Abstract
Purpose
Insulin-like growth factor 1 (IGF1) gene single nucleotide polymorphism (rs5742612) has been associated with adolescent idiopathic scoliosis (AIS) in several studies with limited sample size and inconsistent outcomes. So we perform this meta-analysis to assess the precise association between IGF1 gene single nucleotide polymorphism (rs5742612) and AIS.
Methods
We systematically searched Pubmed, Embase, Web of Science and Cochrane Library up to January 19, 2016 to obtain relevant studies using our research strategy. Four articles all belonging to case–control studies were included in our meta-analysis.
Results
A total of four studies containing 763 cases and 559 controls satisfied the inclusion criteria after judgment by two reviewers. No significant associations were detected between IGF1 gene single nucleotide polymorphism (rs5742612) and AIS (T vs. C, OR = 1.10, 95 % CI 0.91–1.34, p = 0.32; TT vs. CC: OR = 1.28, 95 % CI 0.82–2.02, p = 0.28; TC vs. CC: OR = 1.29, 95 % CI 0.82–2.06, p = 0.27; TT/TC vs. CC: OR = 1.28, 95 % CI 0.83–1.98, p = 0.27; TT vs. TC/CC: OR = 1.06, 95 % CI 0.82–1.36, p = 0.66).
Conclusions
IGF1 gene single nucleotide polymorphism (rs5742612) is not significant associated with susceptibility to AIS in either Asian or Caucasian populations. However, IGF1 gene rs5742612 may be associated with severity of AIS. Further studies with larger sample size and different population groups involving the relationship are required to confirm the potential association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adolescent idiopathic scoliosis (AIS) is defined as a complex three-dimensional deformity of the spine, which involves the spinal imbalance of sagittal and coronal planes, and vertebrae rotation deformity [1]. In addition, AIS is also the most common form of scoliosis in adolescents, especially in girls around puberty, of which the incidence ranges from 2 to 4 % worldwide [2–4] and accounts for 80 % [5] in scoliosis. The susceptibility of AIS is influenced by different kinds of factors, such as heritage and genetic factors [6–8], environment [9], hormonal disturbance [10], developmental neuromuscular dysfunction [11] and so on. Though the exact etiology and pathogenesis of AIS remains unknown [1], genetic factors are considered to play an essential role, such as insulin-like growth factor 1 (IGF1) gene, estrogen receptor 1 (ESR1) gene, ladybird homeobox 1 (LBX1) gene, matrilin 1 (MATN1) gene and so on [12]. In addition, some genetic factors may be associated with curve severity of AIS, such as ESR1 gene [13] and transforming growth factor beta 1 (TGFB1) gene [14].
With regard to hormonal disorders, growth hormone and IGF1 axis plays a critical part in modulating children’s growth and development [15–17]. Growth hormone and IGF1 have extensive physiological functions that can contribute to cell proliferation, regulate substance metabolism, and promote organ development [15]. Meanwhile, IGF1 can regulate the growth-promoting actions of growth hormone [15]. Moreover, some studies showed that IGF1 could directly promote osteoblast proliferation and collagen synthesis [16, 18], thus the irregular expression of IGF1 gene may affect the progress of AIS [16]. In addition, some studies suggested that the T/C polymorphism (rs5742612) of IGF1 gene at the promoter region of IGF1 gene might influence levels and functional activities of IGF1 protein [19, 20].
Recently, several researches have studied the association between the IGF1 rs5742612 single nucleotide polymorphism (SNP) and AIS [15, 16, 21–23], but Moon et al.’s result [23] showed a significant association which was inconsistent with the result of other studies. The result from one single study might be less convincing to reach the conclusion. Therefore, we conduct the meta-analysis to evaluate and synthesize the currently available data on the association between the IGF1 (rs5742612) SNP and susceptibility to AIS, and find out the precise association between them.
Materials and methods
The meta-analysis was based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) criteria [24]. Detailed research protocol was established before conducting this meta-analysis. All published studies up to January 19, 2016 on humans were collected.
Search strategy
We systematically searched Pubmed, Embase, Web of Science and Cochrane Library by using the following keyword search string: (scoliosis OR AIS) AND (IGF1 OR rs5742612) AND (polymorphism OR variant OR mutation) to gain relevant data. There were not any restrictions on language or publication date in our study.
Identification of eligible records
Two independent authors (M. Guan and H. Wang) identified and screened eligible studies simultaneously, and any disagreements were resolved by discussion and consensus. We also used a two-level search strategy to get more reliable articles. First, all available databases were used to search relevant studies by using the search (e.g., title, abstract, keywords). Second, full text trials were used to identify available studies following first screening.
