Abstract
Postmortem changes (PMCs) not only affect the results of experimental studies, but also determine postmortem intervals in forensic sciences. The present study aimed to assess the effects of two methods of laboratory animals’ euthanasia on PMCs in rats. In this experimental study, 10 female rats were randomly assigned to two equal groups and were euthanized using the inhalation of CO2 (gas-treated group) or over-dose intramuscular injection of ketamine/xylazine (drug-treated group). Kidney and liver tissue samples were collected at baseline and 2, 4, 6, 8, 10, 12, 24, and 48 h after euthanasia and were subjected to histopathological examinations. The expression of liver-specific microRNA-122 (miR-122) was also assessed in each time point via a SYBR green real-time polymerase chain reaction (PCR) assay. Finally, miR-122 target genes and related functional pathways were identified through bioinformatics analysis. The progression of PMCs in the drug-treated group was faster than the gas-treated group. The expression of miR-122 was significantly (P < 0.0001) upregulated in the drug-treated group at 4, 10, and 24 h in comparison to the gas-treated group; however, it was downregulated at 6, 8, and 48 h after euthanasia. The biosynthesis of amino acids, glycolysis/gluconeogenesis, and carbon metabolism as well as the glucagon, Hedgehog, cGMP-PKG, and neurotrophin signaling pathways were identified as the significant pathways related to miR-122 target genes. The findings indicated that methods of euthanasia for laboratory animals could cause changes at microscopic and molecular levels. Therefore, researchers should consider this issue in the design phase of their studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After death, a range of alterations known as postmortem changes (PMCs) naturally occur in the corpse. PMCs are defined as various pathophysiological, molecular, and biochemical changes that occur in dead body tissues and progress along a timeline. Various external and internal factors such as age, sex, physiological and pathological status, temperature, humidity, microorganisms, and activity of insects can speed up or slow down the decomposition process. Understanding the common PMCs and determining the variables that affect this process allow researchers to more accurately identify the postmortem intervals (PMIs) and provide a framework for estimating the time of occurrence of death (Li et al. 2003; Karadzic et al. 2010; Sampaio-Silva et al. 2013; Lv et al. 2014). Traditional methods for estimation of PMIs are based on some physiological changes such as algor mortis, livor mortis, and rigor mortis. Although these methods often provide an estimation of the PMIs, one of the symptoms sometimes contradicts with others; therefore, they are not completely reliable (Sampaio-Silva et al. 2013; Melotti et al. 2014).
Since PMIs are vitally important in criminal and legal cases, determining biological markers with stable expressions may be helpful (Zhang et al. 2013; Lv et al. 2014). Degradation of molecular markers such as proteins, RNAs, and DNAs provides a more accurate estimate of PMIs (Gomaa et al. 2013; Lv et al. 2014; Melotti et al. 2014; Ebuehi et al. 2015). Although RNAs seem to degrade rapidly and are not good indicators for PMCs, recent studies have suggested that RNA degradation in tissues is based on certain rules, and these molecules are relatively stable after death (Lv et al. 2017). Besides, microRNAs (miRs) as a group of short 18–24 nucleotides remain extremely stable after death (Fordyce et al. 2013). The short sequence of miRs makes them more resistant to extreme heat and pH changes in comparison to other types of RNAs such as mRNAs. Additionally, their critical roles in biological processes and response to various cellular stresses have made miRs good diagnostic biomarkers for therapeutic purposes (Kakimoto et al. 2015). The most abundant miR in the liver tissue that plays an important role in the development of liver and metabolism is miR-122, which has been estimated to account for more than 72% of all liver miRs and is expressed in constant levels. Since miR-122 largely accumulates in liver, an increase in its blood level can be present as a consequence of hepatocyte damages (Bandiera et al. 2015; Roderburg et al. 2015). Therefore, the quantitative measurement of such miRs after death can be useful for the study of PMCs (Lv et al. 2017).
