Abstract
Purpose
We aimed to study generational changes in the dimensions of cervical and lumbar bony spinal canals in Western Switzerland.
Methods
A total of 254 patients were retrospectively included, 144 of whom were born during 1940–1949 and 110 during 1970–1979. Cervical spine CTs were performed as part of the spinal clearance procedure following trauma (n = 135) or while investigating neurological symptoms (CT angiography, n = 119). Three independent observers digitally measured the cross-sectional area (CSA) at pedicle levels from C0 to C7 and the anteroposterior diameter (APD) at C3, C5, and C7. In addition, lumbar spine CSAs and APDs were measured on whole body trauma or abdominal CTs, which were also available for 134 patients.
Results
Mean CSAs at pedicle levels were numerically smaller in the younger patient group in both cervical and lumbar spine, with the difference reaching statistical significance at all lumbar levels (p ≤ 0.024) except L5. Cervical APDs showed no difference between groups. Subgroup analysis revealed that younger CT angiography patients had a significantly smaller CSA at C1 (p = 0.018) and a similar trend at C4 (p = 0.053). There was moderate positive correlation between cervical and lumbar CSAs, taking C4 and L3 as reference (r = 0.509, p < 0.01).
Conclusions
Younger generation patients have smaller bony spinal canals also in the cervical spine even though this difference is less marked than at the lumbar level. There is, nevertheless, moderate positive correlation between these two anatomical regions. Perinatal factors that adversely influence spinal growth, such as increased maternal age and smoking, could explain these generational changes, given that body height has increased during the same time period. The lesser difference observed in the cervical spine could be due to later closure of the neurocentral synchondrosis at this level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stenosis of the cervical spinal canal may remain asymptomatic but is associated with the development of cervical spondylotic myelopathy, a disabling condition whose natural history and prognosis remain poorly understood [1]. Cervical spinal stenosis develops as a result of intervertebral disc degeneration, osteophyte formation, and the presence of a congenitally smaller bony spinal canal [2–4]. The coexistence of degenerative disc disease and developmentally smaller bony canal has been reported as leading to the earlier development of myelopathy [2, 4–7]. Ethnic variations in cervical spinal canal dimensions have been reported, with some evidence suggesting that Asian subjects tend to have smaller mid-sagittal anteroposterior diameters (APDs) than European or American patients, the latter being 10–20 % larger [8].
Previous research on the evolution of lumbar spinal canal dimensions suggested that despite improved nutrition and overall living conditions, the cross-sectional area (CSA) of the bony canal measured at pedicle levels has decreased in one generation in Western Switzerland [9]. Indeed, subjects born in the 70s (1970–1979) were found to have significantly smaller bony canals as compared to those born in the 40s (1940–1949), despite the gradual increase in body height documented during the same time period. Perinatal factors known to negatively impact spinal growth, such as increased maternal age at birth and smoking habits [10, 11], have been postulated as possible explanations for this surprising finding.
The aim of the present study was to evaluate whether the same trend could also be found for the cervical spinal canal of patients born one generation apart, applying the same selection criteria for patient age (i.e., born in the 40s or 70s) in the same geographical area.
Materials and methods
This single-center retrospective study was approved by the institutional ethics committee, with waiver of informed consent (protocol number 121/15).
Study population
We first retrieved from our institutional database 421 consecutive cervical spine computed tomography (CT) scans performed over a 5-year period (January 2009 to December 2013), either as part of the spinal clearance procedure following trauma, or while investigating central neurological symptoms (suspected stroke, transient ischemic attack, or epilepsy) by CT angiography. These CTs were performed in patients born one generation apart, i.e., either during 1940–1949 (a period of harsher living conditions), or during 1970–1979 (a period of economic prosperity). Patients with non-national sounding surnames (n = 159) were excluded to minimize bias due to documented ethnic variations in spinal canal dimensions [8, 12, 13]. After application of other exclusion criteria, such as examinations that did not cover the entire cervical spine from the occiput (C0) to C7 (n = 3), displaced fractures altering the dimensions of the bony spinal canal (n = 3), or previous cervical spine surgery (n = 2), a total of 254 patients (168 males, 86 females) were finally retained. One hundred and forty-four patients (99 males, 45 females) were born during 1940–1949 (older group), while 110 (69 males, 41 females) during 1970–1979 (younger group). Non-contrast cervical spine CTs were performed in 135 subjects (94 males, 41 females) for trauma, while 119 (74 males, 45 females) were CT angiographies. The following patient subgroups were defined for the study purposes: older trauma (n = 65), younger trauma (n = 70), as well as older and younger CT angiography (n = 79, and n = 40, respectively).
