are expressed as mean ± standard deviation. Two-sample Student’s t tests and Fisher’s exact tests were performed to identify differences in means and in proportions, respectively, between positive culture and negative culture groups. P value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 20.0 (Chicago, IL, USA).
Results
A total of 32 patients were enrolled, including 20 males and 12 females with a mean age of 57.8 ± 12.3 years (range 35–77 years). Of 28 patients who had degenerative cervical spondylosis, 9 patients presented with radiculopathy and 19 patients presented with myelopathy. Four patients had a history of acute cervical trauma prior to the onset of symptoms. One patient fell from height. One fell from sitting place. The other two fell down when riding a bicycle. Two patients presented with myelopathy, one with incomplete spinal cord injury and the other with complete cord injury. None of the trauma patients reported symptoms of radiculopathy or myelopathy prior to their injuries. The mean age was 56.2 ± 12.2 years for degenerative patients and 68.7 ± 5.4 for trauma patients (P = 0.055). The mean visual analogue scale (VAS) for neck pain was 2.9 ± 2.1. One patient (3.1%) had diabetes and 13 (40.6%) patients were smokers. No patients received epidural or facet joint injections. MCs were present in 21 of 31 (67.7%) patients, with one trauma patient’s MRI being unavailable. 31 patients (96.9%) received ACDF, among whom 8 patients (25%) received single-level ACDF, and 23 (71.9%) patients received multiple-level ACDF. One patient received combined ACDF and ACCF. The demographic and clinical characteristics of patients are presented in Table 1.
A total of 66 intervertebral discs were excised. C5/6 was the most common spinal level being operated on (37.9% of all fused discs), followed by C4/5 (30.3%), C6/7 (16.7%), and C3/4 (15.2%). The MRI data were collected in all but one trauma patient who had C4/5 subluxation. MCs were present in 18/28 degenerative patients and in all three trauma patients who had MRI data (P = 0.533). Two trauma patients had degenerative MR appearance without fracture or dislocation, and the other had both degenerative change in C3/4 and rupture of ossified anterior longitudinal ligament in C5/6. MCs were present in 30/65 fused segments (46.2%). Of all the MCs in fused segments, type 2 (M2) were most prevalent (27.7%), followed by type 1 (M1) (13.8%). Disc herniation types included bulge in one disc (1.5%), protrusion in 29 discs (44.6%), extrusion in 21 discs (32.3%), and sequestration in 14 discs (21.5%). Disc herniation types included bulge in one disc (1.5%), protrusion in 29 discs (44.6%), extrusion in 21 discs (32.3%), and sequestration in 14 discs (21.5%). Of the six discs in three trauma patients who had MRI data, there were one disc bulge (16.7%), two disc protrusion (33.3%), and three disc extrusion (50.0%). Spinal cord high-signal intensity was present in 34 segments (52.3%). All the three trauma patients who had MRI data had high-signal intensity of the spinal cord. Segment ROM were 7.7° ± 4.4° for all the motion segments available for measurement. Clinical characteristics of discs are presented in Table 1.
Positive disc cultures were noted in eight patients (25%). The muscle biopsy (control) cultures were negative in 28 patients and positive in four patients (12.5%), three of whom had a negative disc culture, and one had a disc culture which was positive for a different bacterium [Staphylococci haemolyticus (S. haemolyticus)] from that of control (P. acnes). None of the four trauma patients had positive disc cultures, but one of them had positive muscle culture (Table 3). One patient had two infected discs, so positive cultures were noted in nine (13.6%) of the total discs excised. Seven discs were positive for CNS (10.6% of total discs/77.8% of positive cultured discs), of which three were positive for Staphylococcus epidermidis (S. epidermidis), two for Staphylococci capitis (S. capitis), and two for S. haemolyticus. Two discs were positive for P. acnes (3.0% of total discs/22.2% of positive cultured discs). None of the disc cultures had an additional organism growth. Clinical details of patients who had positive disc or muscle cultures are presented in Table 2.
The average age was significantly lower in patients who had positive disc cultures than those with negative ones (49.8 versus 60.5 years; P = 0.030) (Table 3). There was no significant association between disc cultures and patient gender (P = 0.204), symptom (P = 0.602), smoker (P = 0.219), presence of MCs (P = 0.786), number of segments treated (P = 0.546), or muscle cultures (P = 1.000) (Table 3). There was no significant difference in VAS between patients with positive disc cultures and patients with negative ones (Table 3).
