Abstract
Zinc plays an important role in the biosynthesis and storage of insulin. Consequently, its deficiency may have a deleterious impact on the progression of diabetes and its associated consequences. Thus, this study was conducted to investigate the effect of hawthorn Crataegus azarolus on blood biochemical parameters, tissue zinc status, and oxidative stress biomarkers in streptozotocin-induced diabetic rats fed a zinc-insufficient diet. Thirty-two male albino Wistar rats were divided into four groups: two groups were fed a zinc-sufficient diet (one non-diabetic and the other diabetic), while the other two groups of diabetic rats were fed a zinc-insufficient diet. One group was not treated, and the other was treated with an extract of Crataegus azarolus (150 mg/kg body weight). Body weight and food intake were regularly recorded. After 4 weeks of dietary manipulation, the fasting animals were sacrificed. Zinc-deficient feed decreased body weight, insulin, and tissue zinc levels (in the femur, liver, kidney, and pancreas), as well as glutathione concentrations, lactic dehydrogenase, catalase, superoxide dismutase, glutathione peroxidase, and glutathione reductase activities. It was also noted that inadequate dietary zinc intake increased concentrations of glucose, cholesterol, triglycerides, urea, uric acid, creatinine, lipid peroxidation levels, and transaminase activities. However, oral administration of hawthorn extract was observed to improve all of the previously mentioned parameters, bringing them approximately back to their normal levels. The present study showed that Crataegus azarolus supplementation, presumably acting as an antioxidant, can be a natural source for reducing the development of diabetes caused by zinc deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a complex group of chronic diseases with different etiologies, in which hyperglycemia is the common criterion that triggers defects in insulin secretion and/or its mechanism of action, causing disorders in various organs and systems (Jiménez et al. 2020). Globally, 537 million adults (20–79 years) were living with diabetes in 2021, and this number is predicted to rise to over 643 million by 2030 and 784 million by 2045 (Sun et al. 2022). Type 2 diabetes mellitus (T2DM) is the most prevalent type of diabetes mellitus, accounting for over 90% of all morbidity and mortality (Ojuade et al. 2021). Diabetes is associated with deficiencies in insulin signaling transduction pathway components (Rahmati et al. 2021). In diabetes, constantly raised glucose levels contribute mostly to the process of overproduction of reactive oxygen species (ROS) through various mechanisms, especially glucose auto-oxidation, activation of polyol, hexamine pathways, protein kinase C, and advanced glycation end products (AGEs), which consequently contribute to the increase in oxidative stress (Abdulmalek et al. 2021). If cellular antioxidant defenses do not adjust ROS production, oxidative damage (lipid peroxidation, protein degradation, disruption of DNA) and possibly cell death may occur (Vélez-Alavez et al. 2013). Oxidative stress may take place when antioxidant mechanisms are not functioning correctly, as in dietary deficiencies of vitamins such as vitamin C and vitamin E or essential trace elements such as zinc, selenium, and manganese (Hamdiken et al. 2018). Zinc performs various biological functions in different aspects. First, it is essential for more than 300 enzymes for their catalytic activation, thus participating in several enzymatic and metabolic cellular processes in the human body. Second, zinc binds to over 2500 proteins, equivalent to 10% of the total human proteome, and maintains the structural integrity for many of them (Choi et al. 2018). The trace element plays a crucial role in the stabilization of insulin hexamer and insulin stockpiling in the pancreas and expands insulin condensation. Zinc actually favors the phosphorylation of insulin receptors by improving the transport of glucose into cells. Nevertheless, zinc deficiency increases the destruction of the islet cell in type 1 diabetes mellitus (T1DM) through cytokine autoimmune attack (Li 2014). The element plays a significant role in antioxidant defense as a cofactor of the superoxide dismutase enzyme, by modulating glutathione metabolism and metallothionein expression, competing with iron and copper in the cell membrane, and inhibiting the nicotinamide adenine dinucleotide phosphate-oxidase enzyme (Gholamhoseinian et al. 2020). Therefore, insufficient intake, reduced absorption, and expanded loss of zinc result in zinc deficiency. Recent studies show that most Crataegus species have beneficial effects on human health. Extracts from aerial parts exhibit several biological activities, such as anti-inflammatory (Wang et al. 2019), antihyperglycemic (Aierken et al. 2017), vasorelaxing (Yang and Liu 2012), and hypolipidemic properties (Hao et al. 2011). In addition, researchers have shown that leaves of Crataegus azarolus are rich in phenolic compounds and exhibit substantial antioxidant and antimicrobial activities (Yahyaoui et al. 2019). It has also been demonstrated that extracts from the fruits and leaves of Crataegus are safe for human consumption (Daniele et al. 2006). Thus, the present investigation was conducted to examine the modulatory effects of Crataegus azarolus administration for the prevention of diabetes pathology development by evaluating body weight gain, zinc status, carbohydrate metabolism, and the antioxidant system in rats fed a zinc-deficient diet.
Materials and methods
Animals
Male albino Wistar rats, weighing approximately 200 to 250 g and aged 10 to 12 weeks, were sourced from the Pasteur Institute (Algiers, Algeria). The rats were allowed to acclimate to the laboratory environment for a period of 2 weeks before the commencement of the experiments. Individual plastic cages with suitable bedding were provided for housing the rats. Standard food and tap water were provided ad libitum unless specified otherwise. The temperature in the animal housing facility was maintained at approximately 22 ± 2 °C. A 12/12-h light/dark cycle was followed, with lights turned on at 6 am.
Preparation of extract
The aerial parts of Crataegus azarolus were collected in September from the Ain-Berda commune in the Annaba region of Eastern Algeria. The plant material was authenticated at the Department of Biology at Badji Mokhtar University in Annaba, Algeria. After thorough washing, the samples were dried in a well-ventilated area at room temperature. Once completely dry, the leaves were ground into a fine powder using a domestic blender. The resulting powder was stored in airtight containers, protected from light, at room temperature until the start of the experiment.