Inclusion criteria
The following list showed the inclusion criteria of our meta-analysis:
-
1.
The patients should meet the criteria for AIS;
-
2.
Studies with a relationship between rs5742612 and AIS;
-
3.
Included studies should have full text articles;
-
4.
Only case–control studies were included;
-
5.
Studies should have available data.
Methodological quality assessment
Two independent authors (M. Guan and H. Wang) assessed the methodological quality of studies using the Newcastle–Ottawa Scale (NOS). Discrepancies were settled by debate until agreement was reached. Only studies with a score of 5 or more stars were deemed of high methodological quality.
Data extraction
-
1.
First author;
-
2.
Publication date;
-
3.
Ethnicity which patients were belonged to and country where the study was implemented;
-
4.
Genotyping method;
-
5.
Genotype distribution and frequency;
-
6.
Study design (total numbers, numbers of cases and controls, sex and age).
Statistical analysis
The Revman 5.3 software was used in performing the meta-analysis. The association was assessed by using p value and odds ratio (OR) with a 95 % confidence interval (CI). For rs5742612, the allelic comparison (T vs. C) and co-dominant genotypic comparison (TT vs. CC and TC vs. CC), dominant (TT + TC vs. CC), and recessive (TT vs. TC + CC) genetic models were examined.
The inconsistency index I 2 and Chi square were used to evaluate the heterogeneity. The fixed-effect model was used to assess the pooled OR when the value of I 2 was less than 50 % [25] and heterogeneity was not statistically significant. Otherwise, the random-effect model was adopted. Pearson’s goodness-of-fit Chi square test was used to evaluate Hardy–Weinberg equilibrium (HWE). Funnel plots [26], Begg’s test and Egg’s test were performed for evidence of publication bias. Every single study was removed one by one to examine the effect of the respective data, and then deleted the article of high heterogeneity.
Results
Study characteristics
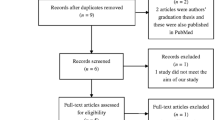
Based on the search strategy, a total of 20 articles were obtained (Pubmed: 4; Embase: 6; Web of Science: 7; Cochrane Library: 3). On second scanning, four literatures [15, 16, 22, 23] satisfying the eligibility criteria were selected in this meta-analysis, which included 1322 subjects with 763 cases and 559 controls. The selection process was reflected in Fig. 1. Moreover, three studies were carried out in Asian populations, whereas another study was conducted in Caucasian population. Study characteristics and allele/genotype frequencies of selected studies are demonstrated in Tables 1 and 2. In addition, Nikolova et al.’s study [22] detected the deviation from HWE in the control group.
Association of rs5742612 and AIS
Table 3 displayed the general outcomes of the summary ORs and I 2 statistics. Overall, the pooled analysis indicated that there was no statistical significant evidence that the IGF1 (rs5742612) SNP have association with AIS (T vs. C, OR = 1.10, 95 % CI 0.91–1.34, p = 0.32, Fig. 2; TT vs. CC: OR = 1.28, 95 % CI 0.82–2.02, p = 0.28, Fig. 3; TC vs. CC: OR = 1.29, 95 % CI 0.82–2.06, p = 0.27, Fig. 4).
Forest plot of the association between IGF1 single nucleotide polymorphism (rs5742612) and AIS risk for the allelic comparison (T vs. C). Meta-analysis was performed using an inverse variance (IV) fixed-effect model. The odds ratio for each study is represented in the plot as a black square with the area corresponding to study weight. The 95 % confidence interval (CI) is represented as a horizontal line
Forest plot of the association between IGF1 single nucleotide polymorphism (rs5742612) and AIS risk for the codominant genotypic comparison (TC vs. CC) Meta-analysis was performed using an inverse variance (IV) fixed-effect model. The odds ratio for each study is represented in the plot as a black square with the area corresponding to study weight. The 95 % confidence interval (CI) is represented as a horizontal line
Forest plot of the association between IGF1 single nucleotide polymorphism (rs5742612) and AIS risk for the codominant genotypic comparison (TT vs. CC) Meta-analysis was performed using an inverse variance (IV) fixed-effect model. The odds ratio for each study is represented in the plot as a black square with the area corresponding to study weight. The 95 % confidence interval (CI) is represented as a horizontal line
The present meta-analysis adopted a leave-one-out analysis to evaluate the sensitivity of our study. The overall statistical significance did not reverse when any single study was removed, indicating that the results were stable despite including or excluding Nikolova et al.’s study [22] with HWE-deviated controls. Therefore, we confirmed that our meta-analysis study had relatively stable and credible characteristics.
