Abstract
Purpose
Chronic low back pain has been associated with intervertebral disc (IVD) degeneration, which is characterized by the accumulation of extracellular matrix (ECM)-degrading proteases and inflammatory molecules in the degenerate tissue. IVD degeneration could be the outcome of natural organismal ageing and/or of the exposure of the disc to cumulative stressful environmental stimuli and is accompanied by an increased population of senescent cells in the tissue. On the other hand, senescent cells are known to secrete proteolytic enzymes and inflammatory molecules, which can contribute to ECM catabolism. The aim of this study was to investigate the transcriptional profile of selected metalloproteinases (MMPs) and inflammatory mediators in human nucleus pulposus IVD cells that became senescent using three different approaches: serial subculturing, exposure to ionizing radiation and p16INK4a overexpression.
Methods
Gene expression was assessed using quantitative RT-PCR and protein levels were determined by western blot analysis. The proliferative potential of the cells, as well as the percentage of senescent cells in the population were estimated by nuclear BrdU incorporation and by senescence-associated β galactosidase staining, respectively.
Results
All senescent cells showed a similar regulation of MMP-1, -2, -3, -9, interleukin (IL) 6, IL8 and interferon γ at the level of transcription, with only some quantitative differentiations observed in p16INK4a-overexpressing cells.
Conclusions
Data described here suggest that senescent cells may have similar functions in IVD homeostasis, irrespective of the origin of senescence induction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Even though intervertebral disc (IVD) degeneration has been associated with the occurrence of low back pain, this widespread spinal pathology still remains nowadays a challenging condition to treat. Disc degeneration in vivo may be the result either of ageing or of exposure of the organism to repetitive external insults [1]. Nevertheless, whatever the origin of degeneration is, it is always accompanied by the accumulation of extracellular matrix (ECM)-degrading molecules and inflammatory mediators. For example, a number of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinase (TIMPs) and disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs) have been identified in degenerate discs [2,3,4,5,6,7], while elevated levels of cytokines, such as interleukins (ILs), tumour necrosis factor α (TNFα) and interferon γ (IFNγ) have been described for lumbar disc herniation and other IVD disorders [8,9,10]. In addition, degenerate IVDs are characterized by the presence of a high number of senescent cells, as identified by their positive senescence-associated β galactosidase (SA-β Gal) staining and by the overexpression of p16INK4a, a cell cycle inhibitor that is also a marker of replicative and stress-induced senescence [11,12,13,14]. On the other hand, it is known for several cell types that senescent cells express and secrete a common set of catabolic and pro-inflammatory proteins—collectively termed senescence-associated secretory phenotype (SASP) [15]—including several MMPs and inflammatory cytokines. Accordingly, it may be suggested that the accumulation of senescent cells may contribute to the ECM catabolism and inflammation observed during IVD degeneration.
The origin of senescent cells in the IVD is not thus far fully elucidated. In previous publications, their presence has been associated with a decrease of the mean telomere length of IVD cells, implying that cells have become senescent after serial replications (a process called replicative senescence) [16, 17]. However, given that IVD cells have a very low proliferation rate in the tissue [18], cellular senescence seems also to be stress-induced [19], since it has been well established that a series of genotoxic (i.e., DNA damaging) stresses can trigger premature cellular senescence. However, it has not been tested yet whether IVD cells that have become senescent by different means may have similar expression levels of catabolic factors, such as MMPs and inflammatory cytokines, and thus exert comparable changes on local tissue homeostasis. We thus compared early passage nucleus pulposus cells, replicative senescent cells and cells that became senescent after exposure to ionizing radiation—a classical genotoxic insult. In addition, we tested here for the first time, to the best of our knowledge, the effect of p16INK4a overexpression on this cell model, as in all types of cellular senescence increased expression of this particular cell cycle inhibitor stabilizes the senescent state. For that reason, we used an artificial system (lentiviral overexpression of p16INK4a) to solely investigate the role of p16INK4a irrespective of the other parameters of senescence induction after serial subculturing or exposure to ionizing radiation. MMP-1, -2, -3, -9, IL6, IL8 and IFNγ mRNA levels were selected to be studied, since these molecules represent major SASP components at the same time that they have been detected to be upregulated in pathological conditions of the IVD [2, 3, 8, 9].