Despite many research on human bodies in forensic science, the accuracy in estimation of the time of death still needs further improvement. Multiple circumstantial and environmental factors, including cause of death, temperature, moisture, disposition of the body, insect activity, scavenger activity, trauma, etc., may affect the decomposition rate. Due to a variety of anatomical and physiological similarities to humans, animals are considered valuable research tools in translational research. Therefore, the determination of PMCs and estimating PMIs in laboratory animals might be helpful to study what happens in the human body (Prabhakar 2012; Brooks 2016). The frequency and trends of the use of animals in forensic science research demonstrated that 4% of original research within the main forensic science journals utilizes animals or animal tissue (Mole and Heyns 2019). To investigate the PMCs in laboratory animals, they must be euthanized in a timely manner to prevent/alleviate animal suffering. Euthanasia, the act of inducing painless death, is a critical event in a laboratory animal’s life, which can impact the research results. Assessing the effects of euthanasia in animal models allows forensic pathologists to identify the molecular and pathological patterns that occur during death (Li et al. 2003; Sampaio-Silva et al. 2013; Ibrahim et al. 2019; Brockbals et al. 2021; Welson et al. 2021). Hence, the present study aims to assess the effects of euthanasia via exposure to a rising concentration of CO2 or a high-dose intramuscular injection of ketamine and xylazine cocktail on PMCs in kidney and liver tissues as well as the expression of liver-specific miR-122 at different time points after death.
Materials and methods
Animals
All animal experiments were approved by the local Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Approval ID: IR.SUMS.REC.1395.S1241). In this study, 10 adult female Sprague Dawley rats (Center of Comparative and Experimental Medicine, Shiraz University of Medical Sciences, Shiraz, Iran) aging 8–10 weeks and weighing 250–300 g were randomly divided into two groups (n = 5). Group 1 was euthanized via exposure to a rising concentration of CO2 (gas-treated group), and group 2 was euthanized via high-dose intramuscular injection of ketamine (300 mg/kg, Alfasan, Woerden, Holland) and xylazine (30 mg/kg, Alfasan, Woerden, Holland) cocktail (drug-treated group).
The liver and kidney tissues were harvested from dead animals and held at 25 ºC. To evaluate PMCs, specific parts of the liver and kidney tissues were collected at baseline and 2, 4, 6, 8, 10, 12, 24, and 48 h after animal euthanizing. Portions of the left lateral liver lobes and sagittal sections of the kidney were fixed in 10% buffered formaldehyde and subjected to histopathological evaluations. Other liver slice was also used for molecular analysis at each time point. The workflow of the methods used in this study has been depicted in Fig. 1.
Histopathological evaluations
To evaluate the histopathological alterations, fixed tissue samples were dehydrated by the series of alcohols and were embedded in paraffin using a tissue processor machine (DID SABZ Co., Iran). The specimen slices were cut at a thickness of 5 µm by a microtome (DID SABZ Co., Iran) and were placed on clean glasses. Then, the prepared slides were stained with hematoxylin and eosin (Merck, Germany). Finally, the tissue slides were assessed under a light microscope (Nikon Eclipse Ni, Tokyo, Japan) by a pathologist who was blinded to the study.
MiRNAs and primer design
A literature review was done to select the appropriate miRNAs that were exclusively expressed in the liver. MiR-122 can be detected in very low levels in different tissues although it is exclusively expressed in the liver (Sharapova et al. 2016). Primers used for real-time polymerase chain reaction (PCR) assays were designed using the Oligo 7 software (Molecular Biology Insights, Inc., Cascade, CO, USA) and were validated by NCBI BLAST. The information related to the primers has been presented in Table 1.
Total RNA extraction
In this study, total RNA was merely extracted from the liver tissues. In brief, 1 mg of the liver sample was homogenized and mixed with 1 mL of cold QIAzol lysis reagent (Cat. No. 79306, Qiagen, USA). The mixture was vortexed vigorously for 1 min and was incubated at room temperature for 5 min. Next, 0.2 mL of chloroform (CAS 67–66-3, Merck, Germany) was added and mixed by inverting for 1 min. The mixture was then centrifuged at 14,000 RPM at 4 °C for 15 min, and the supernatant was carefully removed and transferred into a new RNase, DNase, and pyrogen free micro-tube. After that, total RNA was directly precipitated via 1 mL of cold absolute ethanol (CAS 64–17-5, Merck, Germany) and 2 μL of glycogen (20 mg/mL, cat. no. G024, ABM Inc., Canada). After the RNA pellet was rinsed with 75% ethanol and centrifuged, it was air-dried and solved in 100 μL of diethylpyrocarbonate (DEPC)-treated water (cat. no. CH8141, Cinnagen, Iran). The extracted RNA samples were stored at −70 °C until further processing.