We assessed the publication bias of our meta-analysis through the Begg’s test and Egg’s test in the overall populations and the results illustrated that there was no evidence of obvious asymmetry, revealing minimal evidence of publication bias. The number of involving studies (n < 10) was insufficient for significant elucidation of funnel plots with respect to publication bias.
Discussion
Nowadays, the precise etiology of AIS has been still unknown. Some studies suggested that genetic risk factors played a role in AIS, in which the IGF1 gene attracted much attention. With regard to IGF1 rs5742612 SNP, the study from Yeung et al. [16], which included 503 AIS patients and 227 healthy subjects, first demonstrated that the genetic polymorphisms associated with IGF1 was unlikely to be commonly associated with AIS. In addition, Yang et al. [15] performed the SNP genotyping of IGF1 study including 87 AIS cases and 87 controls with ethnical and sexual match and the outcomes showed that IGF1 rs5742612 SNP could have no correlation to the etiology of AIS in Chinese population. However, in Moon et al.’s study [23], with 68 AIS cases and 68 healthy controls, the result illustrated that the IGF1 rs5742612 SNP was associated with susceptibility to AIS in Korean population. The latest study by Nikolova et al. [22] indicated that there was no statistically significant association between the IGF1 rs5742612 SNP and susceptibility to AIS with 105 AIS patients and 210 healthy controls in Bulgarian population. In summary, the four studies showed that the association between IGF1 gene (rs5742612) and the susceptibility to AIS were inconsistent.
No significant association in either allele or genotype frequencies of IGF1 gene rs5742612 polymorphism between AIS patients and healthy controls was found in our meta-analysis study involving 763 cases and 559 controls and the result was coherent with individual studies [15, 16, 22] included in the present meta-analysis. While, these findings were inconsistent with those reported by Moon et al.’s study [23]. Interestingly, Moon et al.’s study [23] demonstrated that no significant association between IGF1 gene rs5742612 and susceptibility of AIS was observed in the T allele, but significant association was found in the co-dominant genotypic comparison (TT vs. CC and TC vs. CC), the dominant genotypic comparison (TT + TC vs. CC) and the recessive genotypic comparison (TT vs. TC + CC). This phenomenon was not observed in three other studies which showed that no significant association was found in different genotypic models.
Although there is a lack of direct association with AIS predisposition, some evidences indicated that the IGF1 rs5742612 polymorphism may devote to curve severity of AIS, which was demonstrated in Yeung et al.’s study [16]. In this study, a much higher Cobb’s angle was found in patients with the TT genotype compared to those with the TC and CC genotypes. Moreover, Moon et al. [23] also provided the evidence that IGF1 gene rs5742612 was associated with the curve severity in AIS. However, Takahashi et al. [21] and Nikolova et al. [22] concluded that polymorphism (rs5742612) of IGF1 gene was not associated with the AIS curve severity. Therefore, further study needs to identify the role of rs5742612 in risk of the curve severity of AIS.
Besides, some other genes may also play a part in the curve severity of AIS. ESR1 gene rs9340799 may not be a susceptible variant of AIS, but it may play a role in the associated with AIS severity, progression and treatment [13]. Similarly, TGFB1 gene rs1800469 may be associated with the curve severity of AIS and not be a likely predisposition variant for AIS predisposition in the Chinese population [14].
Although above genes showed statistically significant association with the severity of AIS, the predictive values of the associations for clinical use as diagnostic criteria should be further investigated. Noshchenko et al. [27] reviewed 25 studies showed significant association between genes and the severity of AIS. However, the predictive values were limited for clinical use as diagnostic criteria for selection of treatment strategy, especially preventive surgical intervention [27]. Moreover, Zhu et al.’s result [28] showed that though there was no association between suppressor of cytokine signaling 3 (SOCS3) rs4969198 and the susceptibility or curve severity of AIS, it was still associated with abnormal growth pattern of AIS, suggesting that SOCS3 gene might be a disease-modifying gene of AIS.
Quality of evidence
The quality of four studies contained in our meta-analysis was relatively lacking, but adequate data were presented to enable an evaluation of the general methodological quality. All four studies with high methodological quality were evaluated by the modified NOS.
The reason of the gender discrepancy exhibited in AIS incidence is still unclear. However, the gender discrepancy may result in a difference of AIS susceptibility. In our meta-analysis, four studies did not focus on the sex discrepancy of AIS incidence. As a result, no analysis of sex discrepancy was performed in our meta-analysis and further studies might contain the issue.
