Abstract
Purpose
First, to determine whether scoliosis development could be limited or reversed by growth when a novel modular hinged implant was fixed to the convexity of a scoliosis created by contralateral rib and laminar tethering and unilateral rib resection in a sheep model. Second, to assess the effect and performance of the implant in normal non-tethered sheep.
Methods
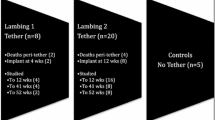
At 5 weeks, 20 Scottish Blackface lambs underwent surgery to create a right sided scoliosis by (i) tethering the left lamina of T5–L1 and the left lower six ribs and (ii) resecting a segment of their right lower six ribs [1, 2]. Twelve weeks later, through an antero-lateral thoracotomy, a mobile bi-planar hinged implant was inserted onto the right side of the spine of eight animals (group 1). For comparison, 12 sheep were tethered only but had no implant insertion (group 2). In addition, seven had no tethering but were implanted (group 3) and normal growth patterns were observed in five that had no surgery (group 4). Curve progression was assessed by plain radiography and CT over a 1-year period.
Results
Before implant insertion the trial animals had a scoliosis of 35º ± 16º and a lordosis of 44º ± 20º (n = 8, mean ± SD). Surgery immediately reduced these values to 25º ± 14º, p < 0.01 and 35º ± 18º, p < 0.001, with scoliosis continuing to decrease during the next three months. Spinal flexibility was retained. In the un-tethered sheep, a scoliosis of 10º ± 6º was created on the opposite side to the implant (p < 0.05) with no significant change in alignment in the sagittal plane (1º ± 6º). The implant did not cause any adverse effect on growth or affect neurological function.
Conclusions
In the un-tethered animals the effect of the implant was to create a scoliotic deformity and in the tethered to improve deformity while maintaining spinal motion. We believe that the results are promising and that devices of similar construct may be of use in children with scoliosis, potentially changing current methods of clinical care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For a number of years the gold standard operative treatment for early onset scoliosis has been deformity control by single or dual growth rods. These however, have to be lengthened regularly and are associated with a high rate of complications from inadequate fixation and spontaneous spinal fusion [3–5]. This has also applied to recent modifications such as the Shilla technique in which pedicle screws are connected loosely to a rod allowing sliding [6] and use of vertical expandable prosthetic titanium ribs (VEPTR) with rib and spinal anchors to correct scoliosis by distraction [7]. Metallosis at the screw rod interface, multiple lengthening and usually spinal fusion at skeletal maturity are commonplace [6].
For these reasons there has recently been much interest in the use of growth modulating non-fusion strategies in the management of early onset spinal deformity. A number of different devices including staples and tethers have been tested on animals to evaluate their growth modulating effects and ability to achieve a favourable change in spinal shape [8–10]. Applied to the vertebral bodies laterally, they potentially give correction in the coronal and rotational planes but are much less effective in controlling sagittal deformity [8]. Vertebral body stapling has been used clinically with some success [9], but the more rigid the construct the stiffer the spine becomes and this leads to premature fusion of the instrumented segments [10]. An optimal device would allow a near physiologic range of motion, give good three dimensional control of spinal shape in the instrumented spinal segments and potentially give effective control of deformity without precipitating premature fusion.
We have developed a semi-rigid mechanical tether that is designed to give partial correction of coronal and rotational deformity at the time of device application to the spine and to give further correction of deformity in the coronal and rotational planes with growth. The aim of this study was to prove that it is possible to control the shape of a growing spine and maintain spinal motion using a novel mechanical device, evaluating its safety and efficacy in an ovine model.
Although not previously used as a model for the study of growth modulating implants designed to treat human spinal deformity, sheep have similar spine biomechanics and size to young humans and are an accepted model for spine disease research [11, 12]. The sheep spine is predominantly loaded by axial compression due to stabilising muscle forces [13], and since the rate of growth at mammalian growth plates responds similarly to mechanical stimuli across a range of species [14], data from sheep studies will be similar to those expected in a child. The sheep may therefore be considered a reasonable model for evaluation of growth modulating devices for treatment of human spinal deformities.
In this project, we specifically wished to determine (i) the extent to which the device would give partial correction of deformity when applied to a surgically induced spinal deformity (ii) whether further deformity correction would occur with growth (iii) whether the device could induce deformity when applied to the normal sheep spine and (iv) to determine if the device could fit onto a range of spinal deformities without the need for a wide range of implants or custom designed implants.