There was no significant association between disc cultures and spinal level (P = 0.366), type of MCs (P = 0.466), or cord high-signal intensity (P = 0.810) (Table 4). In 30 discs with adjacent MCs, the rate of positive culture was 33.3% in type 1 MCs and 4.8% in other types of MCs, but statistical significance was not reached (P = 0.069) (Table 5). There was no significant difference in segment ROM between positive disc cultures and negative ones (P = 0.396) (Table 4). However, there was a significant association between positive disc cultures and type of herniation (P = 0.031) (Table 4). Positive disc cultures were detected in only 1 of 30 discs (3.3%) with disc bulge or protrusion, but in 8 of 35 discs (22.9%) with disc extrusion or sequestration.
Discussion
There is a great interest in the possibility that low virulence bacteria may be one of the causes of degenerative disc disease and back pain. Since Stirling [1] first reported a high positive culture rate of anaerobic bacteria from disc tissues in patients with sciatica, there was growing evidence, indicating that these bacteria could appear in painful degenerative discs without causing symptoms of clinical infection. The positive culture rate covered a wide range from 1.6 to 82% [1,2,3,4,5,6, 9,10,11, 13,14,15,16,17,18] and the pooled estimate of the prevalence was 34% [8]. However, all past studies mainly focus on the lumbar spine, and no study has yet been reported exclusively on the cervical spine. In the present study, we found positive disc cultures in 25% of the patients who underwent anterior cervical fusion and in 13.6% of the total discs excised, which was comparable with the results of other studies conducted on the lumbar spine.
The most prevalent organism identified in disc tissues in the previous studies [1,2,3,4, 6, 13,14,15,16,17,18] was P. acnes, followed by CNS. However, organisms other than P. acnes were reported by some other authors more common. Li [10] reported that 22 patients with lumbar disc herniation underwent discectomy and found positive disc cultures in three cases, two CNS, one particles chain bacterium, with no P. acnes found. Fritzell [21] identified bacteria in two of ten patients (20%), either Bacillus cereus or Citrobacter braakii/freundii. Rigal [9] reported six positive cultures in 379 samples, two P. acnes, two S. epidermidis, one Citrobacter freundii, and one Saccharopolyspora hirsuta. As for the cervical spine, P. acnes was the predominant culture result in both studies which included a subgroup of cervical discs [5, 14]. However, they did not exclude patient who had accepted prior injection or surgery at the operated spinal level. In the present study, CNS was more common than P. acnes in cervical discs. We think these differences in the culture results of the current literature and the present study may be related to differences in patient selection, culture method, identification method, and contamination control.
The rate of positive cultures depends to some extent on culture and identification methods. Stirling [1] used two methods to examine the presence of microorganisms in intervertebral discs. The first is the enrichment method by which disc tissue was first cultured in Robertson’s cooked meat enrichment broth, and then subcultured onto blood agar plates and incubated under anaerobic conditions. The other is the direct method by which large samples were cultured directly by impression onto blood agar plates, and embedded into nutrient agar. The rate of positive cultures using either technique was similar (53 and 55%, respectively). Fritzell [21] used PCR amplification of the 16S rRNA gene followed by gene sequencing, to detect bacterial DNA in degenerative discs. They identified bacteria in two of ten patients (20%). Most researchers used a combination of Stirling enrichment method and the PCR method [15, 16, 18], or either one [3, 9, 11]. However, a few studies found positive cultures rates even close to that of Stirling using the Stirling method alone. The highest rate so far was 81.8% by the PCR method [6]. In contrast, neither of the two studies reporting very low positive cultures rate (3.7 and 1.6%, respectively) used PCR as an identification method. PCR is theoretically more sensitive to detect the bacterial DNA than the Stirling method, which was widely used for disc anaerobic culture. However, PCR method only might be too sensitive to detect any bacteria. In the study by Rajasekaran [6], 81.8% of the samples were positive for P. acnes, including both normal discs without any degenerative change on MRI. Therefore, in the present study, we basically followed the Stirling enrichment procedure first and then investigated and identified any resulting colony by PCR method.
The focus of an ongoing debate is whether these detected bacteria are true infection or intraoperative contamination. Several studies have confirmed P. acnes as a common pathogen in vertebral and disc infection related to surgical procedures, implants, and epidural analgesia [22,23,24,25]. The route of infection was assumed to be occasional skin contamination during the invasive operation: bacteria were cultured not only from disc fragments but also from skin, ligamentum flavum, muscle, wound washings, air samples, and laminar flow [11, 12], with P. acnes being identified most frequently [12]. These results led to continued skepticism on the notion that the low virulence anaerobic bacteria isolated from the disc tissues were true infections and was one of the possible causes of disc degeneration.