Qualitative phytochemical screening
The crude water extract of the plant underwent qualitative phytochemical screening to determine the presence of active chemical constituents. The screening followed the method described by Trease and Evans (Evans and Evans 2009).
Detection of alkaloids
Five grams of the powdered plant material was mixed with 50 mL of 1% HCl. After maceration and filtration, a few drops of Mayer’s reagent were added. The formation of a white precipitate indicated the presence of alkaloids.
Detection of flavonoids
Ten grams of the powdered plant material was macerated in 150 mL of 1% HCl for 24 h. After filtration, the filtrate was made basic by adding a few drops of NH4OH. The appearance of a pale yellow color in the upper part of the tube indicated the presence of flavonoids.
Detection of tannins
One milliliter of the water extract was mixed with 10 mL of distilled water and filtered. Three drops of ferric chloride (FeCl3) reagent were added to the filtrate. A blue-black or green precipitate confirmed the presence of gallic tannins or catechol tannins, respectively.
Detection of saponins
Five milliliters of the water extract was vigorously shaken with 10 mL of distilled water for 2 min. The presence of a stable foam that persisted for at least 15 min indicated the presence of saponins.
Detection of anthocyanins
The detection of anthocyanins involved observing color changes in a 10% extract with changes in pH. A few drops of HCl were added to the extract, followed by a few drops of NH4OH. A positive test was indicated by a pink-red coloration that turned purplish-blue.
Quantitative phytochemical screening
Total polyphenol content determination
An aliquot of 100 µL of the extract was mixed with 2.5 mL of Folin–Ciocalteu phenol reagent (10 × dilution) and allowed to react for 5 min. Then, 2.5 mL of saturated Na2CO3 solution was added, and the mixture was allowed to stand for 30 min. The absorbance of the reaction mixture was measured at 725 nm. The total polyphenol concentration was calculated using the equation Y = 0.0073x − 0.3165 (R2 = 0.990) and expressed as milligrams of gallic acid equivalent (GAE) per gram of dry weight (mg GAE/g DW) (Wong et al. 2006).
Determination of total flavonoid content
The flavonoid content was determined using the aluminum trichloride method with catechin as the reference compound. A volume of 125 µL of the extract was mixed with 75 µL of a 5% NaNO2 solution and allowed to stand for 6 min. Then, 150 µL of aluminum trichloride (10%) was added and incubated for 5 min, followed by the addition of 750 µL of NaOH (1 M). The final volume of the solution was adjusted to 2500 µL with distilled water. After 15 min of incubation, the mixture turned pink, and the absorbance was measured at 510 nm. The total flavonoid concentration was calculated using the equation Y = 0.0029x + 0.131 (R2 = 0.995) and expressed as milligrams of quercetin equivalent (QE) per gram of dry weight (mg QE/g DW) (Zhishen et al. 1999).
Determination of total tannin content
A volume of 0.1–0.5 mL of the crude extract was taken and placed in tubes covered with aluminum foil. Then, 3 mL of 4% vanillin (w/v) in methanol was added, and the tubes were vigorously shaken. Immediately, 1.5 mL of concentrated HCl was pipetted into the tubes, and they were shaken again. After allowing the tubes to stand for 20 min at room temperature, the absorbance was measured at 500 nm against a blank. The total tannin content was calculated using the equation Y = 0.0018x + 0.0737 (R2 = 0.992) and expressed as milligrams of catechin equivalent (CE) per gram of dry weight (mg CE/g DW) (Julkunen-Tiitto 1985).
Determination of DPPH radical scavenging activity
The DPPH radical scavenging activity was determined following the method described by Brand-Williams et al. (Brand-Williams et al. 1995). A DPPH solution (0.070 mg/mL) was prepared and mixed with sample solutions at various concentrations (25 to 100 µg/mL). A control solution containing only methanol and DPPH was also prepared. The solutions were incubated for 30 min at room temperature, and the absorbance was measured at 517 nm.
Quercetin was used as a standard for calibration, and its concentrations ranged from 10 to 100 µg/mL. The radical scavenging capacity was evaluated by measuring the decrease in absorbance at 517 nm. The percentage of inhibition of the samples was calculated using the following equation:
Curves were constructed by plotting the percentage of inhibition against the concentration in micrograms per milliliter. The equation of the curve allowed for the calculation of the half maximal inhibitory concentration (IC50), which corresponds to the sample concentration that reduced the initial DPPH• absorbance by 50%. A lower IC50 value indicates higher antioxidant activity. All analyses were performed in triplicate to ensure the accuracy and reproducibility of the results.
Induction of experimental diabetes
Experimental diabetes was induced by administering a fresh streptozotocin solution intraperitoneally at a dose of 50 mg/kg body weight. Streptozotocin was dissolved in citrate buffer (0.1 M, pH 4.5). To prevent hypoglycemia induced by streptozotocin, the streptozotocin-treated animals were given a 10% glucose solution overnight. After 1 week, the diabetic state was confirmed by measuring blood glucose levels from the tail vein using a glucose meter (VitalCheck®MM1200). Rats with glucose levels over 14 mmol/L were considered diabetic animals.
Diet preparation
The basal diet for the animals was prepared following the method described by Southon et al. (Southon et al. 1988). It consisted of cornstarch (326 g/kg diet), sucrose (326 g/kg diet), protein from Soja (168 g/kg diet), lipids from corn oil (80 g/kg diet), fiber from cellulose (40 g/kg diet), vitamin mix (from Sigma), and mineral mix (40 g/kg diet). The mineral mix was formulated to contain either adequate (54 mg/kg) or inadequate (1.2 mg/kg) amounts of zinc, as determined by atomic absorption spectroscopy. The mineral mix included calcium hydrogen orthophosphate (13 g/kg diet), disodium hydrogen orthophosphate (7.4 g/kg diet), calcium carbonate (8.2 g/kg diet), potassium chloride (7.03 g/kg diet), magnesium sulfate (4 g/kg diet), ferrous sulfate (0.144 g/kg diet), copper sulfate (0.023 g/kg diet), potassium iodide (0.001 g/kg diet), manganese sulfate (0.180 g/kg diet), and zinc carbonate (0.1 g/kg diet). The zinc-deficient diet did not contain any additional zinc carbonate.