Materials and methods
Study design
The studies comprised four groups of animals as shown in Table 1. In group 1, eight sheep had a surgically induced right sided scoliosis as previously described [1, 2]: (i) tethering the left lamina of T5–L1 and the left lower six ribs, and (ii) resecting a segment of the right lower six ribs. These animals were implanted with the growth modulating device 3 months after tethering and the results compared with those from 12 sheep (group 2) that were tethered but received no implant (Table 1). In a third group of seven animals the device was implanted at 10 weeks onto a normal spine (group 3) and the growth characteristics compared with those from five controls who had no surgery (group 4). Six was considered a valid number of sheep in the experimental groups to provide sufficient data to produce reproducible results. Two group 1 and one group 2 spare animals allowed for any unforeseen mobidity/mortality.
Ethical approval was obtained from the University of Edinburgh Ethical Review Committee and the work was performed on a UK Home Office project licence (PIL 60/3832) under veterinary supervision.
Animals
The study group comprised 32 Scottish Blackface lambs purchased together with their ewes from a commercial flock and acclimatised in purpose-built small ruminant accommodation for at least 1-week before surgery. Housing was indoors and consisted of two opposite lines of five hurdled pens. The lambs and their ewes were confined in single pens with water and hay supplied ad libitum. The lambs were allowed to suckle their ewes until immediately before induction of anaesthesia. Pre-operative physical examination indicated that the animals were healthy. Shearing and abdominal bandaging was performed before surgery in all animals to customise the ewes to the post-operative ‘appearance’ and smell of their lambs.
Implant design
The device undergoing testing was a flexible modular anterior tether composed of four loosely fitting hinges linked together and orientated at an angle relative to their attachment members. The construct was fixed to the lateral aspect of the vertebral bodies via hydroxyapatite-coated screws (Fig. 1).
The implant maybe conceptualised as five blocks connected with four hinges (Fig. 2), with the hinges controlling the growth and position of the five blocks (vertebrae). To minimise the number of screws required to fix the implant a linked chain approach was adopted, with each hinge component spanning three vertebrae and locked to its neighbour with a single screw and locknut. This enabled the control of nine vertebrae with only five screws.
The device was designed to change shape as it is lengthened by growth to correct coronal and rotational deformity, yet retain a near physiologic range of motion allowing spinal mobility to be maintained. This was achieved due to an asymmetric looseness in the hinges, constructed as modified ball and socket joints. The ranges of angular motion about each orthogonal axis of the hinge are shown in Fig. 3.
The major hinge axis lies at 45° to the implant axis and the plane of the intervertebral disc.
The device was manufactured from cobalt chrome (Wines Medical, Weald, Kent, TN14 6NP, UK), and coated with a graphite-like coating (Teer Coatings Ltd, West Stone House, Berry Hill Industrial Estate, Droitwich, WR9 9AS, UK) applied to minimise wear by decreasing friction and consequent release of cobalt and chromium ions. To fit different size animals and possibly future patients from early childhood to adolescence, two hinge attachment lengths were available of slotted design.
Since the hinges of the implants are orientated at an angle to their attachment members, the device could bend in the coronal plane and rotate resulting in a 3D shape similar to a scoliosis deformity. Each was designed however to be reversible to allow fitment in a mirror image configuration giving rotation towards the apex of the deformity whether positioned above or below the apex. The hinge components connect together via a bolted joint on the vertebral body screws and a further variable-angle locking component was inserted to define the angle between each link. Thus, when the device is applied to a spine partial correction of deformity may be achieved but a physiologic degree of flexion and extension retained. As growth occurs and the instrumented spine segments lengthen, the hinges would be expected to open, straightening the construct and the spine. The device was thus designed to treat a wide variety of moderately flexible curves with different morphologies and different deformity lengths using only four different modular hinge components.