HWE is a principle to detect whether observed genotypic frequencies and allele frequencies between parents and their offspring are in equilibrium in a population. Population stratification would lead to the deviation of HWE, which may result in a mixed association. In four studies, only Nikolova et al.’s study [22] were not consistent with HWE, which may influence the overall outcomes. But after excluding the research, the overall statistical significance did not reverse. Therefore, we confirmed that our meta-analysis study had relatively stable and credible characteristics.
Sufficient sample sizes were needed to ensure adequate statistical power. However, the sample size of three studies [15, 22, 23] in the present meta-analysis was relatively small and it may decrease the statistical strength to detect the correlation, possibly bring about debating the consequences and influencing the conclusions.
When compared with different ethnic and population groups, the results of our meta-analysis demonstrated that there were no significant associations between IGF1 rs5742612 polymorphisms and susceptibility to AIS in either Chinese [15, 16] or Bulgarian [22] populations, which was inconsistent with Korean population [23]. Since the diversity and potential differences in AIS susceptibility among populations, our meta-analysis outcomes may not be generalizable and further researches in other ethnic and population are necessary to provide more evidences.
Conclusion
In summary, we adopted all the available studies reporting the association between IGF1 rs5742612 polymorphism and AIS in this meta-analysis, and our study illustrated that IGF1 rs5742612 polymorphism was not significantly associated with susceptibility to AIS. However, IGF1 rs5742612 may be associated with the severity of AIS. Further researches should contain larger sample size and different populations to improve the quality of meta-analysis and confirm the potential association.
References
Yagi M MM, Asazuma T (2014) Pathogenesis of adolescent idiopathic scoliosis. JBJS Rev 2(1). doi:10.2106/JBJS.REV.M.00037
Kane WJ (1977) Scoliosis prevalence: a call for a statement of terms. Clin Orthop Relat Res (126):43–46
Suh SW, Modi HN, Yang JH, Hong JY (2011) Idiopathic scoliosis in Korean schoolchildren: a prospective screening study of over 1 million children. Eur Spine J 20:1087–1094. doi:10.1007/s00586-011-1695-8
Weinstein SL (1999) Natural history. Spine (Phila Pa 1976) 24:2592–2600
Negrini S, Aulisa AG, Aulisa L, Circo AB, de Mauroy JC, Durmala J, Grivas TB, Knott P, Kotwicki T, Maruyama T, Minozzi S, O’Brien JP, Papadopoulos D, Rigo M, Rivard CH, Romano M, Wynne JH, Villagrasa M, Weiss HR, Zaina F (2012) 2011 SOSORT guidelines: orthopaedic and Rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis 7:3. doi:10.1186/1748-7161-7-3
Chen S, Zhao L, Roffey DM, Phan P, Wai EK (2014) Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis in East Asians: a systematic review and meta-analysis. Spine J Off J N Am Spine Soc 14:2968–2975. doi:10.1016/j.spinee.2014.05.019
Chan V, Fong GC, Luk KD, Yip B, Lee MK, Wong MS, Lu DD, Chan TK (2002) A genetic locus for adolescent idiopathic scoliosis linked to chromosome 19p13.3. Am J Hum Genet 71:401–406. doi:10.1086/341607
Miller NH, Justice CM, Marosy B, Swindle K, Kim Y, Roy-Gagnon MH, Sung H, Behneman D, Doheny KF, Pugh E, Wilson AF (2012) Intra-familial tests of association between familial idiopathic scoliosis and linked regions on 9q31.3–q34.3 and 16p12.3–q22.2. Hum Hered 74:36–44. doi:10.1159/000343751
Ji XR, Yang ZD, Yang XH, Liu DD, Ni HJ, Li M (2013) Change of selenium in environment and risk of adolescent idiopathic scoliosis: a retrospective cohort study. Eur Rev Med Pharmacol Sci 17:2499–2503
Zhang Y, Gu Z, Qiu G (2014) The association study of calmodulin 1 gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Biomed Res Int 2014:168106. doi:10.1155/2014/168106
Lowe TG, Edgar M, Margulies JY, Miller NH, Raso VJ, Reinker KA, Rivard CH (2000) Etiology of idiopathic scoliosis: current trends in research. J Bone Jt Surg Am 82-A:1157–1168
Gorman KF, Julien C, Moreau A (2012) The genetic epidemiology of idiopathic scoliosis. Eur Spine J 21:1905–1919. doi:10.1007/s00586-012-2389-6