Materials and methods
Cells and cell culture conditions
Human nucleus pulposus IVD cells were isolated from the discs of consenting orthopaedic patients (Supplementary Table 1), as reported previously [20]. In brief, the nucleus pulposus was aseptically separated from the inner and outer annulus fibrosus regions based on morphology, cut into small pieces and subjected to collagenase digestion. The quality of nucleus pulposus-annulus fibrosus separation was assessed by RT-PCR for the gene collagen I (used as a marker for annulus fibrosus cells) and the genes aggrecan and collagen II (used as markers for nucleus pulposus cells) (data not shown). Nucleus pulposus cells were retrieved by centrifugation and seeded into culture dishes in DMEM supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Biochrom AG, Berlin, Germany) and 20% (v/v) FBS (Gibco BRL, Invitrogen, Paisley, UK) until they became confluent. Human embryonic kidney 293 cells containing the SV40 T-antigen (HEK293T), an established cell line for efficient viral growth, were routinely cultured in DMEM containing 10% (v/v) FBS. Primary nucleus pulposus IVD cell cultures and HEK293T cells were maintained at 37 °C and 5% CO2 and subcultured using a trypsin/citrate (0.25%/0.30%, w/v) solution.
Human nucleus pulposus IVD cells of passage 3–6 isolated from at least two different donors were used for all experimental procedures with no significant differences in cellular responses. Cells were plated and left to proliferate until confluence and (a) were serially subcultured to reach replicative senescence (approximately at passage 30), (b) exposed to a 60Co gamma source (Gamma Chamber 4000A, Isotope Group, Bhabha Atomic Research Company, Trombay, Bombay, India) as described earlier [21, 22] and subcultured after 16 h for three times to reach stress-induced premature senescence, and (c) were infected with viruses carrying an empty vector or a vector for p16INK4a overexpression. Early passage cells plated under the same conditions at the beginning of treatments and left untreated throughout the experiment (except for routine medium change and subculturing whenever necessary) served as the untreated control (young cells). Given the similarity of results among different donors, averages of at least three experiments with cells deriving from one representative donor are presented.
Construction of lentiviral vectors, viruses’ production in HEK293T cells and infection of nucleus pulposus cells
For stable overexpression of p16INK4a the lentiviral expression vector pLenti7.3/V5-GW/lacZverA from Invitrogen (Paisley, UK) (gift from Dr. Lesley Probert) was modified. The plasmid was digested by the restriction endonuclease EcoRV and religated using T4 DNA Ligase (New England Biolabs, Beverly, MA, USA). This modification resulted in the elimination of the lacZ gene and the attB1 and attB2 sequences designated as pLenti7.3/V5 EFGP NO attB. Plasmid pLenti6gw u6laminshrna verB (also gift from Dr. Lesley Probert) was digested by the EcoRV/KpnI restriction endonucleases for the isolation of a DNA fragment including early SV40 promoter, EM7 promoter and the blasticidin resistance gene. This fragment was inserted in the EcoRV/KpnI digested pLenti7.3/V5 EFGP NO attB plasmid. The resulting plasmid pLenti7 Bsd(R) contained the blasticidin resistance gene as the selection marker under the control of SV40 and EM7 promoters (Fig. 1a). Subsequently, p16INK4a open reading frame was amplified using primers (5′-CGGGATCCAGCAGCATGGAGCCGGC-3′) and (5′-CGGAATTCTCAATCGGGGATGTCTGAG-3′) and was then subcloned into the aforementioned plasmid at BamHI/EcoRI sites, resulting in pLenti7 Bsd(R) p16 plasmid (Fig. 1b). pLenti7 Bsd(R) and pLenti7 Bsd(R) p16 plasmids were used for the production of lentiviruses in HEK293T cells, using the ViraPower LentiViral Expression System (Invitrogen). Supernatants were collected and used for the infection of human nucleus pulposus IVD cells. Infected cells were selected with 3 μg/ml blasticidin for a total time period of 15 days. RNA and protein were extracted 3 days after the antibiotic selection period.
