Abstract
Background
Both posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) are accepted surgical techniques for the treatment of degenerative lumbar spondylolisthesis (DLS). However, it is still unclear one technique offers distinct advantages over the other.
Objective
A retrospective study was performed to compare perioperative complications and functional outcomes of patients undergoing TLIF versus PLIF for DLS.
Methods
A total of 226 consecutive patients who underwent surgery for treatment of DLS at three institutions were evaluated from January 2012 to December 2014. In this series, 125 patients underwent PLIF and 101 received TLIF. The operative time, blood loss, allogeneic blood transfusion rate and perioperative complications (including re-operative rate, nerve root injury, dural tear, wound infection) were compared between the two groups. Pain (VAS) and functional outcomes of patients (Kirkaldy-Willis criteria) were quantified before surgery and 1 week after surgery.
Results
Patients involved in the two groups had similar baseline demographic, clinical and radiographic characteristics. The PLIF group was associated with a higher incidence of post-operative iatrogenic nerve root dysfunction [12 cases (9.6 %) versus 2 cases (1.9 %), P = 0.018] and dural tears [15 cases (12 %) versus 4 cases (3.9 %), P = 0.030]. The re-operation rate was significantly higher in patients undergoing PLIF [13 cases (10.4 %) versus 2 cases (1.9 %), P = 0.011]. In addition, intra-operative blood loss, operative times, and allogeneic blood transfusion rates were higher in the PLIF group when compared to the TLIF group (P < 0.05). The wound infection rate of the PLIF group was similar to that of the TLIF group (7.2 versus 5.0 %, P = 0.486). VAS scores were decreased from 7.08 ± 1.13 to 2.84 ± 0.89 in the PLIF group, and from 7.18 ± 1.09 to 2.84 ± 0.91 in the TLIF group, respectively (P = 0.32). 85.6 % of patients in the TLIF group had good or excellent functional outcomes within the first post-operative week compared to 83.2 % in the PLIF group (P = 0.64).
Conclusion
Both PLIF and TLIF were equally beneficial in improving short-term functional outcomes for patients with DLS. However, PLIFs were associated with statistically significant higher incidences of nerve root injury, dural tears, allogeneic blood transfusion, increased intra-operative times, blood loss and re-operations. Therefore, caution should be exercised when considering PLIFs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degenerative lumbar spondylolisthesis (DLS), also called lumbar arthritis spondylolisthesis, is a degenerative spinal disorder, which often results in low back and leg pain related to spinal stenosis. Indications for surgical treatment include persistent or recurrent back and/or leg pain, neurogenic claudication and progressive neurological deficit [1]. Surgical procedures include reduction of the misaligned vertebra and reconstitution of the physiological lumbar lordosis by interbody fusion. Because posterior lumbar interbody fusion (PLIF) has the ability to restore spinal alignment, provide indirect decompression of the neural foramina, and affords solid fixation of spinal segments while maintaining load bearing capacity and proper disc height, it is commonly used in the degenerative lumbar disorders [2]. Previous studies have revealed that PLIFs can be associated with nerve injury, dural tear and epidural scarring [3, 4]. Since Harms et al. modified the technique to transforaminal lumbar interbody fusion (TLIF) [5], TLIFs have been used as an alternative technique for surgical treatment of lumbar degenerative diseases [6, 7]. Despite this, however, there exists few direct comparisons of the perioperative complications and clinical outcomes between PLIF and TLIF in adult degenerative spondylolisthesis. In a multi-center study, we retrospectively present a direct comparison of perioperative complications and short-term outcomes of PLIFs and TLIFs.
Materials and methods
Patients
A total of 226 consecutive patients with DLS underwent PLIF or TLIF in three institutions from January 2012 to December 2014. The study protocol was ethically approved by the Human Research Ethics Committee of the three institutions. Informed consent was obtained from all participants. 125 patients received PLIFs and 101 patients received TLIFs. All three institutions performed both procedures and no significant difference was found among them for patients undergoing PLIF or TLIF (P = 0.98). Selection of the procedure was based on patient preference. The risks, advantages and disadvantages of each procedure were discussed with each patient before surgery. Pre-operative variables are denoted in Table 1. Inclusion criteria were radiological evidence of degenerative spondylolisthesis with low back pain and/or leg pain, or neurogenic claudication, which had failed routine conservative treatment for more than 6 months. Patients who underwent three or more levels of intervertebral fusion were excluded.