Chen S, Zhao L, Roffey DM, Phan P, Wai EK (2014) Association between the ESR1 -351A > G single nucleotide polymorphism (rs9340799) and adolescent idiopathic scoliosis: a systematic review and meta-analysis. Eur Spine J 23:2586–2593. doi:10.1007/s00586-014-3481-x
Xu L, Sun W, Qin X, Qiu Y, Zhu Z (2016) The TGFB1 gene is associated with curve severity but not with the development of adolescent idiopathic scoliosis: a replication study in the Chinese population. BMC Musculoskelet Disord 17:15. doi:10.1186/s12891-016-0863-8
Yang Y, Wu Z, Zhao T, Wang H, Zhao D, Zhang J, Wang Y, Ding Y, Qiu G (2009) Adolescent idiopathic scoliosis and the single-nucleotide polymorphism of the growth hormone receptor and IGF-1 genes. Orthopedics 32:411. doi:10.3928/01477447-20090511-08
Yeung HY, Tang NL, Lee KM, Ng BK, Hung VW, Kwok R, Guo X, Qin L, Cheng JC (2006) Genetic association study of insulin-like growth factor-I (IGF-I) gene with curve severity and osteopenia in adolescent idiopathic scoliosis. Stud Health Technol Inform 123:18–24
Rubin K (2000) Pubertal development and bone. Curr Opin Endocrinol Diabetes Obes 7:65–70
McCarthy TL, Centrella M, Canalis E (1989) Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology 124:301–309. doi:10.1210/endo-124-1-301
Kim JG, Roh KR, Lee JY (2002) The relationship among serum insulin-like growth factor-I, insulin-like growth factor-I gene polymorphism, and bone mineral density in postmenopausal women in Korea. Am J Obstet Gynecol 186:345–350
Rietveld I, Janssen JA, Hofman A, Pols HA, van Duijn CM, Lamberts SW (2003) A polymorphism in the IGF-I gene influences the age-related decline in circulating total IGF-I levels. Eur J Endocrinol Eur Fed Endocr Soc 148:171–175
Takahashi Y, Matsumoto M, Karasugi T, Watanabe K, Chiba K, Kawakami N, Tsuji T, Uno K, Suzuki T, Ito M, Sudo H, Minami S, Kotani T, Kono K, Yanagida H, Taneichi H, Takahashi A, Toyama Y, Ikegawa S (2011) Lack of association between adolescent idiopathic scoliosis and previously reported single nucleotide polymorphisms in MATN1, MTNR1B, TPH1, and IGF1 in a Japanese population. J Orthop Res 29:1055–1058. doi:10.1002/jor.21347
Nikolova S, Yablanski V, Vlaev E, Stokov L, Savov AS, Kremensky IM (2015) Association study between idiopathic scoliosis and polymorphic variants of VDR, IGF-1, and AMPD1 genes. Genet Res Int 2015:852196. doi:10.1155/2015/852196
Moon ES, Kim HS, Sharma V, Park JO, Lee HM, Moon SH, Chong HS (2013) Analysis of single nucleotide polymorphism in adolescent idiopathic scoliosis in Korea: for personalized treatment. Yonsei Med J 54:500–509. doi:10.3349/ymj.2013.54.2.500
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:2535. doi:10.1136/bmj.b2535
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. doi:10.1136/bmj.327.7414.557
Ioannidis JP, Trikalinos TA (2007) The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ 176:1091–1096. doi:10.1503/cmaj.060410
Noshchenko A, Hoffecker L, Lindley EM, Burger EL, Cain CM, Patel VV, Bradford AP (2015) Predictors of spine deformity progression in adolescent idiopathic scoliosis: a systematic review with meta-analysis. World J Orthop 6:537–558. doi:10.5312/wjo.v6.i7.537
Zhu F, Qiao J, Qiu X, Xu L, Liu Z, Zhu Z, Qian B, Sun X, Qiu Y (2014) Lack of association between suppressor of cytokine signaling-3 gene polymorphism and susceptibility and curve severity of adolescent idiopathic scoliosis. Eur Spine J 23:2432–2436. doi:10.1007/s00586-014-3452-2
Acknowledgments
This work is supported by National Natural Science Foundation of China (No. 81271347).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest to this work. No benefits in any form have been or wiil be received from a commercial party related directly or indirectly to the subject of this manuscript.
Additional information
M. Guan and H. Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guan, M., Wang, H., Fang, H. et al. Association between IGF1 gene single nucleotide polymorphism (rs5742612) and adolescent idiopathic scoliosis: a meta-analysis. Eur Spine J 26, 1624–1630 (2017). https://doi.org/10.1007/s00586-016-4742-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4742-7