Plasmid maps of the modified overexpression vector carrying the blasticidin resistance gene [Bsd(R)] (a) and of p16INK4a cloned into the overexpression vector (b). p16INK4a gene is under the control of the strong CMV promoter. The expression of the blasticidin resistance gene is under the control of SV40 and EM7 promoters
RNA extraction and qPCR analysis
RNA extraction was performed with Trizol (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA synthesis was carried out with the PrimeScript RT Reagent Kit (Takara, Tokyo, Japan), using 500 ng RNA as template after spectrophotometric determination of the concentration of each sample in a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). qPCR experiments were executed in a MX3000P cycler (Stratagene, La Jolla, CA, USA) using qPCRBIO SyGreen Mix Lo-ROX (PCR Biosystems Ltd, London, UK). Relative gene expression to that of young cells was calculated as described before [20] with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) serving as the reference gene. Gene expression in pLenti7 Bsd(R) p16-infected cells was calculated as a ratio to that of pLenti7 Bsd(R)-infected cells. Primer sequences are shown in Table 1.
Western blot analysis for p16INK4a protein expression
Protein extracts were collected, electrophoretically separated and transferred to PVDF membranes, as reported previously [23]. Western blot analysis was performed against p16INK4a and GAPDH (antibodies were from BD Pharmingen, San Diego, CA, USA and Santa Cruz Biotechnology, Santa Cruz, CA, USA, respectively) using a secondary anti-mouse horseradish peroxidase-conjugated antibody purchased from Sigma (St. Louis, MO, USA). Immunoreactive products were detected by an ECL reagent (Amersham Biosciences, Buckinghamshire, UK).
Cell counting
Cell number of human nucleus pulposus IVD cells 2 and 20 days post-selection of infected with the empty or the p16INK4a overexpression (p16 O/E) vector cells was measured using a hemocytometer after trypsinization of the cells.
Estimation of BrdU incorporation
Immunofluorescence experiments for the estimation of 5-bromo-2′-deoxyuridine (BrdU) incorporation in the nuclei of human nucleus pulposus IVD cells were performed as described before [20]. Briefly, after fixation with 4% (w/v) formaldehyde and permeabilization with 0.2% (v/v) Triton X-100, cells were incubated with 2 N HCl and labelled with an anti-BrdU-FITC antibody (Roche Applied Science, Mannheim, Germany). BrdU-positive and negative nuclei counter-stained with 2 μg/ml DAPI were detected in the cell population under a Zeiss AxioPlan 2 microscope (Carl Zeiss, Jena, Germany).
SA-β Gal staining
SA-β Gal staining was performed as previously reported [20]. Cells were seeded and cultured on glass coverslips before fixation with 3% (w/v) formaldehyde and incubation with the SA-β Gal staining solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide and 1 mg/ml X-Gal in a 40 mM citric acid/sodium phosphate pH 6.0, 150 mM NaCl and 2 mM MgCl2 solution) at 37 °C. SA-β Gal- positive cells were counted using a Zeiss Axioplan 2 phase contrast microscope.
Statistical analysis
Values presented are the means ± standard deviations. Differences in comparison to young cells were considered statistically significant when p < 0.05 (Student’s t test).
Results
p16INK4a overexpression leads nucleus pulposus cells to senescence
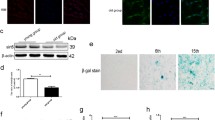
Infected human nucleus pulposus IVD cells with lentiviruses carrying pLenti7 Bsd(R) p16 plasmid showed a significantly higher p16 expression both at the mRNA and protein levels compared to young cells and cells infected with lentiviruses carrying the empty vector (Fig. 2a). p16INK4a overexpression decreased the proliferative capacity of the cells, as shown by the direct cell counting of empty vector- and pLenti7 Bsd(R) p16-infected cells (Fig. 2b). Cell number of p16INK4a-overexpressing cells remained constant for 2 and 20 days post-selection—suggesting an arrest of proliferation during this time period—while cells infected with the virus containing the empty vector demonstrated a progressive increase of their cell number. Reduction of proliferation in p16INK4a-overexpressing cells was confirmed by their diminished ability to incorporate BrdU in the nuclei, evidencing the decrease of novel DNA synthesis (Fig. 2c). At the same time, an increased percentage of SA-β Gal-positive staining was observed for cells infected with lentiviruses containing pLenti7 Bsd(R) p16 plasmid (Fig. 2c), evidencing that the aforementioned lower proliferation rate did not result from a transient cell cycle delay, but rather from the establishment of senescence. Human nucleus pulposus cells after serial subculturing and exposure to ionizing radiation also exhibited increased p16INK4a mRNA levels (data not shown) and were characterized by a percentage of BrdU incorporation and SA-β Gal staining that was always <5 and >60%, respectively, confirming the induction of senescence.