Implantation of device: deformed sheep spine
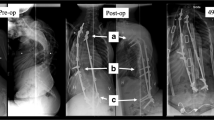
Eight sheep (group 1) with a surgically induced progressive lordoscoliosis [1], (mean age 4 months, mean weight 27 kg) underwent further surgery 3 months after deformity induction surgery to insert the device (Fig. 4). They were anaesthetised and after instillation of 1:200,000 epinephrine solution (5 µg ml−1) subcutaneously at the operative site and administration of pre-operative antibiotic (Cefuroxime 1.5 g IV), a double right thoracotomy was performed through the bed of the seventh and 11th ribs, respectively with conservation of the ribs where possible. The segmental vessels were taken at T5, T7, T9, T11 and L1 and 5 mm hydroxyapatite coated vertebral body screws inserted at these levels. It was possible to insert the L1 screw without taking down the diaphragm. The four hinges were placed over the screws and, when angled, fitted onto the contour of the deformity. It was necessary to trim the rib heads to get the construct to fit onto the spine in a satisfactory position. The construct was tightened to 10 Nm using a calibrated torque wrench after insertion of the sagittal locking components. The wound was closed in layers and a small-bore chest drain brought out at its end. Before recovery morphine (0.2 mg kg−1 in 1.5 ml saline) was injected intrathecally at the L6–S1 level. Twelve control animals (group 2) that had previously undergone deformity induction surgery did not undergo any further intervention.
Implantation of device: normal sheep spine
Seven lambs (group 3) (mean age 10 weeks, mean weight 22 kg) underwent surgery to insert the device as shown in Fig. 5. The pre-operative, anaesthetic and surgical techniques were similar to those described above. Five control animals (group 4) did not undergo any intervention.
Post-operative care
Each lamb was recovered in proximity to its mother at the earliest opportunity to prevent rejection. A radiant heat lamp ensured normothermia and all sheep pairs were monitored on CCTV, rather than by direct observation, to reduce post-operative stress. Most lambs required supplementary feeding for 24 h. During the first 24 h the lambs were evaluated regularly for signs of pain and if necessary morphine (0.2–1.0 mg kg−1) was administered intravenously. In the following days meloxicam (0.6 mg kg−1, Boehringer Ingelheim, UK) and buprenorphine (20 µg kg−1, Animalcare, UK) were administered intravenously as required.
Radiological evaluation
The lambs were weighed and had ventro-dorsal and lateral radiographs preoperatively, immediately postoperatively and at regular intervals thereafter. Light sedation was administered to each animal prior to radiography. Medetomidine 10 mg kg−1 was given IV after which the animal was left in a quiet environment. This was adequate for radiological examination in most animals. Those that required further sedation were given ketamine 0.5–1.0 mg kg−1 IV. After examination, atipamezole 50 mg kg−1 was administered IV to all animals to restore full consciousness. At all time intervals, standardised (1 m tube-film distance) supine ventro-dorsal and lateral radiographs were taken. As near as possible a true ventro-dorsal position was obtained by keeping the neck square and pelvis flat to the table balancing the fore and hind-limbs on each side. Lateral films were taken with the animal naturally supported on its shoulder and hip. In each instance coronal and sagittal Cobb angles were measured and tabulated. Coronal right/left lateral flexion and sagittal flexion/extension views were taken at 7 and 9 months age to determine spinal mobility, by a surgeon placing maximal traction of the fore and hind-legs. CT imaging (Siemens Somatom Esprit CT scanner, US) was performed 3 months after deformity induction surgery prior to surgical insertion of the device and 4 months after application of the device to the normal and deformed sheep spine to assess vertebral rotation [15, 16], alteration in vertebral architecture and spine length. The sheep were sedated, positioned supine and supported to minimise spinal movement with breathing and to maintain their longitudinal axis (and the longitudinal axis of the apical vertebra in the frontal plane) perpendicular to the CT scan. Vertebral rotation was then measured relative to the anterior mid-line of the body (RAML) and the mid-sagittal plane (RMSAG) [1, 16]. The length of the lordoscoliotic deformity was measured in the sagittal plane as the linear distance from the midpoint of the superior endplate of T1 to the midpoint of the inferior endplate of S1.
Statistical analyses
Data are expressed as mean ± standard deviation. Statistical analyses were performed using the Student’s t test with a level of significance of 5 % (p < 0.05).
Results
Surgical induction of scoliosis
All animals undergoing the deformity induction surgery reported in this paper recovered satisfactorily from the procedure, developed progressive lordoscoliotic deformities and put on weight at a similar rate to the normal controls (Fig. 6). Initial care of the lambs, complications and the characteristics of the deformity developed are discussed in detail in paper 1 [1].
