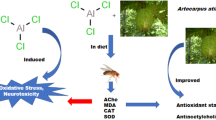
Abstract
Aluminum (Al) toxicity has been associated with multiple neurodegenerative disorders, including Alzheimer’s disease (AD). Since current medications for AD and Al-related toxicity are reported to have significant side effects, the search for therapeutic agents possessing antioxidant and anticholinesterase potential is ongoing. One such agent is rutin, a flavonoid, reported for its potent antioxidant and anti-inflammatory effects. This study investigated the activity of rutin in mitigating Al chloride (AlCl3) toxicity in Drosophila melanogaster. Flies were divided into six groups containing fifty (50) flies each. Group A served as the control; Group B received 40 mM AlCl3; Groups C and D were co-treated with 40 mM AlCl3 + 0.5 mg/kg rutin and 40 mM AlCl3 + 1 mg/kg, respectively; Groups E and F were treated with rutin alone in doses of 0.5 mg/kg and 1 mg/kg, respectively, all through their diet. Negative geotaxis, superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA), and acetylcholinesterase (AChE) activities were evaluated at the end of the study. A marked decrease (P < 0.05) was noticed in survival rate, negative geotaxis, and SOD as well as a significant increase in MDA and AChE in the AlCl3-exposed flies when compared to the control. Conversely, in the groups co-treated with rutin, there was a significant attenuation of the negative effects of AlCl3. Taken together, these findings suggest that rutin protected against AlCl3, thus indicating the possible therapeutic effects of rutin against aluminum toxicity and its related disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum (Al) ranks as the third most prevalent element in the Earth’s crust and is ubiquitous in our surroundings, encompassing the foods we consume (Klein 2019). Al is commonly accessible to both animal and human populations, making intoxication a potential concern. Aluminum can find its way into the body through various means, such as inhaling aerosols or particles, skin contact, vaccination, dialysis, infusions, and ingesting contaminated food, water, or medications. The adverse effects of aluminum toxicity encompass a wide range of issues, including oxidative stress, alterations in the immune system, genetic damage, inflammation, modification of peptides, enzyme dysfunction, disruptions in metabolic processes and iron homeostasis, amyloid formation, and disturbance of cell membranes, as well as cellular responses like apoptosis, necrosis, and abnormal cell growth (Igbokwe et al. 2019). Some of these cases are linked to the fact that Al is a neurotoxic substance, which has been discovered in elevated levels within the brain tissues of individuals with conditions such as Alzheimer’s disease (AD), epilepsy, and autism (Alasfar and Isaifan 2021). Reports indicate that Al exposure inhibits acetylcholine in the brain, thus establishing a link between the potential involvement of Al in the development of AD (Dave et al. 2002). Acetylcholinesterase (AChE), a serine hydrolase enzyme, plays a pivotal role in breaking down the neurotransmitter acetylcholine. Consequently, inhibiting AChE activity has emerged as a promising approach for AD treatment.
Various natural products derived from diverse plant sources are increasingly gaining global recognition as potential AChE inhibitors and offering a potential therapeutic avenue for addressing AD (Taqui et al. 2022). Due to the lack of effective remedies to prevent, reverse, or halt neuronal loss, researchers have turned to plant-derived bioactive compounds as potential treatments for these debilitating conditions; one of such bioactive compounds of interest is rutin. Rutin, a glycoside of the flavonoid quercetin naturally found in various plants and fruits like grapes, apricots, buckwheat, plums, cherries, and oranges, has shown promising effects in various disease conditions and has generated significant attention for its therapeutic potential in different models of neurodegenerative diseases (Enogieru et al. 2018). It possesses antiallergic, anti-inflammatory, antimicrobial, anticancer, and neuroprotective effects. Additionally, rutin exhibits notable antioxidant effects effectively scavenging 1,1-diphenyl radical-2-picrylhydrazyl (DPPH) and efficiently inhibiting lipid peroxidation in experimental studies. Its cytoprotective properties have also been demonstrated in studies involving invertebrate models. Rutin’s remarkable attributes make it a valuable compound in the ongoing quest for new therapeutic substances needed to treat various neurodegenerative disorders (Fidelis et al. 2021).