p16INK4a overexpression leads human nucleus pulposus IVD cells to senescence. a Quantitative RT-PCR and western blot analysis for the estimation of p16INK4a mRNA and protein levels, respectively. Asterisk denotes a statistically significant difference (Student’s t test, p < 0.05) from young cells. b Direct counting of the final number of cells infected with lentiviruses containing the empty and the p16INK4a-overexpressing vectors 2 and 20 days post-selection with blasticidin. c Determination of proliferating cells by nuclear BrdU incorporation and of SA-β Gal-positive cells after p16INK4a overexpression. In the graph means ± standard deviations are presented. Asterisk and number sign represent statistically significant differences from the respective young and empty vector-containing cells for BrdU incorporation and SA-β Gal staining, respectively. p16 O/E: cells infected with the pLenti7 Bsd(R) p16 plasmid
Changes in the transcriptional profile of MMP-1, -2, -3 and -9 in senescent nucleus pulposus IVD cells arising from three different treatments
We then assessed the transcriptional profile of selected MMP-encoding genes in senescent due to p16INK4a-overexpression cells in comparison with that of two well established senescent phenotypes, the replicative senescent cells and the γ-irradiation-induced senescent cells (Fig. 3). The general trend was similar, that is, all MMP-encoding genes investigated showed increased mRNA levels in senescent cells compared to young ones, irrespective of the means originally used to induce senescence. The order of the upregulation’s magnitude was comparable for replicative and γ-irradiation-induced senescent cells, while it differed in the case of cells infected with lentiviruses carrying pLenti7 Bsd(R) p16 plasmid (with the exception of MMP-2 that presented the same transcriptional profile in all senescent cells). More specifically, p16INK4a-overexpressing cells were characterized by higher MMP-1 and -9 mRNA levels and lower MMP-3 upregulation in comparison to senescent cells resulting from the other two treatments.
Real-time PCR analysis for the estimation of MMP-1, -2, -3 and -9 mRNA levels of senescent cells due to ionizing radiation (IS), serial subculturing (RS) and infection with lentiviruses carrying pLenti7 Bsd(R) p16 plasmid (p16 O/E). Graphs present mean values ± standard deviations. Statistically significant differences in comparison to young cells (Student’s t test, p < 0.05) are marked by asterisks
Transcriptional alterations in the inflammatory mediators IL6, IL8 and IFNγ in senescent nucleus pulposus IVD cells
qPCR analysis revealed increased IL6, IL8 and IFNγ mRNA levels in all types of senescence (Fig. 4). Cells becoming senescent after consecutive replications and prematurely senescent cells after exposure to ionizing radiation had both an approx. 20-fold IL6 upregulation in comparison to young cells, while p16INK4a-overexpressing cells showed an approx. 120-fold increase. On the other hand, there was an around 200-fold increase of IL8 mRNA levels in replicative and γ-irradiation-senescent cells and a sixfold increase in pLenti7 Bsd(R) p16-infected cells. Upregulation of IFNγ was comparable for all senescent cells (approx. fivefold induction).
Quantitative PCR analysis for the estimation of IL6, IL8 and IFNγ transcriptional alterations in senescent cells after exposure to γ-irradiation (IS), serial subculturing (RS) and infection with the virus containing pLenti7 Bsd(R) p16 plasmid (p16 O/E). In the graphs means ± standard deviations are depicted. Asterisks represent statistically significant differences in comparison to young cells (Student’s t test, p < 0.05)
Discussion
The population of senescent cells has been shown to increase in the IVD with ageing or with progression of degeneration [11,12,13,14], as has also been reported for several other tissues [15] and age-related pathologies [24]. Senescent cells are characterized by altered expression of genes encoding, amongst others, cytokines, growth factors and proteases (namely the SASP) that may in turn influence the structure of the extracellular microenvironment [25]. The manifestation of the SASP has been attributed to the accumulation of DNA damage and not to senescence itself and it has not always been consistent and firm in all cell models investigated [15]. DNA damage triggers a DNA repair response involving a p53-mediated cell cycle delay [26] that becomes an irreversible growth arrest by the expression of the cell cycle inhibitor p16INK4a [24]. Given that cellular senescence could promote IVD degeneration through the secretion of catabolic enzymes and inflammatory cytokines, here we tested the transcriptional alterations of major SASP factors in human nucleus pulposus IVD cells after inducing senescence with three different modes, i.e., replicative exhaustion and exposure to ionizing radiation (both provoking a DNA damage response) and overexpression of p16INK4a, a key molecule securing the establishment of senescence even in the absence of a functional p53 [27].