Implantation of device: deformed sheep spine
All the animals had a similar natural pre-operative spinal curvature (right scoliosis 2º ± 3º, lordosis 3º ± 8º with no difference between groups). In the operatively treated group (group 1) the range of scoliosis treated was 20º–60º and range of lordosis 13º–75º. The device was fitted across this range of deformities satisfactorily. The device was inserted from T5–L1 (n = 5), T4–L1 (n = 1), T4–T12 (n = 1) and T3–T11 (n = 1). A rescue screw was required in L1 in one animal as the 5 mm screw lost fixation and significant bleeding was encountered from the vertebral body. A screw was placed in T4 in a further animal as technical errors with placement in T5 resulted in loss of fixation. All eight animals recovered satisfactorily from the surgery. The animals grew steadily during the period of study and neither tethering to achieve the scoliosis nor subsequent surgery to implant the device adversely impaired development (Fig. 6). Instrumentation of the deformity with the device resulted in immediate correction of the scoliosis from 35° ± 16° to 25° ± 14°, p < 0.01 and correction of the lordosis from 44° ± 20° to 35° ± 18°, p < 0.001 (paired one tailed t test). This is seen in Fig. 7a as an acute decrease in extent of deformity approximately 120 days from birth. At 5 months post-device insertion (day 289 ± 7 post birth) the mean scoliosis was 20º ± 12° and the mean lordosis was 34° ± 22° (Table 2 and Fig. 7a, b). There was no statistically significant difference between these measurements and the immediate post-implantation measurement (p = 0.09, p = 0.43 respectively, paired one tailed t test) although scoliosis had diminished at day 214 ± 5 after instrumentation to 14° ± 14º (p = 0.04). By comparison, the maximal variation in measurements at any time point in the control (no tether, no implant) animals (group 4) for scoliosis was 3° and for lordosis 5°.
The mean scoliosis in the control (no implant) group (group 2) at the time of instrumentation of the surgical group was 29° ± 21° and the mean lordosis was 48° ± 21°. These figures did not change over the next 5 months (26° ± 25°and 49° ± 23°, both NS). Although at 5 months scoliosis was similar when the implanted and control groups were compared (the implants started from a greater value), lordosis was significantly lower 26° ± 17° vs. 65° ± 23° (p < 0.007, one tailed unequal variance t test). Instrumentation had no statistically significant effect on rotational deformity (Table 3, p = 0.1).
The mean T1–S1 length in the control deformity group (group 2) and the instrumented deformity group pre-instrumentation (group 1) were not different at 40 ± 2 cm and on repeat CT scanning 4 months later, slight growth was evident to 44.5 ± 5.6 and 42.1 ± 2.7 cm, respectively (p < 0.05). The implant did not retard growth over the instrumented segments with increments from 15.9 ± 1 to 16.2 ± 1.6 cm and 15.9 ± 1.2 to 16.6 ± 1.4 cm, respectively (NS). The morphological changes present consequent upon surgically inducing a scoliosis is described in paper 1 [1]. It was not possible to determine changes in morphology in the instrumented animals because of implant artefact.
Implantation of device: normal sheep spine
Seven normal lambs underwent implantation of the device at a mean age of 10 weeks (group 3). One animal developed convulsions postoperatively and died several hours after the procedure. An initial postmortem showed the implants to be in a satisfactory position. A subsequent more detailed postmortem did not reveal a cause of death. A further animal sustained an injury to the thoracic duct during surgery. The surgery was continued as planned and the thoracic duct injury was managed satisfactorily with a small bore chest drain serially aspirated for 5 days, postoperatively. The animals developed normally and gained weight satisfactorily (Fig. 6). There was no significant difference in preoperative and immediate postoperative coronal alignment (2° ± 6° vs. 2° ± 9°). By 10 months however, a scoliosis of 10° ± 6° had developed (p < 0.007, paired two tailed students t test). There was no significant change in alignment in the sagittal plane (1° ± 6° vs. 1° ± 13° either from the pre-operative values or in comparison with the normal controls (Fig. 8a, b).
Implant performance
There were no implant-related adverse events. A moderate degree of top screw pullout without overt loosening was seen in the instrumented deformity group. Screw breakage without adverse effects was seen in one animal in the instrumented deformity group 9 months after implant insertion. Movement of the implants were retained with a similar degree of flexibility in both tethered and untethered spines in the coronal plane (~20º) but slightly less in the sagittal plane in the tethered group (Table 4).