In addition, 134 (98 males, 36 females; 69 from the older group, and 65 from the younger group) of the 254 patients also underwent lumbar spine/abdominal CT, 94 as part of their initial trauma assessment, and 40 while investigating abdominal symptoms. Thirty-two of these 134 patients (24 %) were included in a previous study on lumbar spinal canal dimensions [9].
CT protocols
A 64-detector row CT scanner (LightSpeed VCT; GE Healthcare, Milwaukee, WI, USA) was used for all CT examinations, with the following acquisition parameters: tube voltage, 120 kVp; tube current, 200 or 400 mA for cervical or lumbar/abdominal protocols, respectively; and detector configuration, 64 × 0.625 mm. Images were then reconstructed using the following parameters: section thickness/interval, 1.25/1, 0.625/0.625, or 2.5/2 mm for cervical trauma, CT angiography, or lumbar/abdominal protocols, respectively; and smooth (CT angiography and abdominal protocols) or sharp (cervical and lumbar trauma protocols) convolution kernels.
Image analysis
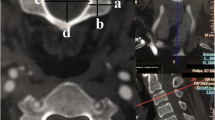
All examinations were anonymized and available for measurements by three independent observers (a student, a resident and an experienced musculoskeletal radiologist) on a picture archiving and communication system (Vue; Carestream Health, Rochester, NY, USA). The radiology resident measured all the cases, while 50 randomly selected cases were measured by the other two to assess interobserver agreement. Furthermore, the first observer measured another random series of 50 cases following a 3-month interval to evaluate intraobserver variability. The CSA of the bony spinal canal was contoured using freehand regions of interest (ROIs) at pedicle levels from C0 to C7 in cervical spine CTs, and when available from L1 to L5 in lumbar spine/abdominal CTs. In addition, the APD was measured at C3, C5, and C7 bony canal levels, as well as L1, L3, and L5. All measurements were performed using the multiplanar reconstruction viewing mode, in a plane perpendicular to the longitudinal axis of the spine and at pedicle levels (Fig. 1), given that degenerative changes influence spinal canal dimensions at disc levels [9, 14–16]. The window level and width of CT images were set to 350 and 2000 Hounsfield units, respectively.
69-year-old male patient belonging to the older generation trauma subgroup. Axial-oblique reformatted CT images of the cervical spine (bone convolution kernel and windowing parameters), either at C4 pedicle levels (a), or at C4–C5 intervertebral foramina levels (b). Note the change in cross-sectional area of the bony spinal canal (red dotted line) partly due to degenerative disc disease and facet joint osteoarthritis at level of intervertebral foramina (b)
Statistical analysis
Continuous variables are presented as means ± standard deviations. Comparison of CSA and APD between groups and subgroups were performed using an unpaired two-tailed Student’s t test on SPSS statistics software (IBM SPSS, Chicago, IL, USA). Pearson’s correlation coefficient was used to assess the relationship between CSAs and APDs measured at mid-cervical and mid-lumbar levels, and interpreted as follows: very weak, <0.20; weak, 0.20–0.39; moderate, 0.40–0.59; strong, 0.60–0.79; and very strong, ≥0.80. Intraclass correlation coefficients (ICCs) were calculated to evaluate both inter- and intraobserver reproducibility of measurements, and interpreted as follows: poor, <0.40; fair, 0.40–0.59; good, 0.60–0.74; and excellent, ≥0.75.
Results
Patient characteristics
The male/female ratio was greater than 1 in all patient groups (1.64–3.93) and subgroups (1.35–2.82). This ratio, however, remained statistically comparable between all groups (p ≥ 0.079) and subgroups (p ≥ 0.062).
Measurement reproducibility
The inter- and intraobserver reproducibility of measurements were excellent, both for CSAs (0.75–0.95 and 0.79–0.98, respectively) and APDs (0.77–0.86 and 0.80–0.84, respectively). Therefore, the values reported below belong to the first observer.
Cervical spine
Taking both subgroups (i.e., trauma and CT angiography) together, younger patients had smaller mean numerical CSA values than older subjects at all levels, although this difference never reached statistical significance (p ≥ 0.087) (Fig. 2a; Table 1). In contrast, mean APDs showed no difference at any level (p ≥ 0.680) (Fig. 2b; Table 1). These results remained unchanged when considering males and females separately.