Drosophila melanogaster, also known as fruit fly or vinegar fly, is an arthropod belonging to the Drosophilae family. Over 100 years ago, William Castle introduced this dipteran insect as a model organism for biological research. It has been extensively employed in studying human genetics, mammalian physiology, microbial pathogenesis, life history evolution, genotoxicity, and cancer. One of the reasons for its widespread use is the significant similarities it shares with humans, making it a valuable tool for studying complex cellular signaling pathways that regulate development and survival (Jennings 2011). Approximately 75% of genes responsible for human diseases have comparable functional homologues in Drosophila melanogaster. This insect species is highly cost-effective to maintain in the laboratory and is recommended as a valuable alternative model for studying diseases, thus surpassing the need for vertebrate models (Abolaji et al. 2013). Accordingly, this study investigated the anticholinesterase and antioxidant potential of rutin against aluminum chloride-induced toxicity in Drosophila melanogaster.
Materials and methods
Chemicals and reagents
All chemicals and reagents used in this study were of analytical grade. Aluminum chloride (CAS no. 7784-13-6) manufactured by GHTECH (China) was purchased from Pyrex Chemicals in Benin City, Edo State. Additional chemicals utilized such as acetylthiocholine iodide and rutin were purchased from Sigma-Aldrich (St. Louis, MO).
Study subject
Drosophila melanogaster (Harwich strain) of both genders of 3–5 days old were obtained from the Drosophila laboratory of the University of Ibadan, Oyo state, Nigeria. The flies were reared on a cornmeal-based diet which contained 1% w/v agar, 1% w/v brewer’s yeast, 1% w/v powdered milk, 2% w/v glucose, and 0.08% v/w nipagin at room temperature (24 °C) under a 12-h dark/light cycle condition at the Drosophila laboratory of Central Research Laboratory, University of Benin, Benin City. The same strain of Drosophila melanogaster was utilized during the experiment.
Experimental design
A controlled experiment was conducted with five (5) treatment groups and one (1) control group to evaluate the effect of the treatment across experimental groups. Group A flies were placed on a cornmeal diet and distilled water, while Group B flies were placed on a cornmeal diet containing AlCl3 alone; Groups C and D were placed on a cornmeal diet containing rutin (0.5 and 1 mg/kg) and AlCl3, respectively; Groups E and F were placed on a cornmeal diet containing rutin alone (0.5 and 1 mg/kg), as described in Table 1.
Survival study
Following appropriate treatment according to the experimental design, the flies were observed daily for mortality and the survival rate was determined by counting the number of dead flies during the 14-day treatment (Abolaji et al. 2017). The data was subsequently analyzed and plotted as a percentage of survival after the treatment period (Akinsanmi et al. 2019; Igharo et al. 2021). The survival assay was carried out in three replicates of each concentration.
Negative geotaxis assay
This assay was used to determine the locomotor performance or climbing activity of the flies. This assay was carried out as previously described (Abolaji et al. 2018). Ten (10) flies from each group were immobilized under ice anesthesia. They were subsequently placed separately in labeled vertical glass columns (length 15 cm; diameter 1.5 cm). After the recovery from the ice exposure, the bottom of the column was gently tapped, and the flies were allowed to climb. The number of flies that climbed up to and above the 6 cm mark of the column in 6 s as well as those that remained below this mark after this time was recorded. The climbing activity was scored by expressing the proportion (%) of flies above the 6 cm mark. After 1 min interval, this procedure was repeated. A total of three repetitions were carried out for each group.
Preparation of samples for biochemical assays
At the end of a 7-day treatment period, the flies (intact whole body) were homogenized and centrifuged, and the supernatants were used for biochemical analysis. The flies were first anesthetized in ice, thereafter weighed, and then homogenized in 0.1 M potassium phosphate buffer of pH 7.4 (1:10) (Oyetayo et al. 2020). They were later centrifuged at 4000 g for 10 min at 4 °C, and the supernatants obtained were used for the following biochemical assays: malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and acetylcholinesterase (AChE) activities.
Superoxide dismutase (SOD) activity
SOD activity was determined as previously described (Misra and Fridovich 1972). The reaction mixture contained 10 µL of the sample, 15% quercetin, 20 mM phosphate buffer (pH 7.8), 0.08 mM EDTA, and 8 mM tetramethylethylenediamine (TEMED). The reaction was left for 3 min, and absorbance was measured at 406 nm. The results were expressed as the amount of protein required to inhibit quercetin auto-oxidation (µmol/min/mg protein) (Misra and Fridovich 1972).