cDNA synthesis and real-time polymerase chain reaction assays
Reverse transcription was performed using the gene-specific primers and the RevertAid first-strand cDNA synthesis kit (Cat. No. K1622, Thermo Scientific, USA). After synthetization of cDNAs, they were diluted at 1:5 ratio in nuclease-free water (cat. no. DW8520C, Cinnagen, Iran) and was used as a template in a subsequent SYBR green real-time PCR assay. Real-time quantification of miR-122 was carried out according to a method described previously (Derakhshanfar et al. 2020). The expression of miR-122 was normalized to the expression of miR-16, miR 221, Let-7a, and U6 snRNA, which are most commonly used as reference genes for the quantification of miRs (Lardizábal et al. 2012, Mahdipour et al. 2015, Schlosser et al. 2015, Sharapova et al. 2016, Moayedi et al. 2020). Finally, the differences in the expression of miR-122 between the study groups were determined using the relative expression software tool (REST 2009, QIAGEN, USA) (Pfaffl et al. 2002) and 2−ΔΔCT formula (Livak and Schmittgen 2001).
Bioinformatics analysis
The TargetScan software (http://www.targetscan.org/) was used for identifying the miR-122 target genes. The functional pathways of the target genes were also identified using the Database of Annotation, Visualization, and Integrated Discovery (DAVID) Tool (https://david.ncifcrf.gov/). DAVID is a tool that rapidly annotates lists of genes or proteins according to shared categorical data for functional pathways, gene ontology, and protein domain (ref: DAVID: Database for Annotation, Visualization, and Integrated Discovery).
Statistical analysis
The Relative Expression Software Tool (REST 2009, Qiagen, USA) was used to evaluate the differences in the expression levels of miR-122 in the study groups. All statistical analyses were performed using Graph Pad Prism 8.0 and SPSS 17.0 software (IMB, Chicago, IL, USA), and P-values less than 0.05 were considered statistically significant.
Results
Histopathological findings in the liver
In samples collected immediately after euthanasia (baseline), the liver tissue in both experimental groups had a normal microscopic structure. The differences were observed at the second hour post euthanasia where the liver tissue in the gas-treated group still had a normal structure, but a series of PMCs including the dilation of central veins and unclear nuclei were seen in the drug-treated group. At the fourth hour post euthanasia, PMCs such as granularity of cytoplasm, degeneration of hepatocytes, dilation of central vein, and dilation of sinusoidal space were observed in the liver of the gas-treated group. At this time, the drug-treated group also showed granularity of cytoplasm, degeneration of hepatocytes, and tissue separation in addition to PMCs observed at the second hour. In the liver tissue samples from the 6 h, tissue separation was also seen in the gas-treated group; however, the liver tissues in the drug-treated group experienced impairment and decomposition.
The marked differences were observed in the samples taken 8 h post euthanasia. Although the liver tissues in the drug-treated group were completely destroyed at this time, tissue separation and impairment and decomposition were observed in the gas-treated group, which was more developed until 24 h post euthanasia. Due to the progression of PMCs, the liver tissue samples obtained at 24 and 48 h post euthanasia were completely destroyed in the gas-treated group. The histopathological findings of the liver tissues have been depicted in Table 2 and Fig. 2.
Histopathological findings in the kidney
Histopathological evaluations of the kidney tissues collected immediately after euthanasia (baseline) showed a normal microscopic structure in both groups. At the second hour post euthanasia, PMCs including atrophy of the glomeruli, detachment of the tubular epithelium, and flattened tubular epithelium were observed in the gas-treated group; however, the drug-treated group presented atrophy of the glomeruli, dilation of tubules, and degeneration of the tubular epithelium. At the fourth hour post euthanasia, PMCs in the gas-treated group were similar to that observed at the previous time point, but uneven capsules and tissue separation were appeared in the kidney of the drug-treated group in addition to PMCs observed at the second hour. In addition to these PMCs, tissue separation and impairment and decomposition were also observed at 6 h post euthanasia in the gas- and drug-treated groups, respectively.
The marked differences were observed at 8 h post euthanasia. Although the kidney tissues in the drug-treated group were completely destroyed at this time, dilation of capillaries and flattened tubular epithelium was observed in the gas-treated group. PMCs were more developed in the gas-treated group until 48 h post euthanasia that the kidney tissues were completely destroyed. The histopathological findings of the kidney tissues have been depicted in Table 3 and Fig. 3.
Quantitative measurement of the expressions of miR-122
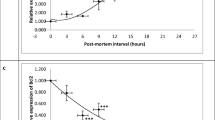
The data from molecular investigations revealed that the expression of miR-122 was significantly (P < 0.0001) upregulated in the drug-treated group at 4, 10, and 24 h, but was downregulated at 6, 8, and 48 h post euthanasia in comparison to the gas-treated group (Fig. 4).