Line charts display the mean cross-sectional areas (CSAs in mm2) (a) and mid-sagittal anteroposterior diameters (APDs in mm) (b) of cervical bony spinal canals in the younger versus the older generation of patients. Error bars represent 95 % confidence intervals; they coincide with the markers in graph a
Subgroup analysis revealed that younger CT angiography patients had a statistically significantly smaller mean CSA than older subjects at C1 (p = 0.018), together with a similar trend at C4 (p = 0.053) (Table 2). Even though mean CSAs were also numerically smaller at all levels in younger trauma patients than in older ones, this difference did not reach statistical significance (p ≥ 0.178) (Table 3). In contrast, mean APDs showed no difference in any subgroup (p ≥ 0.405) (Tables 2, 3).
As expected, gender analysis revealed that female patients had significantly smaller mean CSAs than male subjects at all levels (p ≤ 0.031) (Table 4). Again, mean APDs showed no difference at any level (p ≥ 0.480) (Table 4). When comparing younger and older males versus females separately, these differences proved to no longer be statistically significant in older patients at C3 (p = 0.676) and C4 (p = 0.248) levels.
Lumbar spine
Taking both subgroups together, younger patients had statistically significantly smaller mean CSAs than older subjects at all levels (p ≤ 0.024) except L5 (p = 0.131) (Fig. 3a; Table 5). In contrast, mean APDs showed no difference at any level (p ≥ 0.212) (Fig. 3b; Table 5). As with the cervical spine, these results remained unchanged when considering males and females separately.
Subgroup analysis was limited for abdominal patients due to low statistical power (n = 40). It nevertheless revealed that significant differences in CSA between younger and older subjects were more pronounced in trauma patients (p ≤ 0.036, except at L5).
Cervical and lumbar spine correlation
Plotting CSA values of mid-cervical and mid-lumbar bony spinal canals, taking C4 and L3 as reference levels, showed a moderate positive correlation between these two anatomical regions (r = 0.509, p < 0.01) (Fig. 4). In contrast, there was only a weak positive correlation between mid-cervical and mid-lumbar APDs, taking C5 and L3 as reference (r = 0.294, p < 0.01).
Discussion
In this study, we found that the younger generation patients had, on average, smaller cervical and lumbar bony spinal canals than the older generation patients. These differences, however, reached statistical significance only in the lumbar spine.
Our results confirmed previous findings showing that CSAs of the lumbar spinal canal at pedicle levels were significantly smaller in younger than older generation subjects [9]. Schizas et al. offered possible reasons for this surprising finding in the lumbar spine. They suggested that differences in CSA were primarily related to ante- or perinatal factors, such as increased maternal age at birth and maternal smoking in younger generation subjects, factors already proven to influence spinal canal development adversely [10, 11]. These factors could result in smaller lumbar bony spinal canals at birth not mirrored by smaller adult body heights because there is ample time for catch-up growth in the appendicular skeleton. Those reasons might explain why, despite better overall living conditions expressed by an increase in average body height, the lumbar bony spinal canal of younger generation subjects was significantly smaller in the studied population.
In the present study primarily focused on the cervical spine, there was no statistically significant difference in CSA between the two patient generations despite the change being clearly present in the lumbar spine. Multiple reasons may explain the absence of any statistically significant difference in the cervical spine. Cervical degenerative changes are indeed different from those encountered in the lumbar spine [17, 18]. The presence of posterior uncovertebral and facet joint osteophytes, along with the smaller vertebral dimensions in that particular anatomical region may have the consequence that the pedicle levels on axial-oblique reformatted CT images could include, to a certain degree, those osteophytes and, therefore, contribute to smaller CSAs, something that is less likely to occur in the lumbar spine. This can develop with increasing age, and previous studies tend to support this hypothesis [14, 19].
Another explanation for this discrepancy between the cervical and lumbar spine could be the differential closure of the neurocentral synchondrosis (NCS). It has been postulated that ossification and closure of the NCS first occurs in the cervical spine, preceding that in the lumbar and thoracic spine [20]. Moreover, it was recently described that the NCS closure starts at the cervical level at 6 years of age, followed by the lumbar level at age 12 years, and finally the thoracic region at age 14 years [21]. This early closure of the cervical NCS could lead to a smaller cervical spinal canal size and thus to a smaller difference in CSA between the two patient groups at this level.