Discussion
The growth guiding implant evaluated in this paper is intermediate between a tether with full flexibility and a traditional growing rod construct providing more rigid fixation. It acts as a semi-flexible tether that is designed to fix sagittal plane alignment and give partial correction of deformity at the time of implantation. It permits a near physiological range of motion and controls movement in the coronal and rotational planes coupling deformity correction to elongation of the instrumented spinal segments by growth. Previously described flexible anterior tethers can be inserted in a fashion that would be expected to give both derotation and correction of coronal plane deformity and induce kyphosis. However, due to their flexibility the three dimensional shape of the spine is not defined within fixed parameters and the effect on the sagittal plane is variable [8]. This new device was evaluated using the sheep as an experimental model.
When applied to the normal growing sheep spine the device induced a moderate contralateral scoliosis while maintaining sagittal plane alignment and motion at the instrumented segments. This demonstrated that the device could guide growth to change spinal shape. The scoliosis induced was progressive, was detected on multiple sequential measurements and was greater than the measurement error as seen in Fig. 8. The extent of scoliosis creation was however modest at 10° ± 6° but satisfactory in comparison to the extent of deformity induction by other growth modulation devices. Newton et al. had achieved a 11° ± 2° scoliosis and 16° ± 2° kyphosis using a flexible untensioned anterolateral tether in a porcine model [17] and a 12° ± 5º scoliosis and 5° ± 6º kyphosis in an immature bovine model [18]. Driscoll et al. used a novel anterolateral device to compress the vertebral body growth plate without bridging the disc in an immature porcine model. This induced a coronal plane deformity of 6.5° ± 3.5°, 3 months after instrumentation [19]. The flexible anterolateral tethers in the above studies induced kyphosis of variable extent in addition to scoliosis. In contrast, the device evaluated in this study induced a coronal plane deformity without altering sagittal plane alignment as this is fixed at the time of device insertion.
The surgically induced deformities were more rigid than would be expected in most cases of human scoliosis and therefore less forgiving in terms of device application. However, when applied to the surgically induced spinal deformity the device gave immediate partial correction of both the scoliotic and lordotic deformities with maintenance of spinal mobility. Further initial deformity correction would have been possible but the aim during instrumentation was to get as much correction as could be achieved using minimal force hence minimising the risk of neurological complications. Following the initial deformity correction achieved intraoperatively no further correction occurred with growth. This spinal deformity model produced a lordoscoliosis which continued to progress for only 3 months after the deformity induction procedure (Fig. 7a, b). Thereafter the deformity did not progress and no significant increase in length of the deformed spine segments occurred suggesting that this deformity model is not useful for evaluating scoliosis growth modulation treatments beyond 4 months of age. In addition to this, the deformity was rigid compared to typical human deformities and the corrective growth forces acting via the device were partly dissipated against the spinal tether and tethered ribs. This was probably particularly relevant in terms of our results at 8 months post-implantation when our results were similar to those produced in the tethered only group. Our modelling was however valuable in that it allowed evaluation of not only the capacity of the device to fit onto different degrees of deformity, but its ability to give initial deformity correction and an assessment of any associated adverse events.
The device ably fitted onto both normal and severely deformed spines demonstrating a capacity to treat a wide variety of deformities with a small number of different implant types. This is an important consideration in terms of the clinical utility and commercial viability of this type of device. We also found that the initial correction of the deformity achieved was maintained in both the coronal and sagittal planes apart from the modest late progression in extent of lordosis discussed below. This contrasts with the goat study by Braun et al. [8], in which the efficacy of anterolateral flexible ligament tethers in correction of scoliosis was evaluated Their anterolateral tethers were applied 2 months post-deformity induction surgery and the effects evaluated 4 months later. Although mean scoliosis corrected from 73° to 70° the lordosis progressed from 44° to 59°. This suggests that fully flexible tethers may be less effective in controlling the sagittal plane in comparison to the device evaluated in this study. Also the degree of initial correction of deformity achieved was much greater with the semi-rigid tether compared to the fully flexible tethers [20].
Our results are probably most representative of device function at 5 months post-device implantation as, at this point, the majority of the growth potential of the sheep spine had been achieved and the effects of growth modulation attributable to the device would be expected to be maximal. The final data point 8 months after implantation of the device revealed an increase in the extent of lordosis to approach that of the tethered non-instrumented spines. This probably reflects continued action of the tether but a significant increase in stress placed on the spine by the sheep increasing its abdominal body weight with maturity. The time window during which the ‘tether’ model may be used is a limiting factor to its application but the period of rapid sheep growth does equate with that of peak scoliosis formation in a child.