Surgical procedures
All patients underwent one or two level interbody fusion. The PLIF procedure was performed as previously described [8]. TLIFs were performed in the standard fashion reported by Harms et al. [5]. Interbody fusions were carried out with a PEEK cage filled with local bone graft or allogenic bone in all patients. Posterior segmental spinal pedicle screw instrumentation was used in all cases. The operation time, blood loss, allogeneic blood transfusion rate and perioperative complications (including re-operative rate, nerve root injury, dural tear, wound infection) were recorded in the two groups. All patients used a lumbosacral brace when ambulating for 1 week post-operatively. Pain (VAS) and functional outcomes of the patients were quantified before surgery and 1 week after the surgery. Functional outcome was assessed by using the Kirkaldy-Willis criteria [9].
Statistical analysis
Statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA), and all data were expressed as mean ± standard deviation (SD). The data were analyzed by Student’s t test and Chi square test. A value of P < 0.05 was considered statistically significant.
Results
There were no significant differences between the PLIF and TLIF groups in terms of pre-operative clinical variables including age, gender, spondylolisthesis grade, and vertebral levels involved (Table 1).
Operation time and blood loss
The mean intra-operative time and blood loss volume were statistically higher in the PLIF group than those of TLIF group (241.61 ± 67.31 versus 187.67 ± 45.54 min, P = 0.037; and 482.91 + 403.12 versus 308.06 ± 385.16 ml, P = 0.035, respectively). In PLIF group, the allogeneic blood transfusion rate was 19.2 % (24/125), which was higher than that of TLIF group (4.9 %, 5/101) (P = 0.001).
Perioperative complications
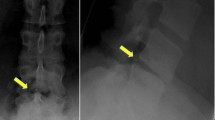
Patients who underwent PLIF had a higher incidence of post-operative iatrogenic nerve root dysfunction [12 cases (9.6 %) versus 2 cases (1.9 %), P = 0.018] and dural tears [15 cases (12 %) versus 4 cases (3.9 %), P = 0.030] than those of TLIF group. Of the patients with nerve root dysfunction, 10 patients in the PLIF group and 2 patients from the TLIF group had resolution of their symptoms after re-operation (Table 2). 10.4 % (13 cases) of PLIF patients and 1.9 % (2 cases) of the TLIF patients required a re-operation because of nerve root injury and wound infection (P = 0.011). 7.2 % of PLIF patients had a wound infection (superficial infection in 6 cases and deep infection in 3 cases), compared to 5.0 % in the TLIF group (superficial infection in 4 patients and deep infection in 1 patient). And one patient in PLIF with deep wound infection underwent re-operation. This was not statistically significant (P = 0.486).
VAS scores and functional outcomes
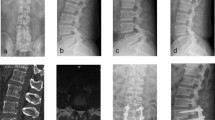
Post-operatively, VAS scores were decreased from 7.08 ± 1.13 to 2.84 ± 0.89 in the PLIF group and from 7.18 ± 1.09 to 2.84 ± 0.91 in TLIF group, respectively (P = 0.32). According to the Kirkaldy-Willis criteria, the functional outcomes in TLIF patients were excellent in 65 cases (52.0 %), good in 42 (33.6 %) and fair in 18 (14.4 %). In TLIF patients, the functional outcomes were excellent in 48 cases (47.5 %), good in 35 cases (34.7 %) and fair in 17 cases (16.8 %) during the first week after surgery. No difference was noted between the two groups (P = 0.64). Illustrative examples of patients who underwent PLIFs or TLIFs are shown in Figs. 1 and 2.