A further explanation might be that degenerative changes tend to develop with age at the mid-cervical spine [14, 22]. This could also explain why there is less difference in CSA between genders in older patient subgroups, particularly at the mid-cervical spine, where osteophytes tend to develop preferentially.
Several studies have looked into the dimensions of the cervical spinal canal because of the correlation between smaller canals and increased risk of injury [2, 23–25]. Some researchers studied cadavers, in particular those originating from museum specimens. Of interest is the study by Lee et al., which showed that older individuals had narrower cervical bony spinal canals [23]. It was a cross-sectional study on specimens collected during the late nineteenth and early twentieth centuries. Even though an attempt was made to correct the spinal canal dimensions to modern body size, the assumption was made that body height and spinal canal size have a parallel growth, something that is not substantiated by research results [24]. There are also several imaging-based studies on cervical spinal canal dimensions but few focused on the changes in dimension with age. Although not specifically looking at the relation between age and diameter, Ulbrich et al., in a magnetic resonance imaging study on healthy individuals, found that the APD changed with age but not in a significant way [24]. Goto et al. studied the APD on lateral cervical spine radiographs of younger and older generation subjects in Japan stratified into six groups differing in birth date by a decade [25]. They found that the younger generation subjects were taller and had a larger arm span. Younger generation subjects also had larger APDs from C3 to C6. Although interesting, this study was limited by the fact that the APDs were measured only on standard radiographs, which are probably not the ideal tool to accurately assess the spinal canal dimensions. We, in fact, observed statistically significant differences in CSA, such as between male and female patients (Table 4), which were not mirrored by the APD. In addition, radiological magnification and technique can introduce bias on standard radiographs, unlike on CT scans. The larger arm span of the younger generation subjects could lead to greater magnification in this group. Our study not only looked at cervical spinal canal dimensions in a cross-sectional manner but provided a better appraisal of dimensions using CSAs on CT scans rather than APDs on standard radiographs. Even though our findings in the cervical spine were less dramatic than those in the lumbar spine, a question has been raised regarding the possibility of narrower cervical bony spinal canals from generation to generation. This fact, along with the aging of the population, could possibly lead to an increase in clinically diagnosed cervical spinal stenosis requiring surgical treatment.
Our study had a few limitations. First, it was not longitudinal but cross-sectional, with the intrinsic limitations of such a study design. Second, the subgroups included could also represent a selection bias. The marked differences in lumbar spinal canal CSAs in the trauma subgroup raises questions on potential confounding factors (e.g., alcohol intake, psychiatric comorbidities) that are difficult to identify and study separately.
In conclusion, younger generation patients tend to have smaller bony spinal canals in both the cervical and lumbar spine in Western Switzerland, even though this difference is less marked at the cervical level. Further research is warranted in the field of cervical spinal canal dimensions, in particular with longitudinal population studies to confirm this trend of spinal canal narrowing. This is of paramount importance for future planning of priority health because the increase in cervical spondylotic myelopathy and its related conditions could represent a significant financial burden for society.
References
Baron EM, Young WF (2007) Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery 60:S35–S41
Dora C, Wälchli B, Elfering A, Gal I, Weishaupt D, Boos N (2002) The significance of spinal canal dimensions in discriminating symptomatic from asymptomatic disc herniations. Eur Spine J 11:575–581
Asgari S (1996) Cervical spondylotic myelopathy. In: Palmer JD (ed) Neurosurgery’96: manual of neurosurgery. Churchill Livingstone, New York, pp 750–754