Determination of catalase activity
Catalase activity was determined following previous methods (Cohen et al. 1970). Ten μL of the sample (1:50 dilution) and 50 mM potassium phosphate buffer (pH 7.0) followed by 300 mM H2O2 were mixed. The loss in absorbance of H2O2 was monitored for 2 min at 240 nm and was subsequently used to calculate catalase activity which was expressed as µmol of H2O2 consumed per minute per milligram of protein (Cohen et al. 1970).
Determination of lipid peroxidation
Lipid peroxidation was determined according to a previous method (Varshney and Kale 1990). The mixture contained 40 µL of the supernatant, 100 µL of 0.67% thiobarbituric acid, 5 µL of 10 mM butyl-hydroxytoluene (BHT), 300 µL of 1% O-phosphoric acid, and 55 µL of distilled water. This was followed by a 45-min incubation time at 90 °C, and the absorbance was measured at 535 nm. The results were expressed as µmol MDA formed per milligram protein (Varshney and Kale 1990).
Determination of acetylcholinesterase activity
Acetylcholinesterase activity was evaluated following the method previously described (Ellman et al. 1961). The reaction mixture contained 30 µL of the sample, 1 mM DTNB, 0.1 M of potassium phosphate buffer (pH 7.4), and 0.8 mM acetylthiocholine. This mixture was monitored for 2 min (at 30-s intervals) at 412 nm. The enzyme activity was then evaluated as µmol of acetylthiocholine hydrolyzed per min per milligram protein (Ellman et al. 1961).
Statistical analysis
Data was analyzed using the GraphPad Prism statistical package (version 7). Statistical significance (P < 0.05) was determined through analysis of variance (ANOVA), followed by Tukey’s multiple comparison post hoc test. Results are presented as mean ± standard error of mean (mean ± SEM).
Results
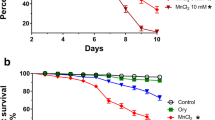
Survival rate of D. melanogaster exposed to AlCl3 and rutin
Figures 1 and 2 illustrate the survival rate in experimental Groups A–F. Figure 1 shows the survival rate of flies from days 0 to 14. On the 14th day (Fig. 2), there was a significant decrease (P < 0.05) for Group B (44.76%) when compared to the control (93.76%), while there was a significant increase (P < 0.05) in Group C (RUT1 + AlCl3; 76.19%) and Group D (RUT1 + AlCl3; 78.76%) when compared to Group B (AlCl3). However, there were no significant differences (P > 0.05) in survival rates between Groups E (RUT1; 90.47%) and F (RUT2; 89.52%) when compared to the control group.
Climbing activity (negative geotaxis) of D. melanogaster exposed to AlCl3 and rutin
Figure 3 illustrates the climbing activity in experimental Groups A–F. There was a significant decrease (P < 0.05) in the climbing activity of Group B when compared to the control, while there was a significant increase (P < 0.05) in Groups C (RUT1 + AlCl3) and D (RUT2 + AlCl3) when compared to Group B (AlCl3). Nevertheless, there were no significant differences (P > 0.05) in climbing activity between Groups E (RUT1) and F (RUT2) when compared to the control group.
Superoxide dismutase (SOD) activity of D. melanogaster treated with AlCl3 and rutin
Figure 4 illustrates the superoxide dismutase (SOD) activity in experimental Groups A–F. A significant decrease (P < 0.05) was noted in Group B when compared to the control. Conversely, there was a significant increase (P < 0.05) in SOD activity in both Groups C (RUT1 + AlCl3) and D (RUT2 + AlCl3) when compared to Group B (AlCl3). However, there were no significant differences (P > 0.05) in SOD activity between Groups E (RUT1) and F (RUT2) when compared to the control group.
Catalase (CAT) activity of D. melanogaster treated with AlCl3 and rutin
Figure 5 illustrates the catalase (CAT) activity in experimental Groups A–F. A significant decrease (P < 0.05) was noted in Group B when compared to the control. Conversely, there was a significant increase (P < 0.05) in CAT activity in Group D (RUT2 + AlCl3) when compared to Group B (AlCl3). However, there were no significant differences (P > 0.05) in CAT activity between Groups E (RUT1) and F (RUT2) when compared to the control group.