The pattern of miR-122 expression in the liver of rats that were euthanized using the inhalation of CO2 (gas-treated group) or over-dose intramuscular injection of ketamine/xylazine (drug-treated group). Asterisk represents a highly significant difference (P < 0.0001) in the expression of miR-122 between the study groups
Bioinformatics analysis
The results of TargetScan showed that miR-122 targeted 216 genes. The functional pathways analysis of the target genes indicated that the biosynthesis of amino acids, glucagon signaling pathway, Hedgehog signaling pathway, cGMP-PKG signaling pathway, glycolysis/gluconeogenesis, carbon metabolism, and neurotrophin signaling pathway were the significant pathways in the DAVID database (Table 4).
Discussion
Various euthanasia methods are currently used to sacrifice animals. CO2 inhalation and ketamine/xylasine cocktail are the most common methods for euthanizing laboratory rodents (Shomer et al. 2020). The findings of the present study revealed that CO2 inhalation and ketamine/xylazine cocktail injection could change the histological patterns of the liver and kidney tissues as well as the hepatic expression of mir-122. Previous studies have shown that the inhalation of CO2 causes some tissue damages including weight gain and vacuolation (Li et al. 2003). Besides, the injection of ketamine and xylazine cocktail is not without harms (Lei et al. 2001). Other studies also demonstrated that different methods of euthanasia may have several effects on the cells and tissues of laboratory animals (Clarke et al. 2017; Shomer et al. 2020). Al-Mousawi et al. (Al-Mousawi et al. 2010) noted that differential expression of inflammatory markers with the use of anesthetics and analgesics should be considered when designing animal studies and interpreting the results, as these appear to have significant modulating impacts. Moreover, Ko et al. (Ko et al. 2019) reported the impacts of different anesthesia/euthanasia methods on the mitogen-activated protein kinase signaling pathway of adult male and female mice’s brains.
Histopathological evaluations in the current study showed that both euthanasia methods did not alter the microscopic structures of liver and kidney at early hours post euthanasia. However, some studies have indicated that some euthanizing agents can alter the histological structures of body tissues. For instance, (Mohamed et al. 2020) reported that pentobarbital overdose during euthanasia caused congestion in interstitial blood vessels, glomerular tuft capillaries, vacuolation in renal tubular epithelium, and degeneration of renal tubules. Additionally, congested central veins and scattered vacuolated hepatocytes were detected in the animals’ livers. In another study, loss of tinctoral quality and mild to moderate edema in the lamina propria of the intestine were observed following the intraperitoneal injection of ethanol and pentobarbital–phenytoin in mice and rats (Allen-Worthington et al. 2015). Grieves et al. also indicated that euthanasia of non-human primates with barbiturate agents like pentobarbital sodium and phenytoin sodium induced histopathological tissue changes in lungs including vascular damage, hemolysis, edema, and necrosis (Grieves et al. 2008). Therefore, injection of ketamine/xylazine cocktail and inhalation of CO2 have been mentioned as better euthanizing methods compared to ethanol and barbiturates such as pentobarbital sodium in terms of histological alterations. However, ethical concerns should be considered regarding CO2 inhalation (Shomer et al. 2020). On the other hands, euthanizing animals using drugs results in an increase in PMCs in tissues, which should be considered by the researchers who study in the field of forensic medicine. To ascertain the optimal euthanasia method for specific studies, counseling a laboratory animal veterinarian and/or performing a pilot study might be helpful (Shomer et al. 2020).
Since miR-122 is an important biomarker in the liver and has been recently used as an indicator of hepatic damages (Sharapova et al. 2016) as well as PMI estimation (Tu et al. 2019), the impact of euthanasia on the hepatic expression of miR-122 was evaluated via the intramuscular injection of ketamine/xylazine cocktail and the inhalation of CO2 in the present study. The findings showed that different methods of euthanasia altered the expression of miR-122. In the same line, (Clarke et al. 2017) indicated that different culling methods used in pre-clinical studies affected the miR-122 levels. They revealed a significant increase in the serum levels of miR-122 in the mice culled with CO2 and to a much greater extent in the mice culled using Pentoject compared to the pre-cull levels. In the latter case, the increase in circulating miR-122 in the control mice influenced the statistical differentiation of the degree of liver injury following exposure to acetaminophen. Up to now, only a few studies have been done on the effects of euthanasia methods on miRs expression. Staib-Lasarzik et al. (2014) did not observe any significant differences between non-anesthetic and anesthetic euthanasia methods regarding the expression of miR-497 in healthy mice and after traumatic brain injury. Hence, further studies are recommended to be conducted on the impacts of euthanasia methods on miRs expression.