Experimental design
After the stabilization of diabetes, the rats were divided into four groups, each consisting of eight rats. The groups were as follows: non-diabetic group (ND), diabetic group fed a sufficient zinc diet (DAZ), diabetic group fed a deficient zinc diet (DZD), and diabetic group treated with an oral dose of 150 mg/kg aqueous extract of C. azarolus (DZD+Az). The duration of the treatment was 4 weeks.
Blood and tissue sample collection
At the end of the treatment period, the rats were euthanized by cervical decapitation. Blood samples were collected and centrifuged at 3000 rpm to obtain serum, which was stored at – 20 °C for subsequent biochemical analysis. The pancreas, liver, and kidney were excised and washed with ice-cold isotonic NaCl saline. They were then blotted dry. The right femur was also collected, and any connective tissues and muscles were removed. The liver fragment, pancreas fragment, right kidney, and femur were weighed and dried at 80 °C for 16 h for zinc concentration determination. Another fragment of the liver and the left kidney were immediately processed to assay malondialdehyde (MDA), glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), and glutathione peroxidase (GSH-Px). Finally, a second fragment of the pancreas was utilized for histological examination.
Measurement of biochemical parameters
The biochemical parameters were measured using a biochemistry analyzer system (ERBA Mannheim XL600). The specific principles for each test were determined based on the ERBA XL600 data sheet. The analyzer system provided accurate and standardized measurements of the biochemical parameters, ensuring reliable results for further analysis and interpretation.
Analysis of zinc in the tissues
The dried kidneys, pancreas, livers, and femurs were subjected to zinc analysis. To prepare the samples, they were heated in silica crucibles at 480 °C for 48 h, resulting in ash. The ash was then dissolved in hot 12 M HCl acid. Zinc analysis was performed using a flame atomic absorption spectrophotometer (Perkin-Elmer Atomic Absorption Spectrometer AAnalyst 400). To ensure accuracy, standard reference materials such as bovine liver and wheat flour were used to verify zinc recovery, which exceeded 96% in the reference materials. Zinc standards were prepared from a 1 mg/mL zinc nitrate standard solution. To prevent zinc contamination from exogenous sources, all tubes used in the analysis were soaked in 10% (v/v) HCl for 16 h and rinsed with doubly distilled water. This meticulous procedure ensured accurate and reliable measurements of zinc levels in the tissues.
Measurement of oxidative stress parameters
Tissue preparation
Approximately 1 g of liver and kidney tissue was homogenized in 2 mL of ice-cold TBS buffer (50 mM Tris, 150 mM NaCl, pH 7.4). The homogenates were then centrifuged at 9000 × g for 15 min at 4 °C. The resulting supernatant was collected for further analysis of various oxidative stress parameters.
Estimation of lipid peroxidation
The level of lipid peroxidation was assessed by measuring the concentration of malondialdehyde (MDA), the end product of lipid peroxidation. MDA reacts with thiobarbituric acid (TBA) to form a TBA reactive substance (TBARS), which produces a red-colored complex with a peak absorbance at 532 nm. The method used for this assessment followed the protocol developed by Buege and Aust (Buege et al. 1978).
Estimation of reduced glutathione
The concentration of reduced glutathione (GSH) was determined using the colorimetric method described by Jollow et al. (Jollow et al. 1974). This method involves the development of a yellow color upon the addition of Ellman’s reagent (5,5′-dithio-bis-2-nitrobenzoic acid) (DTNB) to compounds containing sulfhydryl groups. The GSH concentration was measured in terms of nanomole of GSH per milligram of protein (nmol GSH/mg protein) at a wavelength of 412 nm.
Assay of superoxide dismutase activity
The specific activity of superoxide dismutase (SOD) was determined following the method described by Misra and Fridovich (Misra and Fridovich 1977). Tissue homogenate was added to an EDTA-sodium carbonate buffer (0.05 M, pH 10.2) along with epinephrine (30 mM). The activity was measured at 480 nm for 4 min. SOD activity was expressed as an international unit per milligram of protein (IU/mg protein).
Assay of glutathione peroxidase activity
The activity of glutathione peroxidase (GSH-Px) was determined using the method described by Flohé and Günzler (Flohé and Günzler 1984). This method is based on the reduction of hydrogen peroxide in the presence of reduced glutathione (GSH). The absorbance was measured at 412 nm, and the enzyme activity was expressed as micromole of reduced GSH/minute/milligram of protein (µmole of reduced GSH/min/mg of protein).
Assay of glutathione reductase activity
The estimation of glutathione reductase (GR) activity was based on the method described by Goldberg and Spooner (Goldberg and Spooner 1987). The enzymatic activity was measured photometrically by assessing the consumption of NADPH. The decrease in absorbance at 340 nm indicated the activity of GR.
Assay of catalase activity
Catalase activity was measured using the UV spectrophotometric method described by Aebi (Aebi 1984). The change in absorbance at 240 nm was monitored over 1 min in the presence of high concentrations of hydrogen peroxide (≥ 30 mM). The activity of catalase was determined based on the rate of absorbance change.
Oral glucose tolerance test (OGTT)
The oral glucose tolerance tests were conducted following the Bonner-Weir method (Bonner-Weir 1988). The animals were divided into four groups, each consisting of six animals. Group 1 served as the control, while groups 2, 3, and 4 received oral doses of 100, 150, and 200 mg/kg of aqueous extracts of C. azarolus, respectively. After 14 days of treatment, the OGTT was performed on the animals while they were maintained on a standard diet. Blood samples were withdrawn from the tail vein of fasted rats prior to glucose administration (2 g/kg) and at 30, 60, 90, and 120 min after glucose loading. Blood glucose levels were measured immediately using a vital-check glucometer.