Lipid peroxidation (MDA) activity of D. melanogaster treated with AlCl3 and rutin
Figure 6 illustrates the lipid peroxidation (MDA) activity in experimental Groups A–F. A significant increase (P < 0.05) was noted in Group B when compared to the control. Conversely, there was a significant decrease (P < 0.05) in MDA activity in both Groups C (RUT1 + AlCl3) and D (RUT2 + AlCl3) when compared to Group B (AlCl3). However, there were no significant differences (P > 0.05) in MDA activity between Groups E (RUT1) and F (RUT2) when compared to the control group.
Acetylcholinesterase (AChE) activity of D. melanogaster treated with AlCl3 and rutin
Figure 7 illustrates the AChE activity in experimental Groups A–F. A significant increase (P < 0.05) was noted in Group B (AlCl3) when compared to the control. In contrast, both Groups C (RUT1 + AlCl3) and D (RUT1 + AlCl3) displayed a significant decrease (P < 0.05) in AChE activity compared to Group B (AlCl3). No significant differences (P > 0.05) in AChE activity were evident in Groups E (RUT1) and F (RUT2) when compared to the control group.
Discussion
The fruit fly (Drosophila melanogaster) has been firmly established as a model organism for the study of neurotoxicity and neurodegenerative diseases (Adedayo et al. 2022). Its extensive genetic toolbox allows for precise and highly targeted genome manipulations, leading to significant breakthroughs. Additionally, its relatively short lifespan enables researchers to investigate intricate aspects of brain function more rapidly compared to other model organisms like mice (McGurk et al. 2015). The survival rate in Drosophila refers to the proportion of flies that remain alive or survive within a given population or group over a specified period, often in the context of experiments or studies involving the effects of various factors like toxic substances, diets, or treatments on the fly population’s longevity (Akinsanmi et al. 2019; Farombi et al. 2018). The substantial decline in the survival rate of Drosophila melanogaster subjected to AlCl3 exposure alone serves as clear evidence of the detrimental effects of aluminum chloride; this is consistent with other studies demonstrating that AlCl3 significantly reduces the survival rate of Drosophila melanogaster via oxidative stress (Oyetayo et al. 2020; Oboh et al. 2021).
Negative geotaxis, a natural reflexive behavior in Drosophila, involves the flies climbing upwards along the inner wall of a cylindrical container in response to a stimulus, for instance, a gentle tap at the bottom (Rhondenizer et al. 2008). Investigations confirmed that negative geotaxis in Drosophila represents an age-related decline in climbing primarily due to reduced climbing speed. This decrease in locomotor speed with age is a characteristic feature of declining locomotor function observed in both flies and humans (Gargano et al. 2005; Rhondenizer et al. 2008). For this study, the notable decrease observed in the climbing activity of Drosophila melanogaster exposed to aluminum chloride alone underscores its harmful impact; this aligns with the findings reported previously (Inneh and Eiya 2023; Oyetayo et al. 2020).
Organisms have a defense mechanism to safeguard their tissues by secreting enzymes possessing antioxidant properties which serve to neutralize or eliminate damaging oxygen species (Beyer et al. 1991). SOD regulates oxidative stress, lipid metabolism, inflammation, and oxidation, preventing lipid peroxidation, low-density lipoprotein oxidation, and inflammatory cell adhesion. As a primary antioxidant enzyme, SOD converts superoxide radicals into hydrogen peroxide and oxygen, safeguarding tissues from harmful oxygen species (Beyer et al. 1991; Islam et al. 2022). The findings from this study demonstrate a significant reduction in superoxide dismutase (SOD) levels in flies exposed solely to AlCl3 when compared to the control group. This suggests that aluminum has the potential to disrupt antioxidant functions by inhibiting antioxidant enzymes; this is consistent with other studies (Efosa et al. 2023; Inneh and Enogieru 2023). Rutin exhibited the capacity to augment SOD activity when administered concurrently, implying its potential to enhance the antioxidant properties of SOD; this is consistent with other studies which showed rutin increased SOD activity in rodent models (Arowoogun et al. 2021; Prasad and Prasad 2019; Qu et al. 2019). Catalase (CAT), known as hydrogen peroxide/hydrogen peroxide oxidoreductase, stands as a vital intracellular antioxidant enzyme, safeguarding cells against oxidative stress, located within the peroxisomes of aerobic cells (Shangari and O’Brien 2006). Its primary role involves shielding the cell from the harmful impacts of elevated hydrogen peroxide (H2O2) levels. This is achieved by catalyzing the breakdown of H2O2 into molecular oxygen and water, without generating free radicals in the process (Shangari and O'Brien 2006). The findings from this study demonstrate a reduction in CAT levels in flies exposed solely to AlCl3 when compared to the control group. This suggests that aluminum has the potential to disrupt antioxidant functions by inhibiting the activity of CAT; this is consistent with other studies (Ahmad Rather et al. 2019; Inneh and Enogieru 2023; Liaquat et al. 2019). Rutin exhibited the capacity to augment CAT activity when administered concurrently, implying its potential to enhance the antioxidant properties of CAT; this is consistent with other studies which showed rutin increased CAT activity in rodent models (Iova et al. 2021; Uthra et al. 2022).