Recently, however, the use of new detection techniques provided new evidence that bacteria were preexisting in the intervertebral discs rather than intraoperative contamination. Capoor [4] homogenized disc tissues before anaerobic culture to release bacteria from the biofilm to be cultured, reducing the rate of false-negative. One biopsy fragment was homogenized and cultured quantitatively, and a second was frozen and used for PCR-based quantification of bacterial genomes. The quantification study revealing significant correlation between the bacterial counts obtained by culture and the number of bacterial genomes detected by PCR, minimizing the likelihood that observed findings were due to contamination. Rajasekaran [6] used proteomics analysis to provide evidence of preexisting infection in a degenerated disc by identifying proteins signifying bacterial presence and host defence response proteins (HDRPs). 14 HDRPs were either uniquely present or up-regulated in disc herniation and disc degeneration groups in comparison to the normal disc group. Two recent studies showed visible evidence of bacteria within the disc tissues in situ. Yuan [18] reported that 7 of the 16 samples positive for P. acnes using 16S rDNA PCR had visible Gram-positive and rod-shape microbes stained with hematoxylin–eosin staining and modified Brown–Brenn staining. Capoor [13] observed the presence of P. acnes biofilms in situ within disc materials by confocal laser scanning and fluorescence in situ hybridization. These findings indicated that the identified bacteria in discs were infection rather than microbiological contamination. In our study, we used muscle samples as intraoperative contamination control and blank broth as environmental contamination control. We did not find any bacteria in blank broth. We did find positive muscle control cultures which indicated contamination in four patients, but three of them did not have a positive disc culture and the other had a positive disc culture which was different from the muscle culture. Our results were more consistent with disc infection in situ, but with a 12.5% incidence of positive culture in control, we could not completely exclude the possibility of intraoperative contamination of disc tissues.
One important finding of our study is that there is a significant association between the positive culture and type of herniation in cervical spine. Bacteria culture rate was low in discs that were merely bulging or protruding, yet much higher in discs with extrusion or sequestration, which indicate a full-thickness annulus fissure. Our finding is in consistent with that of others mainly done in lumbar spine [5, 15]. This result suggests that bacteria might invade cervical discs primarily though a radial fissure in the AF and further supported the theory that bacteria is more likely to affect herniated discs. With the outer structures (AF and endplate) of an intervertebral disc intact, the NP is isolated from the circulation, hence minimizing the chance of developing a intra-discal infection and subsequent adjacent bone marrow edema [26]. However, a herniated disc with full-thickness AF fissure can cause new capillarization and focal inflammation around the extruded disc materials [27, 28]. Therefore, it might be possible for very small amount of bacteria in the blood circulation to enter the disc through the fissure during transit bacteremia which occasionally happens without clinical symptoms [29,30,31]. Due to the nature of being avascular and low in oxygen tension, intervertebral disc thereby is suitable culture medium for anaerobic bacteria from the circulation, such as P. acnes and CNS, which can cause a low virulent and slowly developing infection in the disc but can hardly survive in highly vascularized vertebrae [16, 32].
Another interesting result of our study is that younger patients are more likely to have bacteria in their cervical discs. Other studies in lumbar spine also reported similar results [4, 15]. This seems to contradict the theory of the infectious origin of disc degeneration [2,3,4,5,6], because disc degeneration is more common and more severe in the elderly [33, 34]. However, on the other hand, it could be explained by the fact that a prolapsed cervical disc through a complete annulus fissure is more prevalent in younger patients [35]. This would support the concept of bacterial infection in cervical discs being predominantly via a complete annulus fissure.
However, strong evidence is lacking that these skin-resident bacteria can easily enter deep inside intervertebral discs via circulation. Both P. acnes and CNS are commonly found in the skin and the oral mucosa [29, 31, 36]. They may frequently invade the circulatory system during tooth brushing [30]. Theoretically, it is possible for the bacteria to enter the intervertebral disc through an annulus fissure when the disc is herniated and vascularized, or through a damaged endplate. However, none of the current animal models can rigorously confirm this hypothesis. Li [10] injected P. acnes intravenously in rabbits but failed to establish a local infection model in any of the discs which were incised to induce degeneration. Others [37,38,39] directly inoculated a high concentration of bacteria into the disc or endplate and successfully induced degeneration-like changes in the disc and the adjacent marrow. However, this model is not accurate to reflect the infection routes presumed by the theory mentioned above.