The functional pathways analysis of the miR-122 target genes identified several signaling pathways such as biosynthesis of amino acids, glucagon signaling pathway, Hedgehog signaling pathway, cGMP-PKG signaling pathway, glycolysis/gluconeogenesis, carbon metabolism, and neurotrophin signaling pathway as significant pathways. The expression of miR-122 showed a significant increase in the drug-treated group at 4, 10, and 24 h. Therefore, the miR-122 target genes were downregulated in this group. Generally, cell survival and growth need pathways that produce energy, substrates, and precursors for macromolecular synthesis. The dysregulation of genes can disrupt functional pathways. The biosynthesis of amino acids is a pathway that is regulated by miR-122 target genes. Liver is a key organ for protein synthesis, degradation, detoxification, and amino acid metabolism (Dejong et al. 2007). Amino acids are an important component of protein synthesis in cellular metabolism; plays a key role as the intermediated metabolite in the biosynthesis of nucleotides, glucosamine, lipids, glutathione, and polyamines; and regulates cell proliferation and tricarboxylic acid circulating carbon (Tsun and Possemato 2015; Lukey et al. 2017). Therefore, dysfunction of this pathway disrupts cell growth and survival. For example, the ammonia is produced from amino acids, nucleotide, and tissue degradation during the decomposition of a corpse. Since ammonia is not removed by the liver of corpse, it may accumulate over time and rapidly increased in plasma; hence, assessing the concentration of ammonia in plasma can be used as a marker of PMI determination (Donaldson and Lamont 2013).
Glucagon signaling, Hedgehog signaling, and cGMP-PKG signaling pathways are other pathways related to miR-122 target genes. The activity of glucagon is regulated by a G protein-couple receptor in tissues such as liver, intestine, brain, smooth muscles, kidneys, heart, adipose tissue, pancreatic β-cells, and placenta (Charron and Vuguin 2015). Sinclair et al. (2008) in 2008 reported that glucagon receptor (Gcgr) − / − mice enhanced susceptibility to liver injury. Besides, the increase of exogenous glucagon decreased the caspase activation in primary murine hepatocyte cultures through the regulation of cAMP-dependent pathways (Sinclair et al. 2008). A previous study also showed that the expression of glucagon-like peptide 1 receptor in brain was changed after death (Mansur et al. 2018).
Hedgehog signaling pathway also has an essential role in intricate regulation, tissue regeneration, wound healing, and homeostasis maintenance. The activation of this pathway also increases liver injuries. The expression of Hh ligands is stimulated in injured livers by several factors including epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and TGF-β, which induce regeneration in the liver (Omenetti et al. 2007; Jung et al. 2008; Machado and Diehl 2018). The cGMP-PKG signaling pathways are involved in such processes as the regulation of relaxation and contraction of vascular smooth muscle cells, anti-atherosclerosis, anti-cardiac hypertrophy, and anti-vascular injury/restenosis (Francis et al. 2010). The activity of cGMP-dependent protein kinase (PKG) leads to the activation of vacuolar H+-ATPase’s and extrusion of H+ from cytosol hepatocytes into the extracellular environment. The extrusion of H+ from the cytosol inhibits H+-driven Na+/H+ exchanger and Na+/HCO3− cotransporter, which reduces Na+ and apoptosis in hepatocyte cells (Abu-Amara et al. 2012). Therefore, decreased activity of these pathways can cause necrosis and apoptosis of the liver tissue. The activity of cGMP-PKG signaling pathways and the expression of genes involved in this pathway can also change in the early post mortem (Ding et al. 2022).
Glucose homeostasis regulates the energy and metabolism of vital organs. Glucose homeostasis is controlled by glycogenesis, glycogenolysis, glycolysis, and gluconeogenesis in the liver (Han et al. 2016). On the other hand, one-carbon metabolism regulates physiological processes including amino acid homeostasis, biosynthesis of purines and thymidine, redox defense, and epigenetic maintenance (Ducker and Rabinowitz 2017). The alterations in glycogenolytic and glycolytic enzymes can regulate early post-mortem metabolism (Bucław et al. 2021). Thus, dysregulation of these metabolic pathways can cause dysfunctional cells, eventually leading to cell death.