Lipid peroxidation is an important mechanism in free radical-mediated cell injury. It has the potential to directly harm cellular membranes, and the resulting reactive carbonyl byproducts could extend the damage well beyond the initial location of radical generation. It has long been considered to be involved in various toxic tissue injuries and certain disease processes (Cheeseman 1993). Lipid peroxidation plays a significant role in crucial cellular antioxidant mechanisms. The processes by which iron, oxygen, and specific hepatotoxins induce tissue damage often revolve around the creation of intermediate free radical species and the initiation of lipid peroxidation (Protchenko et al. 2021). Malondialdehyde (MDA) serves as a conclusive byproduct resulting from the peroxidation of polyunsaturated fatty acids within cellular systems. Elevated levels of free radicals contribute to an excessive production of MDA. The quantification of MDA levels is widely recognized as a pivotal indicator of oxidative stress and antioxidant status (Gaweł et al. 2004). In this study, the substantial elevation in MDA when exposed to AlCl3 is consistent with earlier studies (Akpanyung et al. 2020; Kumar et al. 2009). Rutin exhibited the capacity to reduce MDA activity when administered concurrently, implying its potential to decrease lipid peroxidation. This is consistent with other studies which showed rutin reduced MDA activity in rodent models (Korkmaz, and Kolankaya 2010; Rotimi et al. 2023).
Acetylcholinesterase (AChE) is recognized as a pivotal biomarker in AD. Inhibitors of AChE hold substantial promise for AD therapy due to AChE’s role in augmenting the neurotoxicity associated with the amyloid component implicated in AD pathogenesis. Consequently, the development of a straightforward and exceptionally sensitive method for monitoring AChE levels and identifying potent AChE inhibitors is of utmost importance and significance (Wang et al. 2023). Acetylcholinesterase plays a pivotal role in terminating impulse transmission by swiftly breaking down the neurotransmitter acetylcholine in multiple cholinergic pathways within the central and peripheral nervous systems (Colović et al. 2013). In this study, we evaluated the effects of AlCl3 on the activity of AChE; it was observed that the activity of AChE increased in flies subjected to aluminum exposure and agrees with other studies with fly models (Zatta et al. 2002; Oboh et al. 2021). On the other hand, rutin was able to lower the activity of this enzyme, which highlights its effectiveness as a promising acetylcholinesterase inhibitor and might hold therapeutic significance in the treatment and/or management of neurodegenerative disorders such as Alzheimer’s disease (Xie et al. 2014; Oboh et al. 2020).
Conclusion
The results of this study suggest that rutin’s protective activity against AlCl3 toxicity is mediated through its potent antioxidant and anticholinesterase properties. Consequently, rutin may have potential applications in the treatment and/or management of neurological disorders associated with aluminum exposure.