There was a considerable degree of variation in the mean extent of scoliosis and lordosis measured at different time points in the study groups during the course of this investigation. Potential sources of error included deviation from true ventro-dorsal and lateral positioning during radiography (most prominent in the animals where a significant rib hump was present) and measurement errors. The former were negligible on repeat measurements in the few animals where duplicate films were taken to improve quality and digital reading was performed by computer of the CT images. The study design attempted to minimise the effects of the above sources of error by making measurements at as many data points as was practical (the sheep had to be anaesthetised on each occasion) and standardising the radiography technique (same technician).
In the instrumented deformity model described here, residual growth potential was not sufficient to drive lengthening of the spine/implant construct and further correction of the Cobb angles. Potentially, further correction might have been achieved by augmenting the endogenous growth potential with distraction using a hybrid arrangement with a magnetically driven device in parallel [21]. If the deformity had been corrected sufficiently at skeletal maturity then potentially, no further intervention would be required allowing the device to be left in situ. In comparison to those treated with fusion alone the deformed spine segments would be expected to remain mobile reducing the risk of symptomatic adjacent segment degenerative change.
In this study, the instrumented spinal segments remained mobile. A degree of top screw pullout was observed to occur with slight lateral drift of the implant. Top screw pullout is a recognised problem with anterior instrumentation for scoliosis correction [22]. This problem may be addressed by modifying the implant design to have two screws in adjacent vertebrae at the top of the construct sacrificing movement at the level between the screws. Also, it was necessary to trim the rib heads in both the normal and deformed sheep spines to fit the device onto the spine satisfactorily which might have caused fusion at the instrumented segments in the longer term. This could be addressed by modification of the design to minimise device impingement on the rib heads and anyway would be less of an issue in the human due to anatomical differences. Probably, with some modification, a device of similar design could relatively easily be inserted thoracoscopically.
Although it would have been valid to have performed a sham procedure (surgical exposure but no instrument insertion) providing additional data, with already four experimental groups it was felt that this would have meant additional animal suffering and animal licensing would not have been granted. In any event, trauma from the exposure in our experimental groups would have had a negative effect on device function rather than producing gain and better outcome.
Driscoll et al. [23], have recently shown inverse disc wedging associated with a novel non-fusion hemi-staple device in a pig model. The effects of the implant on intervertebral disc radiographic appearance and histology were not evaluated in this study, as it was an initial investigation to evaluate the potential of this new concept of device to alter spinal shape. Further study of this type of device should include an assessment of the effects of the device on intervertebral disc health.
In summary, the device was shown to induce a defined deformity with growth when applied to the normal sheep spine and to give immediate partial correction of deformity when applied to a relatively rigid ovine spinal deformity model with minimal remaining residual growth potential. The ongoing effect in the model as presented was less convincing since the intermediate and final values of the mean curves (shown in Fig. 7a, b) were close and for this reason a definite conclusion may not be drawn. However, considering both the effects of the device on the normal sheep spine and in our deformity model it is reasonable to suggest that in a less rigid human curve with significant residual growth potential that partial correction of deformity of ~50 % could be achieved upon application of the device to the spine and that a further degree of correction by growth would occur. In a typical case correction of a 40° curve to 20° on instrumentation with a further 10° correction with growth would yield a very acceptable result of a 10° curve at maturity with maintenance of spinal mobility. This device remains experimental and needs further development before clinical use may be considered. It does however, represent a new approach to mechanical growth modulation in the treatment of scoliosis.