We showed that senescent IVD cells after consecutive replications, exposure to ionizing radiation and overexpression of p16INK4a had a similar profile of upregulated genes encoding the secreted molecules MMP-1,-2, -3, -9, IL6, IL8 and IFNγ. In the same vein, a catabolic phenotype has been also shown to characterize human nucleus pulposus cells that senesced prematurely after exposure to a different stimulus, i.e., oxidative stress [20]. MMPs may provoke the catabolism of the tissue’s ECM, while inflammatory cytokines—known to also have a degenerative effect by increasing the expression of catabolic enzymes—may in addition reinforce senescence [26], thus perpetuating the degenerative process. In favour of our findings, MMP-1, -2, -3 and -9 expression levels and/or activity have been reported to be increased in degenerate and herniated lumbar discs [2, 5, 8, 28]. The different tendency of MMP-3 upregulation in senescent cells deriving from the three treatments in comparison to the other MMPs investigated here may be attributed to the inherent variations of MMP expression in IVD tissues [29]. In addition, IL6 has been detected in cases of herniation, radiculopathy or spinal fusion [8,9,10], IL8 has been identified in surgical disc specimens removed to relieve pain and arthrodesis patients [8] and IFNγ has been found in degenerated discs and low back pain patients [8, 10]. IL6 and IL8 have been considered as major markers of the SASP, as they have been shown to be elevated in many types of senescence [30]. Furthermore, IL6 also enhances the action of other inflammatory molecules in the nucleus pulposus, such as IL1 and TNFα [10]. In contrast to our findings, it has been reported that ectopic expression of p16INK4a in human and mouse fibroblasts leads to senescence that is not accompanied by a SASP at the mRNA or the protein level [24]. Moreover, it has been demonstrated that p16INK4a-overexpressing chondrocytes retain the ability to increase MMP-1 and MMP-13 protein levels, however, without significantly upregulating IL6 and IL8 [31]. This discrepancy may arise from the different cell model used in each study or may reflect differences in the experimental design. Nevertheless, a positive correlation between the increased p16INK4a expression in the degenerate IVDs and that of matrix-degrading enzymes has been reported in cells isolated from control and degenerated discs [12]. Of note, in our study, senescent cells after serial subculture or exposure to ionizing radiation (both inducing a DNA damage response) exhibited quantitatively similar expression levels of the investigated genes, while in senescent cells after p16INK4a induction alone an overexpression of all these genes was observed albeit with different potencies in some cases. However, to characterize in detail the SASP of these cells, other factors associated with IVD degeneration (e.g., ADAMTSs) need to be analyzed as well, which is a part of an ongoing research in our laboratory.
Conclusion
Low back pain that concerns the majority of the population worldwide has been connected to IVD degeneration, a cumulative result of ageing and external degenerative stimuli. Degenerated IVD is characterized by the presence of a high percentage of senescent cells, as a result of the continuous exposure to several external insults characteristic of the harsh environment of this tissue [32]. Findings of this, as well as of previous studies [20], generally indicate that nucleus pulposus IVD cells express a similar transcriptional profile of many catabolic and inflammatory genes known to be linked with IVD degeneration, irrespective of the mechanism triggering senescence. Accordingly, all these types of senescent cells may contribute to the disturbance of the structural integrity of the disc. Thus, understanding the mechanism of induction of cellular senescence, as well as the physiology and behaviour of these cells is of outmost importance for the design of therapies targeting anti-senescence and for cell replacement strategies for the prevention or treatment of disc degeneration.