Histological assay
The pancreas was obtained through dissection and washed with isotonic saline (0.9%). It was then fixed in Bouin solution for 24 h, processed using a series of graded ethanol, and embedded in paraffin. The paraffin sections were sliced into 5 μm thickness and stained with hematoxylin–eosin. Optical microscope images were captured at a magnification of × 100.
Statistical analysis
The data were presented as mean ± SEM. Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. GraphPad Prism software (Version 8.0.1) was utilized for the statistical analysis. Statistically significant differences were considered at p < 0.05.
Results
Phytochemical screening
Table 1 presents the phytochemical analysis of the aqueous extract of C. azarolus.
Quantitative determination of total phenolic, flavonoid, and tannin contents
The quantitative analysis revealed that C. azarolus extract contains 15.53 mg GAE/g DW for total phenolic content, 23.14 mg QE/g DW for total flavonoid content, and 1.29 mg CE/g DW for total tannin content (Table 2).
Body weight gain and food intake
Diabetes significantly reduced body weight (p < 0.001) and increased food intake (p < 0.01). However, treatment with C. azarolus restored the growth rate and food consumption in zinc-deficient diabetic (DZD) rats (Table 3). These findings suggest that C. azarolus may have potential therapeutic effects in restoring body weight and food intake in diabetic rats with zinc deficiency.
Tissue zinc concentrations
Table 3 presents the zinc concentrations in the femur, liver, kidney, and pancreas of the experimental rats. The results indicate that the zinc levels in the femur and liver of diabetic (DAZ) rats were significantly lower than those of non-diabetic (ND) rats (p < 0.05 and p < 0.001, respectively). Moreover, diabetic rats fed a zinc-deficient diet exhibited significantly lower zinc concentrations in the liver, pancreas, and kidney (p < 0.01). Treatment with C. azarolus improved zinc levels in these tissues.
Blood biochemical values
The results in Table 4 demonstrate the impact of diabetes on various biochemical parameters. The diabetic group exhibited significantly higher levels of glucose (p < 0.001), cholesterol (p < 0.01), triglycerides (p < 0.001), urea (p < 0.01), uric acid (p < 0.001), aspartate aminotransferase (p < 0.05), and alanine aminotransferase (p < 0.01) compared to the non-diabetic group. Conversely, insulin (p < 0.01) and lactic dehydrogenase (p < 0.01) levels were significantly lower in the diabetic group. Additionally, zinc deficiency led to significant increases in cholesterol (p < 0.05), aspartate aminotransferase (p < 0.05), urea (p < 0.01), and creatinine (p < 0.05). However, administration of C. azarolus restored these parameters to normal levels, indicating its therapeutic potential in managing diabetes and zinc deficiency.
Oxidative stress parameters
Figures 1, 2, 3, 4, 5, and 6 demonstrate the impact of diabetes on oxidative stress parameters. Diabetes significantly increased levels of malondialdehyde (MDA) (p < 0.01; p < 0.001) and decreased glutathione (GSH) content (p < 0.05; p < 0.01), glutathione peroxidase (GSH-Px) (p < 0.001), catalase (CAT) (p < 0.01), superoxide dismutase (SOD) (p < 0.01; p < 0.001), and glutathione reductase (GR) (p < 0.05) activities in the liver and kidney. Additionally, zinc deficiency resulted in a slight increase in MDA levels in the liver and kidney, accompanied by a decrease in GSH, GSH-Px, SOD, GR, and liver catalase. However, treatment with C. azarolus extract improved these oxidative stress parameters.
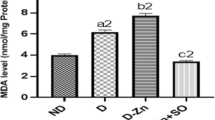
Malondialdehyde (MDA) level of non-diabetic (ND) rats, diabetic adequate-zinc (DAZ) rats, diabetic zinc-deficient (DZD) rats, and diabetic zinc-deficient rats given Crataegus azarolus (DZD+Az). Values are mean ± SEM, number of samples = 8. a1p < 0.01, a2p < 0.001: DAZ vs. ND. c2p < 0.001: DZD+Az vs. DZD
Reduced glutathione (GSH) concentration of non-diabetic (ND) rats, diabetic adequate-zinc (DAZ) rats, diabetic zinc-deficient (DZD) rats, and diabetic zinc-deficient rats given Crataegus azarolus (DZD+Az). Values are mean ± SEM, number of samples = 8. ap < 0.05, a1p < 0.01: DAZ vs. ND. c2p < 0.001: DZD+Az vs. DZD
Glutathione peroxidase (GSH-Px) activity of non-diabetic (ND) rats, diabetic adequate-zinc (DAZ) rats, diabetic zinc-deficient (DZD) rats, and diabetic zinc-deficient rats given Crataegus azarolus (DZD+Az). Values are mean ± SEM, number of samples = 8. a2p < 0.001: DAZ vs. ND. c2p < 0.001: DZD+Az vs. DZD
Superoxide dismutase (SOD) activity of non-diabetic (ND) rats, diabetic adequate-zinc (DAZ) rats, diabetic zinc-deficient (DZD) rats, and diabetic zinc-deficient rats given Crataegus azarolus (DZD+Az). Values are mean ± SEM, number of samples = 8. a1p < 0.01, a2p < 0.001: DAZ vs. ND. c2p < 0.001: DZD+Az vs. DZD
Glutathione reductase (GR) activity of non-diabetic (ND) rats, diabetic adequate-zinc (DAZ) rats, diabetic zinc-deficient (DZD) rats, and diabetic zinc-deficient rats given Crataegus azarolus (DZD+Az). Values are mean ± SEM, number of samples = 8. ap < 0.05: DAZ vs. ND. cp < 0.05, c1p < 0.01: DZD+Az vs. DZD
Oral glucose tolerance test (OGTT)
To assess the antihyperglycemic effect of C. azarolus extract, an OGTT was performed. Figure 7 shows that glucose administration led to a significant increase in blood glucose levels at 30 and 60 min in all experimental groups. However, at 90 and 120 min, the experimental groups treated with C. azarolus extract exhibited lower blood glucose concentrations compared to the control group. These results suggest that C. azarolus extract has the potential to improve glucose metabolism and may be beneficial in managing hyperglycemia. Further investigations are necessary to elucidate the underlying mechanisms of the antihyperglycemic effect of C. azarolus extract.