References
Burke JG, Vettorato E, Schöffmann G, Clutton RE, Drew TS, Gibson JNA (2015) Creation of an ovine model of progressive structural scoliosis using a unilateral laminar tether. Eur Sp J. doi:10.1007/s00586-014-3609-z
Braun JT, Ogilivie JW, Akyuz E, Brodke DS, Bachus KN (2006) Creation of an experimental idiopathic-type scoliosis in an immature goat model using a flexible posterior asymmetric tether. Spine 31:1410–1414
Tis JE, Karlin LI, Akbarnia BA, Blakemore LC, Thompson GH, McCarthy RE, Tello CA, Mendelow MJ, Southern EP (2012) Growing spine committee of the scoliosis research society. J Pediatr Orthop 32(7):647–657
Cahill P, Marvil S, Cuddihy L, Schutt C, Idema J, Clements DH, Antonacci MD, Asghar J, Samdani AF, Betz RR (2010) Autofusion in the immature spine treated with growing rods. Spine 35:E1199–E1203
Sankar W, Skaggs D, Yazici M, Johnston CE, Shah SA, Javidan P, Kadakia RV, Day TF, Akbarnia BA (2011) Lengthening of dual growing rods and the law of diminishing returns. Spine 36:806–809
Singh V, Simpson J, Rawlinson J, Hallab N (2013) Growth guidance system for early onset scoliosis: comparison of experimental and retrieval wear. Spine 38:1546–1553
Shah SC, Birknes JK, Sagoo S, Thome S, Samdani AF (2009) Vertical expandable prosthetic titanium rib (VEPTR): indications, technique and management review. Surg Technol Int 18:223–229
Braun JT, Akyuz E, Udall H, Ogilvie JW, Brodke DS, Bachus KN (2006) Three-dimensional analysis of two fusionless scoliosis treatments: a flexible ligament tether versus a rigid-shape memory alloy staple. Spine 31:262–268
Betz RR, Ranade A, Samdani AF, Chafetz R, D’Andrea LP, Gaughan JP, Asghar J, Grewal H, Mulcahey MJ (2010) Vertebral body stapling: a fusionless treatment option for a growing child with moderate idiopathic scoliosis. Spine 35(2):169–176
Akbarnia BA (2007) Management themes in early onset scoliosis. J Bone Joint Surg 89-A(SUPPL I):42–54
Lenke LG, Dobbs MB (2007) Management of juvenile idiopathic scoliosis. J Bone Joint Surg 89-A(SUPPL I):54–63
Thompson GH, Lenke LG, Akbarnia BA, McCarthy RE, Campbell RM (2007) Early onset scoliosis: future directions. J Bone Joint Surg 89-A(SUPPL I):163–166
Block AJ, Wexler J, McDonnell EJ (1970) Cardiopulmonary failure of the hunchback. A possible therapeutic approach. JAMA 212:1520–1522
Stokes IA, Aronsson DD, Dimock AN, Cortright V, Beck S (2006) Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res 24:1327–1334
Lam GC, Hill DL, Le LH, Raso JV, Lou EH (2008) Vertebral rotation measurement: a summary and comparison of common radiographic and CT methods. Scoliosis 3:16
Aaro S, Dahlborn M (1981) Estimation of vertebral rotation and the spinal and rib cage deformity in scoliosis by coumputer tomography. Spine 6(5):460–467
Newton P, Fricka K, Lee SS, Farnsworth CL, Cox TG, Mahar AT (2002) Asymetrical flexible tethering of spine growth in an immature bovine model. Spine 27:689–693
Newton P, Faro FD, Farnsworth CL, Shapiro GS, Mohamad F, Parent S, Fricka K (2005) Multilevel spinal growth modulation with an anterolateral flexible tether in an immature bovine model. Spine 30:2608–2613
Driscoll M, Aubin C, Moreau A, Wakula Y, Sarwark JF, Parent S (2012) Spinal growth modulation using a novel intravertebral epiphyseal device in an immature porcine model. Eur Spine J 21:138–144
Braun JT, Akyuz E, Ogilvie JW, Bachus KN (2005) The efficacy and integrity of shape memory alloy staples and bone anchors with ligament tethers in the fusionless treatment of experimental scoliosis. J Bone Joint Surg 87-A:2038–2051
Dannawi Z, Altaf F, Harshavardhana NS, El Sebaie H, Noordeen H (2013) Early results of a remotely-operated magnetic growth rod in early-onset scoliosis. Bone Joint J 95-B(1):75–80
Rohlmann A, Richter M, Zander T, Klöckner C, Bergmann G (2006) Effect of different surgical strategies on screw forces after correction of scoliosis with a VDS implant. Eur Spine J 15:457–464
Driscoll M, Aubin CE, Moreau A, Wakula Y, Amini S, Parent S (2013) Novel Hemi-staple for the fusionless correction of pediatric scoliosis: influence on intervertebral discs and growth plates in a porcine model. J Spinal Disord Tech. [Epub ahead of print]
Acknowledgments
Grant funding was received from The Medical Research Council, UK. Special thanks are due to Joan Docherty and staff of the University of Edinburgh for ensuring welfare of the animals and laboratory assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burke, J.G., Vettorato, E., Schöffmann, G. et al. Modulation of spinal shape with growth following implantation of a novel surgical implant. Eur Spine J 24, 1522–1532 (2015). https://doi.org/10.1007/s00586-014-3610-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3610-6