References
Vo NV, Hartman RA, Patil PR, Risbud MV, Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA, Kang JD (2016) Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res 34:1289–1306
Sivan SS, Hayes AJ, Wachtel E, Caterson B, Merkher Y, Maroudas A, Brown S, Roberts S (2014) Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur Spine J 23(Suppl 3):S344–S353
Le Maitre CL, Freemont AJ, Hoyland JA (2004) Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol 204:47–54
Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA (2009) Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheumatol 60:482–491
Bachmeier BE, Nerlich A, Mittermaier N, Weiler C, Lumenta C, Wuertz K, Boos N (2009) Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J 18:1573–1586
Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD (2013) Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J 13:331–341
Wuertz K, Vo N, Kletsas D, Boos N (2012) Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-kappaB and MAP kinases. Eur Cell Mater 23:103–119 (discussion 119–120)
Cuellar JM, Golish SR, Reuter MW, Cuellar VG, Angst MS, Carragee EJ, Yeomans DC, Scuderi GJ (2010) Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J 10:212–218
Andrade P, Hoogland G, Garcia MA, Steinbusch HW, Daemen MA, Visser-Vandewalle V (2013) Elevated IL-1beta and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur Spine J 22:714–720
Risbud MV, Shapiro IM (2014) Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 10:44–56
Gruber HE, Ingram JA, Norton HJ, Hanley EN Jr (2007) Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine 32:321–327 (Phila Pa 1976)
Le Maitre CL, Freemont AJ, Hoyland JA (2007) Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther 9:R45
Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM (2006) Senescence in human intervertebral discs. Eur Spine J 15(Suppl 3):S312–S316
Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y (2016) Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle 15:1674–1684
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6:2853–2868
Jeong SW, Lee JS, Kim KW (2014) In vitro lifespan and senescence mechanisms of human nucleus pulposus chondrocytes. Spine J 14:499–504
Kim KW, Chung HN, Ha KY, Lee JS, Kim YY (2009) Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J 9:658–666
Johnson WE, Roberts S (2003) Human intervertebral disc cell morphology and cytoskeletal composition: a preliminary study of regional variations in health and disease. J Anat 203:605–612
Kletsas D (2009) Senescent cells in the intervertebral disc: numbers and mechanisms. Spine J 9:677–678
Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D (2015) Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater 30:89–102 (discussion 103)
Liakou E, Mavrogonatou E, Pratsinis H, Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG, Kletsas D (2016) Ionizing radiation-mediated premature senescence and paracrine interactions with cancer cells enhance the expression of syndecan 1 in human breast stromal fibroblasts: the role of TGF-beta. Aging (Albany NY) 8:1650–1669
Papadopoulou A, Kletsas D (2011) Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int J Oncol 39:989–999
Mavrogonatou E, Kletsas D (2009) High osmolality activates the G1 and G2 cell cycle checkpoints and affects the DNA integrity of nucleus pulposus intervertebral disc cells triggering an enhanced DNA repair response. DNA Repair (Amst) 8:930–943
Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J (2011) Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem 286:36396–36403
Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118
Tasdemir N, Lowe SW (2013) Senescent cells spread the word: non-cell autonomous propagation of cellular senescence. EMBO J 32:1975–1976
Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE (2006) p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443:453–457
Rutges JP, Kummer JA, Oner FC, Verbout AJ, Castelein RJ, Roestenburg HJ, Dhert WJ, Creemers LB (2008) Increased MMP-2 activity during intervertebral disc degeneration is correlated to MMP-14 levels. J Pathol 214:523–530
Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N (2002) 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J 11:308–320
Purcell M, Kruger A, Tainsky MA (2014) Gene expression profiling of replicative and induced senescence. Cell Cycle 13:3927–3937
Philipot D, Guerit D, Platano D, Chuchana P, Olivotto E, Espinoza F, Dorandeu A, Pers YM, Piette J, Borzi RM, Jorgensen C, Noel D, Brondello JM (2014) p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res Ther 16:R58
Urban JP (2002) The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans 30:858–864
Acknowledgements
We would like to thank Dr. Lesley Probert for generously providing us plasmids pLenti7.3/V5-GW/lacZverA and pLenti6gw u6laminshrna verB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vamvakas, SS., Mavrogonatou, E. & Kletsas, D. Human nucleus pulposus intervertebral disc cells becoming senescent using different treatments exhibit a similar transcriptional profile of catabolic and inflammatory genes. Eur Spine J 26, 2063–2071 (2017). https://doi.org/10.1007/s00586-017-5198-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5198-0