Pancreatic histopathologic results
The histological examination of the pancreas (Fig. 8) revealed that non-diabetic rats exhibited intact pancreatic islets. In contrast, diabetic rats with adequate-zinc (DAZ) and zinc-deficient diabetic (DZD) rats displayed depleted islet cells. Notably, treatment with the aqueous extract of C. azarolus in zinc-deficient diabetic (DZD+Az) rats preserved the pancreatic islet cells.
Effect of Crataegus azarolus on histopathological changes in the pancreas after 4 weeks of treatment: a section of pancreas tissue from non-diabetic (ND) rats showing normal architecture; b section of pancreas tissue from diabetic adequate-zinc (DAZ) rats showing reduced B-cell size (read arrowhead) and degenerative vascular changes in pancreatic islets (red arrow); c section of pancreas tissue from diabetic zinc-deficient (DZD) rats indicating more severe degenerative vascular changes in pancreatic islets (red double arrow); d section of pancreas tissue from diabetic zinc-deficient rats given Crataegus azarolus (DZD+Az) showing a preserved pancreatic islet population (black arrowhead) compared to the DZD group
Discussion
Oxidative stress plays a crucial role in the development of DM. It is responsible not only for the destruction of islet β cells and insulin signaling pathways, but it may also contribute to serious complications such as cardiovascular and nephropathy (Zhang et al. 2019). Recent studies have shown that some medicinal plants are a good source of traditional medicines, and many modern medicines are produced from these plants (Dar et al. 2019). However, the use of plants in medicine is primarily based on their biologically active compounds, which have numerous therapeutic properties, including antioxidant, antidiabetic, anti-inflammatory, and antihypercholesterolemic properties (Neeta et al. 2015; Pal et al. 2010). Therefore, several studies have been conducted to identify the therapeutic properties of plants. In this regard, the current study evaluated the potential antidiabetic and antioxidant effects of the aerial aqueous extract of C. azarolus on diabetes under nutritional zinc deficiency conditions. The results obtained from the estimation of total phenolic, flavonoid, and total tannin content indicate the richness of C. azarolus in these chemical compounds, highlighting its potent biological antioxidant properties. This is evident for aqueous extract, as there is a close relationship between phenolic and flavonoid contents and antioxidant activity. Furthermore, the IC50 value for the C. azarolus extract was close to those used in standards, which is consistent with previous studies of other species of the Crataegus genus collected from many countries (Yahyaoui et al. 2019; Alirezalu et al. 2018). The reduction of body weight was undoubtedly due to the disturbance of the metabolic state and suggests that weight loss might be due to the body’s inability to use carbohydrates as a source of energy and the high rate of catabolism of fat and protein mass. Moreover, diabetic rats fed a zinc-deficient diet had lower body weight gain and food intake than rats fed a zinc-adequate diet, which is consistent with published reports (Derouiche and Kechrid 2016; Tebboub and Kechrid 2021; Derai and Kechrid 2014). Zinc is required for the normal growth and development of animal species and humans (Hendy et al. 2001). The treatment of zinc-deficient diabetic animals with C. azarolus ameliorated body weight. The noticeable increase in body weight of animals might be due to the rise in food intake and protein synthesis. Additionally, it was documented that Crataegus azarolus has the ability to reverse gluconeogenesis and control protein loss (Shih et al. 2013). The present study revealed reduced concentrations of zinc in the different tissues of diabetic rats. It has been postulated that the low level of zinc in patients with diabetes is usually related to excessive urinary output and gastrointestinal malabsorption (Kinlaw et al. 1983). The administration of C. azarolus extract led to the restoration of zinc levels in diabetic rats with zinc deficiency. These findings suggest that zinc deficiency may contribute to the development and progression of diabetes, and C. azarolus may have a beneficial effect in restoring zinc levels in diabetic rats, which is consistent with some published investigations (Tebboub and Kechrid 2021; Beloucif et al. 2021). This was unlikely due to the antioxidant effect of this plant extract against the development of the diabetic state. It is possible that treatment with this aqueous extract positively influences oxidative stress harm through downregulating hyperglycemia and preventing kidney dysfunction, resulting in the reduction of zinc urinary losses. In the present study, animals with diabetes that were fed an inadequate-zinc diet and those under an adequate-zinc diet showed no differences in blood glucose levels when the time of feeding was strictly controlled, and the amount of food eaten by each animal before an overnight fast was known to be similar. This is despite the fact that zinc deficiency often exacerbates fasting hyperglycemia related to decreased circulating insulin. However, blood glucose levels were found to decrease in diabetic zinc-deficient rats that were treated with C. azarolus. This finding was correlated with histological studies of the pancreas, where the plant extracts preserved islet cells. The hypoglycemic effect of C. azarolus might be due to the presence of some α-glucosidase inhibitors, including the polyphenols mainly containing quercetin, the epigallocatechin gallate (EGCG), and flavonoids (Abu-Gharbieh and Shehab 2017; He et al. 2016). Phytochemical screening indicated the richness of this plant in these