Discussion
Degenerative lumbar spondylolisthesis was first described by Junghanns as pseudospondylolisthesis in 1935 [10]. Pathophysiologically, it is characterized by one vertebral body slipping over the other, potentially resulting in central spinal canal stenosis and/or instability. The incidence of DLS in the general population is approximately 4.1 % [11, 12]. Although most studies recommend conservative treatment [13], increasing evidence demonstrates that surgery may be usually necessary if patients experience no improvement with conservative measures [14]. Sengupta et al. [15] suggested three distinct indications for surgical treatment of DLS, including: (1) persistent or recurrent back and/or leg pain or neurogenic claudication with reduction of quality of life despite a reasonable trial of nonoperative treatment (a minimum of 3 months); (2) progressive neurologic deficit; (3) bladder or bowel symptoms. The primary purpose of surgery is to correct sagittal and coronal imbalances as well as decompress the neural elements, relieving both the patient’s back and leg symptoms. Because decompression without concomitant stabilization may cause worsening of the inherent instability associated with spondylolisthesis, leading to further misalignment and potential worsening of neurologic symptoms, many surgeons recommend instrumented fusion to stabilize the spine [16]. Since Cloward et al. [17] first described the PLIF technique in 1940, interbody fusion techniques have gained popular for patients with degenerative lumbar disorders. Currently, as a modification of PLIF, TLIF has gained considerable traction as an alternative procedure [18]. However, there remains no conclusive evidence demonstrating differences in perioperative complications or clinical outcomes between PLIF and TLIF for treating DLS.
Based on the current study, the average operation time, blood loss and allogeneic blood transfusion rate were statistically higher in the PLIF group compared to the TLIF group (P > 0.05). This is most likely due to the fact that a PLIF requires bilateral discectomy and interbody bone graft and cage placement, which increase intra-operative times, blood loss, and patients requiring blood transfusions. In this study, VAS scores were significantly improved in both PLIF and TLIF groups post-operatively. However, no statistical difference was found between the two groups (P = 0.32). 85.6 % of patients in TLIF group had good or excellent function outcomes during the first week post-operatively compared to 83.2 % of PLIF patients (P = 0.64). These results were similar to the study of Yan et al. [19].
Although PLIF has demonstrated effective clinical outcomes in patients, several studies have shown that it can be associated with increased neural complications such as nerve injury, dural tear and epidural scarring [3, 4, 20]. Such injuries result from excess medial retraction of the dura when placing the cage [21]. In contrast, TLIFs use a posterior approach to the spine via the far lateral portion of the vertebral foramen, which would reduce the risks associated with PLIF. In the present study, we confirm these hypotheses, as the incidence of post-operative iatrogenic nerve root dysfunction was 9.6 % in the PLIF group and 1.9 % in the TLIF group (P = 0.018). These results are consistent with the findings of Yan et al. [19] (3.3 % in PLIF versus 2.1 % in TLIF) and Sakeb et al. [22] (5.8 % in PLIF versus no case in TLIF). The incidence of dural tear of this study was 12 % in the PLIF group and 3.9 % in TLIF group, which were commensurate with those reported in Sakeb et al.’s study [22] (7.7 % in PLIF versus none in TLIF). Compared to the TLIF group, the wound infection of the PLIF group was higher (7.2 versus 5.0 %). In addition, the re-operative rate of PLIF group was 10.4 % and it was significantly higher than that of PLIF group (1.9 %) in our study. Patients underwent re-operation were resulted from nerve root injury (12 cases in PLIF versus 2 cases in TLIF) and deep wound infection (1 patient in PLIF). Thus, according to the present study, the incidence of intra-operative complications of PLIF was higher than that TLIF.
Although this study demonstrates satisfactory results about PLIF and TLIF in the treatment of DLS, some limitations were presented in it, including retrospective analysis of the data and short-term follow-up for patients. In addition, the surgical procedure was chosen by the patients preference while discussed with the surgeons. A randomized controlled trial and long-term follow-up are needed to further define long-term outcomes.
Conclusion
Both PLIF and TLIF were equally beneficial in improving short-term functional outcomes for patients with DLS. However, PLIFs were associated with statistically significant higher incidences of nerve root injury, dural tears, allogeneic blood transfusion, increased intra-operative times, blood loss and re-operations. For carefully selected patients, TLIFs may be a safer approach in treating DLS compared to PLIFs.