Benzel EC (1993) Cervical spondylotic myelopathy: posterior surgical approaches. In: Cooper PR (ed) Degenerative disease of the cervical spine. American Association of Neurological Surgeons, Park Ridge, pp 91–104
Ferguson RJ, Caplan LR (1985) Cervical spondylitic myelopathy. Neurol Clin 3:373–382
Arnasson O, Carlsson CA, Pellettieri L (1987) Surgical and conservative treatment of cervical spondylotic radiculopathy and myelopathy. Acta Neurochir (Wien) 84:48–53
White AA 3rd, Panjabi MM (1988) Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine (Phila Pa 1976) 13:856–860
Chazono M, Tanaka T, Kumagae Y, Sai T, Marumo K (2012) Ethnic differences in pedicle and bony spinal canal dimensions calculated from computed tomography of the cervical spine: a review of the English-language literature. Eur Spine J 21:1451–1458. doi:10.1007/s00586-012-2295-y
Schizas C, Schmit A, Schizas A, Becce F, Kulik G, Pierzchala K (2014) Secular changes of spinal canal dimensions in Western Switzerland: a narrowing epidemic? Spine (Phila Pa 1976) 39:1339–1344. doi:10.1097/BRS.0000000000000445
Papp T, Porter RW, Craig CE, Aspden RM, Campbell DM (1997) Significant antenatal factors in the development of lumbar spinal stenosis. Spine (Phila Pa 1976) 22:1805–1810
Jeffrey JE, Campbell DM, Golden MH, Smith FW, Porter RW (2003) Antenatal factors in the development of the lumbar vertebral canal: a magnetic resonance imaging study. Spine (Phila Pa 1976) 28:1418–1423
Eisenstein S (1977) The morphometry and pathological anatomy of the lumbar spine in South African negroes and caucasoids with specific reference to spinal stenosis. J Bone Joint Surg Br 59:173–180
Tatarek NE (2005) Variation in the human cervical neural canal. Spine J 5:623–631
Kato F, Yukawa Y, Suda K, Yamagata M, Ueta T (2012) Normal morphology, age-related changes and abnormal findings of the cervical spine. Part II: Magnetic resonance imaging of over 1200 asymptomatic subjects. Eur Spine J 21:1499–1507
Yukawa Y, Kato F, Suda K, Yamagata M, Ueta T (2012) Age-related changes in osseous anatomy, alignment, and range of motion of the cervical spine. Part I: Radiographic data from over 1200 asymptomatic subjects. Eur Spine J 21:1492–1498. doi:10.1007/s00586-012-2167-5
Battié MC, Ortega-Alonso A, Niemelainen R, Gill K, Levalahti E, Videman T, Kaprio J (2014) Lumbar spinal stenosis is a highly genetic condition partly mediated by disc degeneration. Arthritis Rheumatol 66:3505–3510
Weiler C, Schietzsch M, Kirchner T, Nerlich AG, Boos N, Wuertz K (2012) Age-related changes in human cervical, thoracal and lumbar intervertebral disc exhibit a strong intra-individual correlation. Eur Spine J 21(Suppl 6):S810–S818. doi:10.1007/s00586-011-1922-3
Teraguchi M, Yoshimura N, Hashizume H, Muraki S, Yamada H, Minamide A, Oka H, Ishimoto Y, Nagata K, Kagotani R, Takiguchi N, Akune T, Kawaguchi H, Nakamura K, Yoshida M (2014) Prevalence and distribution of intervertebral disc degeneration over the entire spine in a population-based cohort: the Wakayama Spine Study. Osteoarthr Cartil 22:104–110. doi:10.1016/j.joca.2013.10.019
Katz JN, Harris MB (2008) Clinical practice. Lumbar spinal stenosis. N Engl J Med 358:818–825. doi:10.1056/NEJMcp0708097
Dimeglio A (1993) Growth of the spine before age 5 years. J Pediatr Orthop B 1:102–107
Rajwani T, Bhargava R, Moreau M, Mahood J, Raso VJ, Jiang H, Bagnall KM (2002) MRI characteristics of the neurocentral synchondrosis. Pediatr Radiol 32:811–816
Rowland LP (1992) Surgical treatment of cervical spondylotic myelopathy: time for a controlled trial. Neurology 42:5–13
Lee MJ, Cassinelli EH, Riew KD (2007) Prevalence of cervical spine stenosis. Anatomic study in cadavers. J Bone Joint Surg Am 89:376–380
Ulbrich EJ, Schraner C, Boesch C, Hodler J, Busato A, Anderson SE, Eigenheer S, Zimmermann H, Sturzenegger M (2014) Normative MR cervical spinal canal dimensions. Radiology 271:172–182. doi:10.1148/radiol.13120370
Goto S, Umehara J, Aizawa T, Kokubun S (2010) Comparison of cervical spinal canal diameter between younger and elder generations of Japanese. J Orthop Sci 15:97–103. doi:10.1007/s00776-009-1427-7
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
F. Becce and C. Schizas contributed equally to this work.
Rights and permissions
About this article
Cite this article
Monier, A., Omoumi, P., Schizas, S. et al. Dimensional changes of cervical and lumbar bony spinal canals in one generation in Western Switzerland: a computed tomography study. Eur Spine J 26, 345–352 (2017). https://doi.org/10.1007/s00586-016-4386-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4386-7