active constituents. These compounds can effectively reduce insulin resistance and gluconeogenesis and increase hepatic glycogen synthesis and storage (Xin et al. 2021). Oral administration of Crataegus azarolus at a dose of 150 mg/kg diet exhibited a significant antihyperglycemic effect and marked improvement in glucose tolerance (OGTT), which confirmed the effectiveness of C. azarolus extract as a hypoglycemic agent through delaying carbohydrate digestion and thereby lowering blood glucose levels (Abu-Gharbieh and Shehab 2017). Diabetes is generally associated with abnormal lipid metabolism, which is an important risk factor for diabetic vascular disease (Chen et al. 2020). The high concentration of cholesterol and triglycerides is definitely a result of lipid metabolism variations under diabetic conditions, which lead to the suppression of lipoprotein lipase activity in the account of insulin deficiency and insulin resistance (Suryawanshi et al. 2006). Moreover, variations of zinc status exhibited lipid and protein disturbance via highly significant elevation of cholesterol, triglycerides, and creatinine. In other words, zinc deficiency provoked catabolism of lipids and proteins because of the increased demand for energy (Hamdiken et al. 2018; Beloucif et al. 2021). The treatment of zinc-deficient diabetic rats with C. azarolus resulted in an improvement of these altered parameters. An explanation for the reduction in serum total cholesterol and triglyceride levels is a complex process involving multiple steps in cholesterol metabolism. Among these, total flavonoids contribute to the expression of two essential liver enzymes, hydroxy-methylglutaryl coenzyme A reductase (HMG-CoA) and cholesterol-7-alpha-hydroxylase (CYP7α), which are key enzymes for lipid synthesis. Moreover, they play a role in inhibiting cholesterol absorption by downregulating the expression and activity of intestinal acyl-CoA cholesterol acyltransferase (ACAT) (Wu et al. 2020; Kwok et al. 2013). Significant increases in serum GOT and GPT activities were observed in zinc-deficient diabetic rats compared to zinc-sufficient diabetic rats, in accordance with previously published reports (Tebboub and Kechrid 2021; Beloucif et al. 2021). Transaminases appear to be the most sensitive markers to zinc restriction, with their activities being adversely affected by a Zn-deficient diet (Yousef et al. 2002). Greeley and Sandstead (Greeley and Sandstead 1983) found evidence of decreased oxidation of the carbon chain of alanine when zinc was restricted, leading to an accumulation of alanine in the blood. A reduction in serum lactic dehydrogenase (LDH) was also observed in zinc-deficient diabetic rats, and this decrease in LDH activity was certainly due to zinc depletion. LDH is a metalloenzyme that requires zinc as a cofactor for its activity (Derouiche and Kechrid 2016). Considerable recent evidence suggests that cellular redox imbalance leads to oxidative stress and subsequent occurrence and development of diabetes complications by involving certain signaling pathways in β-cell dysfunction and insulin resistance (Zhang et al. 2020). According to the results obtained, a rise in MDA concentration was observed, confirming the detrimental impact of zinc deficiency on lipid peroxidation. Additionally, the levels of glutathione (GSH), catalase (CAT), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and glutathione reductase (GR) activities were reduced in diabetic rats with zinc deficiency. The depletion of glutathione levels may be attributed to its role in scavenging free radicals and its high consumption (Shaheen and Abd El-Fattah 1995). Moreover, the decline in antioxidant enzyme activities observed may be due to the modification of sulfhydryl groups in these enzymes by the generation of reactive oxygen species or by the auto-oxidation of glucose and non-enzymatic glycation of proteins (Hamdiken et al. 2018). However, the administration of C. azarolus resulted in the attenuation and reduction of oxidative stress and cellular damage. The findings indicated a significant decrease in the formation of malondialdehyde, an increase in GSH concentration and an improvement in CAT, GSH-Px, SOD, and GR activities in zinc-deficient diabetic animals treated with C. azarolus. In other words, it has been reported that several polyphenolic compounds isolated from C. azarolus, such as alkaloids, tannins, and flavonoids, exhibit strong antioxidant properties and play a critical role in inhibiting and scavenging free radicals (Jurikova et al. 2012). Additionally, studies have shown that the extract of C. azarolus can increase the expression of several antioxidant genes, including RP-n11, CAT, GSH-Px, SOD, and GR (Zhang et al. 2014). Through a thorough analysis of the results, it is evident that the administration of an aqueous extract of C. azarolus presents a potential therapeutic approach for managing the development and complications of diabetes associated with zinc deficiency, as demonstrated by the observed improvement in growth rate, zinc status, carbohydrate metabolism, and antioxidant system in diabetic rats. However, further clinical investigations are required to validate the safety and efficacy of this extract and its bioactive compounds in humans. The findings of this study suggest that C. azarolus holds promise as a source for developing new therapeutic strategies for diabetes management, particularly for those with zinc deficiency, opening new avenues for further research in this field.
Data availability
All data underlying the reported findings are available in a publicly accessible repository. The data presented in this study are openly available in Research Square at https://doi.org/10.21203/rs.3.rs-2262461/v1.