Neurotrophin signaling pathway has a key role in the survival and differentiation of neurons. Neurotrophins such as the nerve growth factor (NGF) regulate lipid metabolism through activating the sterol regulatory element-binding protein-2 (SREBP2) in the liver. Pham et al. (2016) reported that pro-NGF could stimulate p75NTR, which led to an increase in low density lipoprotein receptor (LDLR) and increased the activity of nuclear factor-B (NF-B) signaling pathway, which increased the viability of hepatocytes. Dysregulation of this pathway can be one of the effective factors in necrosis of the liver tissue. A decrease in the expression and activity of neurotrophins was also reported in the brains of suicide subjects (Dwivedi et al. 2009).
The present study results revealed the effects of two methods of euthanasia on molecular and pathology patterns of the kidney and liver tissues. However, this study had several limitations in the budget and design. Hence, the effects of other euthanasia methods are recommended to be investigated in future in vitro and in vivo studies. Overall, researchers should be aware of the possible pathological and molecular effects of euthanasia on laboratory animals and should consider this issue in designing their studies.
Conclusions
Based on the findings of the present study, euthanasia methods have significant effects on tissues at both microscopic and molecular levels. Consulting a laboratory animal veterinary specialist and/or conducting a pilot study are highly recommended to choose the optimal euthanasia method for laboratory animals. Besides, more experimental studies are warranted to find the possible effects of euthanasia on the histopathological changes of tissues, miRs expression patterns, and functional pathways involved in cells survival.
References
Abu-Amara M, Yang SY, Seifalian A, Davidson B, Fuller B (2012) The nitric oxide pathway–evidence and mechanisms for protection against liver ischaemia reperfusion injury. Liver Int 32(4):531–543
Al-Mousawi AM, Kulp GA, Branski LK, Kraft R, Mecott GA, Williams FN, Herndon DN, Jeschke MG (2010) Impact of anesthesia, analgesia and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury. Shock 34(3):261–268
Allen-Worthington KH, Brice AK, Marx JO, Hankenson FC (2015) Intraperitoneal injection of ethanol for the euthanasia of laboratory mice (Mus musculus) and rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 54(6):769–778
Bandiera S, Pfeffer S, Baumert TF, Zeisel MB (2015) miR-122–a key factor and therapeutic target in liver disease. J Hepatol 62(2):448–457
Brockbals L, Wartmann Y, Mantinieks D, Glowacki LL, Gerostamoulos D, Kraemer T, Steuer AE (2021) Postmortem metabolomics: strategies to assess time-dependent postmortem changes of diazepam, nordiazepam, morphine, codeine, mirtazapine and citalopram. Metabolites 11(9):643–659
Brooks J (2016) Postmortem changes in animal carcasses and estimation of the postmortem interval. Vet Pathol 53(5):929–940
Bucław M, Lepczyński A, Herosimczyk A, Ożgo M, Szczerbińska D, Majewska D, Liput K, Pierzchała M (2021) Post mortem changes in M. iliotibialis lateralis muscle protein profile of emu (Dromaius novaehollandiae). Meat Sci 180:108562
Charron MJ, Vuguin PM (2015) Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. J Endocrinol 224(3):123–130
Clarke JI, Forootan SS, Lea JD, Howell LS, Rodriguez JM, Kipar A, Goldring CE, Park BK, Copple IM, Antoine DJ (2017) Circulating levels of miR-122 increase post-mortem, particularly following lethal dosing with pentobarbital sodium: implications for pre-clinical liver injury studies. Toxicol Res 6(4):406–411
Dejong CH, van de Poll MC, Soeters PB, Jalan R, Olde Damink SW (2007) Aromatic amino acid metabolism during liver failure. J Nutr 137(6):1579–1585
Derakhshanfar A, Moayedi J, Vahedi M, Valizadeh A (2020) Arum conophalloides aqueous extract induced hepatotoxicity in rat; histopathological, biochemical, and mir-122 assessments. MicroRNA 9(3):224–231
Ding Z, Wei Q, Liu C, Zhang H, Huang F (2022) The quality changes and proteomic analysis of cattle muscle postmortem during rigor mortis. Foods 11(2):217–233