References
Kanayama M, Hashimoto T, Shigenobu K, Oha F, Ishida T, Yamane S (2005) Non-fusion surgery for degenerative spondylolisthesis using artificial ligament stabilization: surgical indication and clinical results. Spine 30:588–592
Starkweather A (2006) Posterior lumbar interbody fusion: an old concept with new techniques. J Neurosci Nurs 38(1):13–20
Talia AJ, Wong ML, Lau HC, Kaye AH (2015) Comparison of the different surgical approaches for lumbar interbody fusion. J Clin Neurosci 22(2):243–251
DiPaola CP, Molinari RW (2008) Posterior lumbar interbody fusion. J Am Acad Orthop Surg 16(3):130–139
Harms JG, Jeszenszky D (1998) The unilateral, transforaminal approach for posterior lumbar interbody fusion. Orthop Traumatol 6:88–99
Witoon N, Tangviriyapaiboon T (2014) Clinical and radiological outcomes of segmental spinal fusion in transforaminal lumbar interbody fusion with spinous process tricortical autograft. Asian Spine J 8(2):170–176
Høy K, Bünger C, Niederman B, Helmig P, Hansen ES, Li H, Andersen T (2013) Transforaminal lumbar interbody fusion (TLIF) versus posterolateral instrumented fusion (PLF) in degenerative lumbar disorders: a randomized clinical trial with 2-year follow-up. Eur Spine J 22(9):2022–2029
Liu Z, Liu J, Tan Y, He L, Long X, Yang D, Huang S, Shu Y (2014) A comparative study between local bone graft with a cage and with no cage in single posterior lumbar interbody fusion (PLIF): a multicenter study. Arch Orthop Trauma Surg 134(8):1051–1057
Kirkaldy-Willis WH, Paine KW, Cauchoix J, McIvor G (1974) Lumbar spinal stenosis. Clin Orthop 99:30–50
Stewart TD (1935) Spondylolisthesis without separate neural arch (pseudospondylolisthesis of Junghanns). J Bone Joint Surg Am 17:640–648
Kalichman L, Hunter DJ (2008) Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J 17(3):327–335
Faldini C, Pagkrati S, Acri F, Miscione MT, Francesconi D, Giannini S (2007) Surgical treatment of symptomatic degenerative lumbar spondylolisthesis by decompression and instrumented fusion. J Orthop Traumatol 8(3):128–133
Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN et al (2009) Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. Four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 91(6):1295–1304
Fischgrund JS (2004) The argument for instrumented decompressive posterolateral fusion for patients with degenerative spondylolisthesis and spinal stenosis. Spine 29(2):173–174
Sengupta DK, Herkowitz HN (2005) Degenerative spondylolisthesis: review of current trends and controversies. Spine 30(6):S71–S81
Eismont FJ, Norton RP, Hirsch BP (2014) Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg 22(4):203–213
Cloward RB (1953) The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. J Neurosurg 10:154–168
Wang SJ, Han YC, Liu XM, Ma B, Zhao WD, Wu DS et al (2014) Fusion techniques for adult isthmic spondylolisthesis: a systematic review. Arch Orthop Trauma Surg 134(6):777–784
Yan DL, Pei FX, Li J, Soo CL (2008) Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J 17(10):1311–1316
Hosono N, Namekata M, Makino T, Miwa T, Kaito T, Kaneko N et al (2008) Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: analysis of risk factors. J Neurosurg Spine 9(5):403–407
Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA (2001) Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine 26:567–571
Sakeb N, Ahsan K (2013) Comparison of the early results of transforaminal lumbar interbody fusion and posterior lumbar interbody fusion in symptomatic lumbar instability. Indian J Orthop 47(3):255–263
Acknowledgments
This work is supported by the Department of Science and Technology Program Funds of Jiangxi Province, P. R. China (No. 20123BBG70245, 20121BBG70037) and the Natural Science Foundation of Jiangxi Province, P. R. China (No. 20142BAB215046).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No benefits in any form have been or will be received from any commercial party related directly and indirectly to the subject of this manuscript.
Additional information
J. Liu and H. Deng contributed equally to this study and share the first authorship.
Rights and permissions
About this article
Cite this article
Liu, J., Deng, H., Long, X. et al. A comparative study of perioperative complications between transforaminal versus posterior lumbar interbody fusion in degenerative lumbar spondylolisthesis. Eur Spine J 25, 1575–1580 (2016). https://doi.org/10.1007/s00586-015-4086-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-4086-8