What has been proved by animal studies is that low virulence anaerobic bacteria really can survive in the disc tissues and may induce inflammation. Li [10] made an incision in the lumbar discs of rabbits to induce disc degeneration, followed by inoculation of P. acnes into the operated discs. Six weeks later, live P. acnes was found in 11 out of 18 discs, indicating that a degenerating disc was suitable for survival and growth of the bacteria. These bacteria may also induce disc degeneration and MC-like changes in the adjacent marrow [37,38,39]. Shan [37] directly inoculated P. acnes with a fine needle into the subchondral bone of the vertebrae in rabbits. Six months later, they observed signal intensity changes on MRI resembling MCs in the inoculation group, while the sham surgery group did not show any endplate changes.
Although experimental studies demonstrated that low virulence bacteria were closely related to MCs, results were conflicting from clinical studies which were done in the lumbar spine [2, 3, 5, 7, 14,15,16]. In the setting of cervical spine, we did not find a correlation between MCs and low virulence bacteria infection at the time of surgery. However, we cannot make any conclusion based on this cross-sectional study about the cause–effect relationship between the two for the reason that MCs represent a dynamic process and may change over time [26, 40,41,42]. Therefore, the absence of MCs at the time of surgery does not mean that it would never develop in the future. Usually, new MCs may be observed about 1 year after the herniation [26]. There is a theory that the formation of MCs has two possible causes: one mechanical and the other bacterial [32]. The MCs, especially type 1, probably represent inflammation and edema in the bone marrow surrounding an infected disc [32]. In a longitudinal study, Albert [16] reported that low virulence infection inside the intervertebral discs was a risk factor for newly developing MCs at adjacent endplates regardless of the type of baseline MCs. Therefore, bacteria could be present at the time of biopsy of a herniated disc, but with the absence of MCs. In a randomized clinical controlled trial, a reduction in the volume of M1 was observed after antibiotic treatment compared to the placebo group [7], which provided indirect evidence that MCs were partially related to low virulence infection in the discs. Actually, we did find out that positive culture in cervical discs was more related to M1 than to other types of MCs in segments with MCs, although this correlation was not significant.
In the present study, we did not found low virulence infection of cervical discs in correlation with either neck pain or type of symptoms. In addition, we did not found correlations between infection and either cervical focal ROM or cord high intensity, although Zhou [15] found that lumbar disc infection was related to decreased disc height. Our results show that low virulent infection may be a possible but not the primary cause of clinical symptoms, although these bacteria may lead to the continued destruction of the internal structure of the disc over a long period.
Conclusions
Different from the previous studies which conducted mainly on lumbar spine, our data show a 13.6% low virulence infection rates in degenerative cervical discs, with CNS being more prevalent than P. acnes. The infection is associated with complete annulus tear, and is more common in younger patients, which indicates that the route of infection in cervical discs may be predominantly through an annulus fissure. We cannot make a conclusion about the cause–effect relationship between the bacteria and the MCs due to the dynamic nature of the latter and the cross-sectional design of this study. There is no correlation confirmed between the infections and clinical symptoms, suggesting a limited clinical significance of these low virulence infections on the current management of cervical degenerative disc diseases.