References
Abdulmalek S, Eldala A, Awad D, Balbaa M (2021) Ameliorative effect of curcumin and zinc oxide nanoparticles on multiple mechanisms in obese rats with induced type 2 diabetes. Sci Rep 11:20677. https://doi.org/10.1038/s41598-021-00108-w
Abu-Gharbieh E, Shehab NG (2017) Therapeutic potentials of Crataegus azarolus var. eu-azarolus Maire leaves and its isolated compounds. BMC Complement Altern Med 17:218. https://doi.org/10.1186/s12906-017-1729-9
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Aierken A, Buchholz T, Chen C, Zhang X, Melzig MF (2017) Hypoglycemic effect of hawthorn in type II diabetes mellitus rat model. J Sci Food Agric 97(13):4557–4561. https://doi.org/10.1002/jsfa.8323
Alirezalu A, Salehi P, Ahmadi N, Sonboli A, Aceto S, Hatami Maleki H, Ayyari M (2018) Flavonoids profile and antioxidant activity in flowers and leaves of hawthorn species (Crataegus spp.) from different regions of Iran. Int J Food Prop 21:452–470. https://doi.org/10.1080/10942912.2018.1446146
Beloucif A, Kechrid Z, Bekada AM (2021) Effect of zinc deficiency on blood glucose, lipid profile, and antioxidant status in streptozotocin diabetic rats and the potential role of sesame oil. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02934-5
Bonner-Weir S (1988) Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes 37:616–621. https://doi.org/10.2337/diab.37.5.616
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Fleischer S, Packer L (eds) Methods in enzymology. Academic Press, pp 302–310
Chen Z, Hu H, Chen M, Luo X, Yao W, Liang Q, Yang F, Wang X (2020) Association of triglyceride to high-density lipoprotein cholesterol ratio and incidence of diabetes mellitus: a secondary retrospective analysis based on a Chinese cohort study. Lipids Health Dis 19:33. https://doi.org/10.1186/s12944-020-01213-x
Choi S, Liu X, Pan Z (2018) Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin 39:1120–1132. https://doi.org/10.1038/aps.2018.25
Daniele C, Mazzanti G, Pittler MH, Ernst E (2006) Adverse-event profile of Crataegus spp.: a systematic review. Drug Saf 29(6):523–535. https://doi.org/10.2165/00002018-200629060-00005
Dar R, Shahnawaz M, Qazi P (2019) General overview of medicinal plants: a review. Phytomorphology 69:349–351. https://doi.org/10.31254/phyto.2017.6608
Derai EH, Kechrid Z (2014) Combined effect of vitamins C and E on zinc status, carbohydrate metabolism and antioxidant values in diabetic rats fed zinc-deficient diet. J Metab Nutr 7:55–65. https://doi.org/10.3233/MNM-140005
Derouiche S, Kechrid Z (2016) Zinc supplementation overcomes effects of copper on zinc status, carbohydrate metabolism and some enzyme activities in diabetic and nondiabetic rats. Can J Diabetes 40:342–347. https://doi.org/10.1016/j.jcjd.2016.02.005
El Hendy HA, Yousef MI, Abo El-Naga NI (2001) Effect of dietary zinc deficiency on hematological and biochemical parameters and concentrations of zinc, copper, and iron in growing rats. Toxicology 167:163–170. https://doi.org/10.1016/S0300-483X(01)00373-0
Evans WC, Evans D (2009) General methods associated with the phytochemical investigation of herbal products. In: Evans WC, Evans D (eds) Trease and Evans’ pharmacognosy, 16th ed. W.B. Saunders, pp 135–147
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121. https://doi.org/10.1016/S0076-6879(84)05015-1
Gholamhoseinian A, Shahouzehi B, Mohammadi G (2020) Trace elements content of some traditional plants used for the treatment of diabetes mellitus. Biointerface Res Appl Chem 10(6):6167–6173. https://doi.org/10.33263/BRIAC105.61676173
Goldberg D, Spooner R (1987) Methods of enzymatic analysis. Bergmayer, VCH, New York
Greeley S, Sandstead HH (1983) Oxidation of alanine and β-hydroxybutyrate in late gestation by zinc-restricted rats. J Nutr 113:1803–1810. https://doi.org/10.1093/jn/113.9.1803
Hamdiken M, Bouhalit S, Kechrid Z (2018) Effect of Ruta chalepensis on zinc, lipid profile and antioxidant levels in the blood and tissue of streptozotocin-induced diabetes in rats fed zinc-deficient diets. Can J Diabetes 42:356–364. https://doi.org/10.1016/j.jcjd.2017.08.239
Hao W, Zhang Z, Guo Y, Sun P, Lv X, Zuo Y (2011) Hawthorn fruit increases the antioxidant capacity and reduces lipid peroxidation in senescence-accelerated mice. Eur Food Res Technol 232(4):743–751. https://doi.org/10.1007/s00217-011-1435-7
He K, Song S, Zou Z, Feng M, Wang D, Wang Y, Li X, Ye X (2016) The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother Res 30:283–291. https://doi.org/10.1002/ptr.5529
Jiménez PG, Martín-Carmona J, Hernández EL (2020) Diabetes mellitus. Medicine - Programa De Formación Médica Continuada Acreditado 13:883–890. https://doi.org/10.1016/j.med.2020.09.010
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169. https://doi.org/10.1159/000136485
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217. https://doi.org/10.1021/jf00062a013
Jurikova T, Sochor J, Rop O, Mlcek J, Balla S, Szekeres L, Adam V, Kizek R (2012) Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules 17:14490–14509. https://doi.org/10.3390/molecules171214490
Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ (1983) Abnormal zinc metabolism in type II diabetes mellitus. Am J Med 75:273–277. https://doi.org/10.1016/0002-9343(83)91205-6
Kwok CY, Li C, Cheng HL, Ng YF, Chan TY, Kwan YW, Leung GPH, Lee SMY, Mok DKW, Yu PHF, Chan SW (2013) Cholesterol lowering and vascular protective effects of ethanolic extract of dried fruit of Crataegus pinnatifida, hawthorn (Shan Zha), in diet-induced hypercholesterolaemic rat model. J Funct Foods 5:1326–1335. https://doi.org/10.1016/j.jff.2013.04.020
Li YV (2014) Zinc and insulin in pancreatic beta-cells. Endocrine 45:178–189. https://doi.org/10.1007/s12020-013-0032-x
Misra HP, Fridovich I (1977) Superoxide dismutase: “positive” spectrophotometric assays. Anal Biochem 79:553–560. https://doi.org/10.1016/0003-2697(77)90429-8