Donaldson AE, Lamont IL (2013) Biochemistry changes that occur after death: potential markers for determining post-mortem interval. PLoS ONE 8(11):e82011
Ducker GS, Rabinowitz JD (2017) One-carbon metabolism in health and disease. Cell Metab 25(1):27–42
Dwivedi Y, Rizavi HS, Zhang H, Mondal AC, Roberts RC, Conley RR, Pandey GN (2009) Neurotrophin receptor activation and expression in human postmortem brain: effect of suicide. Biol Psychiatry 65(4):319–328
Ebuehi O, Amode M, Balogun A, Fowora A (2015) Postmortem time affects brain, liver, kidney and heart DNA in male rat. Am J Biochem 5(1):1–5
Fordyce SL, Kampmann M-L, Van Doorn NL, Gilbert MTP (2013) Long-term RNA persistence in postmortem contexts. Investig Genet 4(1):1–7
Francis SH, Busch JL, Corbin JD (2010) cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62(3):525–563
Gomaa MS, Abd El-Khalek AM, Sameer MM (2013) The relationship between the postmortem interval and the DNA degradation in brain and liver of adult albino rats. J Am Sci 9(5):535–540
Grieves J, Dick E Jr, Schlabritz-Loutsevich N, Butler S, Leland M, Price S, Schmidt C, Nathanielsz P, Hubbard G (2008) Barbiturate euthanasia solution-induced tissue artifact in nonhuman primates. J Med Primatol 37(3):154–161
Han H-S, Kang G, Kim JS, Choi BH, Koo S-H (2016) Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med 48(3):218–218
Ibrahim SF, Ali MM, Basyouni H, Rashed LA, Amer EA, Abd El-Kareem D (2019) Histological and miRNAs postmortem changes in incisional wound. Egypt J Forensic Sci 9(1):1–6
Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, Hines IN, Rippe RA, Spahr L (2008) Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology 134(5):1532–1543
Kakimoto Y, Kamiguchi H, Ochiai E, Satoh F, Osawa M (2015) MicroRNA stability in postmortem FFPE tissues: quantitative analysis using autoptic samples from acute myocardial infarction patients. PLoS ONE 10(6):e0129338
Karadzic R, Ilic G, Antovic A, Kostic Banovic L (2010) Autolytic ultrastructural changes in rat and human hepatocytes. Rom J Legal Med 18(4):247–252
Ko MJ, Mulia GE, Van Rijn RM (2019) Commonly used anesthesia/euthanasia methods for brain collection differentially impact MAPK activity in male and female C57BL/6 mice. Front Cell Neurosci 13(96):1–10
Lardizábal MN, Nocito AL, Daniele SM, Ornella LA, Palatnik JF, Veggi LM (2012) Reference genes for real-time PCR quantification of microRNAs and messenger RNAs in rat models of hepatotoxicity. PLoS ONE 7(5):e36323
Lei H, Grinberg O, Nwaigwe C, Hou H, Williams H, Swartz H, Dunn J (2001) The effects of ketamine–xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res 913(2):174–179
Li X, Elwell M, Ryan A, Ochoa R (2003) Morphogenesis of postmortem hepatocyte vacuolation and liver weight increases in Sprague-Dawley rats. Toxicol Pathol 31(6):682–688
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Lukey MJ, Katt WP, Cerione RA (2017) Targeting amino acid metabolism for cancer therapy. Drug Discov Today 22(5):796–804
Lv Y-H, Ma J-L, Pan H, Zeng Y, Tao L, Zhang H, Li W-C, Ma K-J, Chen L (2017) Estimation of the human postmortem interval using an established rat mathematical model and multi-RNA markers. Forensic Sci Med Pathol 13(1):20–27
Lv Y-H, Ma K-J, Zhang H, He M, Zhang P, Shen Y-W, Jiang N, Ma D, Chen L (2014) A time course study demonstrating mRNA, microRNA, 18S rRNA, and U6 snRNA changes to estimate PMI in deceased rat’s spleen. J Forensic Sci 59(5):1286–1294
Machado MV, Diehl AM (2018) Hedgehog signalling in liver pathophysiology. J Hepatol 68(3):550–562
Mahdipour M, van Tol HT, Stout TA, Roelen BA (2015) Validating reference microRNAs for normalizing qRT-PCR data in bovine oocytes and preimplantation embryos. BMC Dev Biol 15(1):1–10
Mansur RB, Fries GR, Subramaniapillai M, Frangou S, De Felice FG, Rasgon N, McEwen B, Brietzke E, McIntyre RS (2018) Expression of dopamine signaling genes in the post-mortem brain of individuals with mental illnesses is moderated by body mass index and mediated by insulin signaling genes. J Psychiatr Res 107:128–135
Melotti A, Mas C, Kuciak M, Lorente-Trigos A, Borges I, Ruiz i Altaba A (2014) The river blindness drug I vermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol Med 6(10):1263–1278