References
Abolaji AO, Kamdem JP, Farombi EO, Rocha JBT (2013) Drosophila melanogaster as a promising model organism in toxicological studies. Arch Basic Appl Med 1:33–38
Abolaji AO, Olaiya CO, Oluwadahunsi OJ, Farombi EO (2017) Dietary consumption of monosodium L-glutamate induces adaptive response and reduction in the life span of Drosophila melanogaster. Cell Biochem Funct 35(3):164–170
Abolaji AO, Adedara AO, Adie MA, Vicente-Crespo M, Farombi EO (2018) Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem Biophys Res Commun 503(2):1042–1048
Adedayo BC, Ogunsuyi OB, Akinniyi ST, Oboh G (2022) Effect of Andrographis paniculata and Phyllanthus amarus leaf extracts on selected biochemical indices in Drosophila melanogaster model of neurotoxicity. Drug Chem Toxicol 45(1):407–416
Ahmad Rather M, Justin-Thenmozhi A, Manivasagam T, Saravanababu C, Guillemin GJ, Essa MM (2019) Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in Wistar rats. Neurotox Res 35:955–968
Akinsanmi AO, Balogun O, Titlayo JO, Longdet IY, John AC (2019) The pro-oxidant effect of dextran sodium sulphate on oxidative stress biomarkers and antioxidant enzymes in Drososphila melanogaster. Afr J Biochem Res 13(6):82–89
Akpanyung EO, Bassey UE, Noah UT, Effiong GS (2020) Effects of ethanol leaf extract of Vernonia amygdalina on some indices of liver function, oxidative stress and lipid profile in aluminium chloride intoxicated male Wistar rats. Biokemistri 32(1):11–21
Alasfar RH, Isaifan RJ (2021) Aluminum environmental pollution: the silent killer. Environ Sci Pollut Res 28(33):44587–44597
Arowoogun J, Akanni OO, Adefisan AO, Owumi SE, Tijani AS, Adaramoye OA (2021) Rutin ameliorates copper sulfate-induced brain damage via antioxidative and anti-inflammatory activities in rats. J Biochem Mol Toxicol 35(1):e22623
Beyer W, Imlay J, Fridovich I (1991) Superoxide dismutases. Prog Nucleic Acid Res Mol Biol 40:221–253
Cheeseman KH (1993) Mechanisms and effects of lipid peroxidation. Mol Aspects Med 14(3):191–197
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34(1):30–38
Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol 11(3):315–335
Dave KR, Syal AR, Katyare SS (2002) Effect of long-term aluminum feeding on kinetics attributes of tissue cholinesterases. Brain Res Bull 58(2):225–233
Efosa JO, Omage K, Azeke MA (2023) Hibiscus sabdariffa calyx protect against oxidative stress and aluminium chloride-induced neurotoxicity in the brain of experimental rats. Toxicol Rep 10:469–480
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Enogieru AB, Haylett W, Hiss DC, Bardien S, Ekpo OE (2018) Rutin as a potent antioxidant: implications for neurodegenerative disorders. Oxid Med Cell Longev 2018
Farombi EO, Abolaji AO, Farombi TH, Oropo AS, Owoje OA, Awunah MT (2018) Garcinia kola seed biflavonoid fraction (Kolaviron), increases longevity and attenuates rotenone-induced toxicity in Drosophila melanogaster. Pestic Biochem Physiol 145:39–45
Fidelis KR, dos Santos Nunes RG, da Silva CS, Oliveira CVB, Costa AR, de Lima Silva JR, ... Barros LM (2021) Evaluation of the neuroprotective effect of rutin on Drosophila melanogaster about behavioral and biochemical aspects induced by mercury chloride (HgCl2). Comp Biochem Physiol Part C Toxicol Pharmacol 249:109119
Gargano JW, Martin I, Bhandari P, Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40(5):386–395
Gaweł S, Wardas M, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci lekarskie (Warsaw, Poland: 1960) 57(9–10):453–455
Igbokwe IO, Igwenagu E, Igbokwe NA (2019) Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol 12(2):45
Igharo OG, Abadaike LI, Osunbor JO, Owie IC, Aruomaren AI, Igharo EL, Ikeke KI (2021) Effect of dietary inclusion of chitosan on survival and selected cellular antioxidants in Drosophila melanogaster. J Appl Sci Environ Manag 25(6):1093–1098
Inneh CA, Eiya BO (2023) Anticholinesterase activity and antioxidant effect of vitamin E in aluminium chloride induced toxicity in Drosophila melanogaster. J Afr Assoc Physiol Sci 11(1):17–28
Inneh C, Enogieru A (2023) Anticholinesterase and antioxidant potential of aqueous leave extract of Phyllantus amarus in aluminium chloride-induced Alzheimer’s disease in Drosophila melanogaster. Physiology 38(S1):5685820
Iova GM, Calniceanu H, Popa A, Szuhanek CA, Marcu O, Ciavoi G, Scrobota I (2021) The antioxidant effect of curcumin and rutin on oxidative stress biomarkers in experimentally induced periodontitis in hyperglycemic Wistar rats. Molecules 26(5):1332