Neeta M, Mukta N, Bilwa K (2015) Comparative qualitative phytochemical analysis of Sesamum indicum L. Int J Curr Microbiol App Sci 4(3):172–181. https://doi.org/10.20546/ijcmas.2015.403.020
Ojuade FI, Olorundare OE, Akanbi OB, Afolabi SO, Njan AA (2021) Antidiabetic and antihyperlipidemic effects of aqueous extract of Parquetina nigrescens in streptozotocin–nicotinamide induced type 2 diabetic rats. Heliyon 7:e07363. https://doi.org/10.1016/j.heliyon.2021.e07363
Pal A, Khanum F, Bawa AS (2010) Nutritional, medicinal and industrial uses of sesame (Sesamum indicum L.) seeds - an overview. Agric Conspec Sci 75(4):159–168
Rahmati M, Keshvari M, Mirnasouri R, Chehelcheraghi F (2021) Exercise and Urtica dioica extract ameliorate hippocampal insulin signaling, oxidative stress, neuroinflammation, and cognitive function in STZ-induced diabetic rats. Biomed Pharmacother 139:111577. https://doi.org/10.1016/j.biopha.2021.111577
Shaheen AA, Abd El-Fattah AA (1995) Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int J Biochem Cell Biol 27:89–95. https://doi.org/10.1016/1357-2725(94)00053-0
Shih CC, Lin CH, Lin YJ, Wu JB (2013) Validation of the antidiabetic and hypolipidemic effects of hawthorn by assessment of gluconeogenesis and lipogenesis related genes and AMP-activated protein kinase phosphorylation. Evid Based Complementary Altern Med 2013:597067. https://doi.org/10.1155/2013/597067
Southon S, Kechrid Z, Wright AJ, Fairweather-Tait SJ (1988) Effect of reduced dietary zinc intake on carbohydrate and Zn metabolism in the genetically diabetic mouse (C57BL/KsJ db+/db+). Br J Nutr 60:499–507. https://doi.org/10.1079/BJN19880122
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Suryawanshi NP, Bhutey AK, Nagdeote AN, Jadhav AA, Manoorkar GS (2006) Study of lipid peroxide and lipid profile in diabetes mellitus. Indian J Clin Biochem 21:126. https://doi.org/10.1007/BF02913080
Tebboub I, Kechrid Z (2021) Effect of curcuma on zinc, lipid profile and antioxidants levels in blood and tissue of streptozotocin-induced diabetic rats fed zinc deficiency diet. Arch Physiol Biochem 127:162–169. https://doi.org/10.1080/13813455.2019.1623820
Vélez-Alavez M, Labrada-Martagón V, Méndez-Rodriguez LC, Galván-Magaña F, Zenteno-Savín T (2013) Oxidative stress indicators and trace element concentrations in tissues of mako shark (Isurus oxyrinchus). Comp Biochem Physiol a: Mol Integr Physiol 165:508–514. https://doi.org/10.1016/j.cbpa.2013.03.006
Wang SZ, Wu M, Chen KJ, Liu Y, Sun J, Sun Z, Ma H, Liu LT (2019) Hawthorn extract alleviates atherosclerosis through regulating inflammation and apoptosis related factors: an experimental study. Chin J Integr Med 25(2):108–115. https://doi.org/10.1007/s11655-018-3020-4
Xin C, Zhao M, Wang J, Wang Z (2021) Hawthorn polyphenols, D-chiro-inositol, and epigallocatechin gallate exert a synergistic hypoglycemic effect. J Food Biochem 45:e13771. https://doi.org/10.1111/jfbc.13771
Yahyaoui A, Arfaoui MO, Rigane G, Hkir A, Amari K, Ben Salem R, Ammari Y (2019) Investigation on the chemical composition and antioxidant capacity of extracts from Crataegus azarolus L.: effect of growing location of an important Tunisian medicinal plant. Chem Afr 2(2):361–365. https://doi.org/10.1007/s42250-019-00054-1
Yang B, Liu P (2012) Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J Sci Food Agric 92(8):1578–1590. https://doi.org/10.1002/jsfa.5671
Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175:223–234. https://doi.org/10.1016/s0300-483x(02)00049-5
Wong SP, Leong LP, Koh WH (2006) Antioxidant activities of aqueous extracts of selected plants. Food Chem 99(4):775–783. https://doi.org/10.1016/j.foodchem.2005.07.058
Wu M, Liu L, Xing Y, Yang S, Li H, Cao Y (2020) Roles and mechanisms of hawthorn and its extracts on atherosclerosis: a review. Front Pharmacol 11:118. https://doi.org/10.3389/fphar.2020.00118
Zhang B, Yue R, Chen Y, Yang M, Huang X, Shui J, Peng Y, Chin J (2019) Gut microbiota, a potential new target for Chinese herbal medicines in treating diabetes mellitus. Evid Based Complementary Altern Med 2019:2634898. https://doi.org/10.1155/2019/2634898
Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y (2020) Oxidative stress and diabetes: antioxidative strategies. Front Med 14:583–600. https://doi.org/10.1007/s11684-019-0729-1
Zhang Y, Ti S, Liu S, Zhao J, Chen W, Wang H (2014) Effect of hawthorn on Drosophila melanogaster antioxidant-related gene expression. Trop J Pharm Res 13(3):353. https://doi.org/10.4314/tjpr.v13i3.6
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
The authors express their gratitude to the Algiers Pasteur Institute for providing the rats used in this study and to ONAB EL-Harouch in Skikda, Algeria, for their assistance with diet component supplementation. Their contributions were essential to the successful completion of this research project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by a grant from the Ministry of Higher Education and Scientific Research, Algeria (Grant Number: D01N01UN230120190003).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were approved by the Ethical Committee of Annaba University, Algeria (PNR-ANDRS 8/u23/332). The Guide for the Care and Use of Laboratory Animals was followed.
Informed consent
All the authors read the manuscript and approved for submission.
Consent for publication
For this type of study, consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Triki, R., Boughediri, K., Chelaghmia, M.L. et al. The therapeutic potential of “Crataegus azarolus” on zinc, lipid profile, and antioxidant status in streptozotocin-induced diabetic rats with zinc-deficient diet. Comp Clin Pathol 33, 7–19 (2024). https://doi.org/10.1007/s00580-023-03517-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03517-7