Moayedi J, Hashempour T, Musavi Z, Arefian E, Naderi M, Heidari MR, Dehghani B, Hasanshahi Z, Merat S (2020) Evaluation of miR-122 serum level and IFN-λ3 genotypes in patients with chronic HCV and HCV-infected liver transplant candidate. MicroRNA 10(4):1–6
Mohamed AS, Hosney M, Bassiony H, Hassanein SS, Soliman AM, Fahmy SR, Gaafar K (2020) Sodium pentobarbital dosages for exsanguination affect biochemical, molecular and histological measurements in rats. Sci Rep 10(1):1–13
Mole CG, Heyns M (2019) Animal models in forensic science research: justified use or ethical exploitation? Sci Eng Ethics 25(4):1095–1110
Omenetti A, Yang L, Li Y-X, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM (2007) Hedgehog-mediated mesenchymal–epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest 87(5):499–514
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30(9):36–46
Pham DD, Do HT, Bruelle C, Kukkonen JP, Eriksson O, Mogollón I, Korhonen LT, Arumäe U, Lindholm D (2016) p75 neurotrophin receptor signaling activates sterol regulatory element-binding protein-2 in hepatocyte cells via p38 mitogen-activated protein kinase and caspase-3. J Biol Chem 291(20):10747–10758
Prabhakar S (2012) Translational research challenges: finding the right animal models. J Investig Med 60(8):1141–1146
Roderburg C, Benz F, Vargas Cardenas D, Koch A, Janssen J, Vucur M, Gautheron J, Schneider AT, Koppe C, Kreggenwinkel K (2015) Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver Int 35(4):1172–1184
Sampaio-Silva F, Magalhães T, Carvalho F, Dinis-Oliveira RJ, Silvestre R (2013) Profiling of RNA degradation for estimation of post morterm interval. PLoS ONE 8(2):e56507
Schlosser K, McIntyre LA, White RJ, Stewart DJ (2015) Customized internal reference controls for improved assessment of circulating MicroRNAs in disease. PLoS ONE 10(5):e0127443
Sharapova T, Devanarayan V, LeRoy B, Liguori M, Blomme E, Buck W, Maher J (2016) Evaluation of miR-122 as a serum biomarker for hepatotoxicity in investigative rat toxicology studies. Vet Pathol 53(1):211–221
Shomer NH, Allen-Worthington KH, Hickman DL, Jonnalagadda M, Newsome JT, Slate AR, Valentine H, Williams AM, Wilkinson M (2020) Review of rodent euthanasia methods. J Am Assoc Lab Anim Sci 59(3):242–253
Sinclair EM, Yusta B, Streutker C, Baggio LL, Koehler J, Charron MJ, Drucker DJ (2008) Glucagon receptor signaling is essential for control of murine hepatocyte survival. Gastroenterology 135(6):2096–2106
Staib-Lasarzik I, Kriege O, Timaru-Kast R, Pieter D, Werner C, Engelhard K, Thal SC (2014) Anesthesia for euthanasia influences mRNA expression in healthy mice and after traumatic brain injury. J Neurotrauma 31(19):1664–1671
Tsun Z-Y, Possemato R (2015) Amino acid management in cancer. Elsevier, Semin Cell Dev Biol
Tu C, Du T, Ye X, Shao C, Xie J, Shen Y (2019) Using miRNAs and circRNAs to estimate PMI in advanced stage. Legal Med 38:51–57
Welson NN, Gaber SS, Batiha GE-S, Ahmed SM (2021) Evaluation of time passed since death by examination of oxidative stress markers, histopathological, and molecular changes of major organs in male albino rats. Int J Legal Med 135(1):269–280
Zhang H, Zhang P, Ma K-J, Lv Y-H, Li W-C, Luo C-L, Li L-L, Shen Y-W, He M, Jiang J-Q (2013) The selection of endogenous genes in human postmortem tissues. Sci Justice 53(2):115–120
Acknowledgements
Hereby, the authors would like to thank Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
Funding
This study was financially supported by grant no. 95–01-45–13692 from the Vice-chancellor for Research Affairs of Shiraz University of Medical Sciences, Shiraz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All animal experiments were approved by the local Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (Approval ID: IR.SUMS.REC.1395.S1241). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Derakhshanfar, A., Kian, M., Dehghan, Z. et al. Comparison of the effects of two methods of euthanasia on post mortem changes in rats: histopathological and molecular findings. Comp Clin Pathol 31, 815–826 (2022). https://doi.org/10.1007/s00580-022-03385-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-022-03385-7