References
Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TS (2001) Association between sciatica and Propionibacterium acnes. Lancet 357(9273):2024–2025
Arndt J, Charles YP, Koebel C, Bogorin I, Steib J-P (2012) Bacteriology of degenerated lumbar intervertebral disks. J Spinal Disord Tech 25(7):E211–E216
Aghazadeh J, Salehpour F, Ziaeii E, Javanshir N, Samadi A, Sadeghi J, Mirzaei F, Naseri Alavi SA (2017) Modic changes in the adjacent vertebrae due to disc material infection with Propionibacterium acnes in patients with lumbar disc herniation. Eur Spine J 26:3129–3134
Capoor MN, Ruzicka F, Machackova T, Jancalek R, Smrcka M, Schmitz JE, Hermanova M, Sana J, Michu E, Baird JC, Ahmed FS, Maca K, Lipina R, Alamin TF, Coscia MF, Stonemetz JL, Witham T, Ehrlich GD, Gokaslan ZL, Mavrommatis K, Birkenmaier C, Fischetti VA, Slaby O (2016) Prevalence of Propionibacterium acnes in intervertebral discs of patients undergoing lumbar microdiscectomy: a prospective cross-sectional study. PLoS One 11(8):e0161676
Coscia MF, Denys GA, Wack MF (2016) Propionibacterium acnes, coagulase-negative Staphylococcus, and the “biofilm-like” intervertebral disc. Spine (Phila Pa 1976) 41(24):1860–1865
Rajasekaran S, Tangavel C, Aiyer SN, Nayagam SM, Raveendran M, Demonte NL, Subbaiah P, Kanna R, Shetty AP, Dharmalingam K (2017) ISSLS PRIZE IN CLINICAL SCIENCE 2017: is infection the possible initiator of disc disease? An insight from proteomic analysis. Eur Spine J 26(5):1384–1400
Albert HB, Sorensen JS, Christensen BS, Manniche C (2013) Antibiotic treatment in patients with chronic low back pain and vertebral bone edema (Modic type 1 changes): a double-blind randomized clinical controlled trial of efficacy. Eur Spine J 22(4):697–707
Urquhart DM, Zheng Y, Cheng AC, Rosenfeld JV, Chan P, Liew S, Hussain SM, Cicuttini FM (2015) Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med 13:13
Rigal J, Thelen T, Byrne F, Cogniet A, Boissiere L, Aunoble S, Le Huec J-C (2016) Prospective study using anterior approach did not show association between Modic 1 changes and low grade infection in lumbar spine. Eur Spine J 25(4):1000–1005
Li B, Dong Z, Wu Y, Zeng J, Zheng Q, Xiao B, Cai X, Xiao Z (2016) Association between lumbar disc degeneration and Propionibacterium acnes infection: clinical research and preliminary exploration of animal experiment. Spine (Phila Pa 1976) 41(13):E764–E769
Carricajo A, Nuti C, Aubert E, Hatem O, Fonsale N, Mallaval FO, Vautrin AC, Brunon J, Aubert G (2007) Propionibacterium acnes contamination in lumbar disc surgery. J Hosp Infect 66(3):275–277
McLorinan GC, Glenn JV, McMullan MG, Patrick S (2005) Propionibacterium acnes wound contamination at the time of spinal surgery. Clin Orthop Relat Res 437:67–73
Capoor MN, Ruzicka F, Schmitz JE, James GA, Machackova T, Jancalek R, Smrcka M, Lipina R, Ahmed FS, Alamin TF, Anand N, Baird JC, Bhatia N, Demir-Deviren S, Eastlack RK, Fisher S, Garfin SR, Gogia JS, Gokaslan ZL, Kuo CC, Lee Y-P, Mavrommatis K, Michu E, Noskova H, Raz A, Sana J, Shamie AN, Stewart PS, Stonemetz JL, Wang JC, Witham TF, Coscia MF, Birkenmaier C, Fischetti VA, Slaby O (2017) Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS One 12(4):e0174518
Rao PJ, Phan K, Reddy R, Scherman DB, Taylor P, Mobbs RJ (2016) DISC (Degenerate-disc Infection Study With Contaminant Control): pilot study of australian cohort of patients without the contaminant control. Spine (Phila Pa 1976) 41(11):935–939
Zhou Z, Chen Z, Zheng Y, Cao P, Liang Y, Zhang X, Wu W, Xiao J, Qiu S (2015) Relationship between annular tear and presence of Propionibacterium acnes in lumbar intervertebral disc. Eur Spine J 24(11):2496–2502
Albert HB, Lambert P, Rollason J, Sorensen JS, Worthington T, Pedersen MB, Norgaard HS, Vernallis A, Busch F, Manniche C, Elliott T (2013) Does nuclear tissue infected with bacteria following disc herniations lead to Modic changes in the adjacent vertebrae? Eur Spine J 22(4):690–696
Agarwal V, Golish SR, Alamin TF (2011) Bacteriologic culture of excised intervertebral disc from immunocompetent patients undergoing single level primary lumbar microdiscectomy. J Spinal Disord Tech 24(6):397–400
Yuan Y, Zhou Z, Jiao Y, Li C, Zheng Y, Lin Y, Xiao J, Chen Z, Cao P (2017) Histological identification of Propionibacterium acnes in nonpyogenic degenerated intervertebral discs. Biomed Res Int 2017:6192935
Modic MT, Masaryk TJ, Ross JS, Carter JR (1988) Imaging of degenerative disk disease. Radiology 168(1):177–186
Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR (1988) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166(1 Pt 1):193–199
Fritzell P, Bergstrom T, Welinder-Olsson C (2004) Detection of bacterial DNA in painful degenerated spinal discs in patients without signs of clinical infection. Eur Spine J 13(8):702–706
Carragee EJ (1997) Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 79(6):874–880
Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR (2007) Propionibacterium acnes vertebral osteomyelitis: seek and ye shall find? Clin Orthop Relat Res 461:25–30
Hernandez-Palazon J, Puertas-Garcia JP, Martinez-Lage JF, Tortosa JA (2003) Lumbar spondylodiscitis caused by Propionibacterium acnes after epidural obstetric analgesia. Anesth Analg 96(5):1486–1488
Hahn F, Zbinden R, Min K (2005) Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J 14(8):783–788
Albert HB, Manniche C (2007) Modic changes following lumbar disc herniation. Eur Spine J 16(7):977–982
Doita M, Kanatani T, Harada T, Mizuno K (1996) Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine 21(2):235–241
Ito T, Yamada M, Ikuta F, Fukuda T, Hoshi S, Kawaji Y, Uchiyama S, Homma T, Takahashi HE (1996) Histologic evidence of absorption of sequestration-type herniated disc. Spine 21(2):230–234
Bomstein MM, Hakimi B, Persson GR (2008) Microbiological findings in subjects with asymptomatic oral lichen planus: a cross-sectional comparative study. J Periodontol 79(12):2347–2355
Roberts GJ, Holzel HS, Sury MR, Simmons NA, Gardner P, Longhurst P (1997) Dental bacteremia in children. Pediatr Cardiol 18(1):24–27
Barbeau J, ten Bokum L, Gauthier C, Prevost AP (1998) Cross-contamination potential of saliva ejectors used in dentistry. J Hosp Infect 40(4):303–311
Albert HB, Kjaer P, Jensen TS, Sorensen JS, Bendix T, Manniche C (2008) Modic changes, possible causes and relation to low back pain. Med Hypotheses 70(2):361–368
Boden SD, Mccowin PR, Davis DO, Dina TS, Mark AS, Wiesel S (1990) Abnormal magnetic-resonance scans of the cervical-spine in asymptomatic subjects—a prospective investigation. J Bone Joint Surg Am 72A(8):1178–1184
Healy JF, Healy BB, Wong WHM, Olson EM (1996) Cervical and lumbar MRI in asymptomatic older male lifelong athletes: frequency of degenerative findings. J Comput Assist Tomogr 20(1):107–112
Kelsey JL (1978) Epidemiology of radiculopathies. Adv Neurol 19:385–398
Kennedy HF, Morrison D, Kaufmann ME, Jackson MS, Bagg J, Gibson BES, Gemmell CG, Michie JR (2000) Origins of Staphylococcus epidermidis and Streptococcus oralis causing bacteraemia in a bone marrow transplant patient. J Med Microbiol 49(4):367–370
Shan Z, Zhang X, Li S, Yu T, Mamuti M, Zhao F (2017) The influence of direct inoculation of Propionibacterium acnes on Modic changes in the spine: evidence from a rabbit model. J Bone Joint Surg Am 99(6):472–481
Dudli S, Liebenberg E, Magnitsky S, Miller S, Demir-Deviren S, Lotz JC (2016) Propionibacterium acnes infected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J Orthop Res 34(8):1447–1455
Shan Z, Zhang X, Li S, Yu T, Liu J, Zhao F (2017) Propionibacterium acnes incubation in the discs can result in time-dependent Modic changes: a long-term rabbit model. Spine (Phila Pa 1976) 42(21):1595–1603
Mitra D, Cassar-Pullicino VN, McCall IW (2004) Longitudinal study of vertebral type-1 end-plate changes on MR of the lumbar spine. Eur Radiol 14(9):1574–1581
Hutton MJ, Bayer JH, Powell JM (2011) Modic vertebral body changes: the natural history as assessed by consecutive magnetic resonance imaging. Spine (Phila Pa 1976) 36(26):2304–2307
Mann E, Peterson CK, Hodler J, Pfirrmann CWA (2014) The evolution of degenerative marrow (Modic) changes in the cervical spine in neck pain patients. Eur Spine J 23(3):584–589
Acknowledgements
The authors would like to acknowledge Prof. Michael Adams for the assistance in revising this manuscript.
Funding
Medical and health research project of Zhejiang Province (2015KYB448).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
Written informed consent was obtained from all participants in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, X., Zhang, X. et al. Low virulence bacterial infections in cervical intervertebral discs: a prospective case series. Eur Spine J 27, 2496–2505 (2018). https://doi.org/10.1007/s00586-018-5582-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5582-4