Islam MN, Rauf A, Fahad FI, Emran TB, Mitra S, Olatunde A, ... Mubarak MS (2022) Superoxide dismutase: an updated review on its health benefits and industrial applications. Crit Rev Food Sci Nutr 62(26):7282–7300
Jennings BH (2011) Drosophila–a versatile model in biology medicine. Mater Today 14(5):190–195
Klein GL (2019) Aluminum toxicity to bone: a multisystem effect? Osteoporosis Sarcopenia 5(1):2–5
Korkmaz A, Kolankaya D (2010) Protective effect of rutin on the ischemia/reperfusion induced damage in rat kidney. J Surg Res 164(2):309–315
Kumar A, Dogra S, Prakash A (2009) Protective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioral and biochemical alterations in rats. Behav Brain Res 205(2):384–390
Liaquat L, Sadir S, Batool Z, Tabassum S, Shahzad S, Afzal A, Haider S (2019) Acute aluminum chloride toxicity revisited: study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci 217:202–211
McGurk L, Berson A, Bonini NM (2015) Drosophila as an in vivo model for human neurodegenerative disease. Genetics 201(2):377–402
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Oboh G, Adebayo AA, Ademosun AO, Olowokere OG (2020) Rutin restores neurobehavioral deficits via alterations in cadmium bioavailability in the brain of rats exposed to cadmium. Neurotoxicol 77:12–19
Oboh G, Oladun FL, Ademosun AO, Ogunsuyi OB (2021) Anticholinesterase activity and antioxidant properties of Heinsia crinita and Pterocarpus soyauxii in Drosophila melanogaster model. J Ayurveda Integr Med 12(2):254–260
Oyetayo BO, Abolaji AO, Fasae KD, Aderibigbe A (2020) Ameliorative role of diets fortified with curcumin in a Drosophila melanogaster model of aluminum chloride-induced neurotoxicity. J Funct Foods 71:104035
Prasad R, Prasad SB (2019) A review on the chemistry and biological properties of rutin, a promising nutraceutical agent. Asian J Pharm Pharmacology 5(S1):1–20
Protchenko O, Baratz E, Jadhav S, Li F, Shakoury-Elizeh M, Gavrilova O, ... Philpott CC (2021) Iron chaperone poly rC binding protein 1 protects mouse liver from lipid peroxidation and steatosis. Hepatol 73(3):1176–1193
Qu S, Dai C, Guo H, Wang C, Hao Z, Tang Q, ... Zhang Y (2019) Rutin attenuates vancomycin-induced renal tubular cell apoptosis via suppression of apoptosis, mitochondrial dysfunction, and oxidative stress. Phytother Res 33(8):2056–2063
Rhondenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M (2008) Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol 43(8):739–748
Rotimi DE, Elebiyo TC, Ojo OA (2023) Therapeutic potential of rutin in male infertility: a mini review. J Integr Med
Shangari N, O’Brien PJ (2006) Catalase activity assays. Curr Protoc Toxicol 27(1):7–7
Taqui R, Debnath M, Ahmed S, Ghosh A (2022) Advances on plant extracts and phytocompounds with acetylcholinesterase inhibition activity for possible treatment of Alzheimer’s disease. Phytomedicine Plus 2(1):100184
Uthra C, Reshi MS, Jaswal A, Yadav D, Shrivastava S, Sinha N, Shukla S (2022) Protective efficacy of rutin against acrylamide-induced oxidative stress, biochemical alterations and histopathological lesions in rats. Toxicol Res 11(1):215–225
Varshney R, Kale RK (1990) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58(5):733–743
Wang F, Liu M, Niu X, Xia L, Qu F (2023) Dextran-assisted ultrasonic exfoliation of two-dimensional metal-organic frameworks to evaluate acetylcholinesterase activity and inhibitor screening. Anal Chim Acta 1243:340815
Xie Y, Yang W, Chen X, Xiao J (2014) Inhibition of flavonoids on acetylcholine esterase: binding and structure–activity relationship. Food Funct 5(10):2582–2589
Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T (2002) In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res Bull 59(1):41–45
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Research Ethics Committee, College of Medical Sciences, University of Benin, with approval no. CMS/REC/2023/356.
Informed consent
For this type of study, informed consent is not required.
Consent for publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iyiegbu, M.E., Enogieru, A.B. Antioxidant and anticholinesterase activity of rutin in aluminum chloride-exposed Drosophila melanogaster. Comp Clin Pathol 33, 445–452 (2024). https://doi.org/10.1007/s00580-024-03564-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-024-03564-8