Abstract
Inflammation remains a complex process in host defense system and the pathology caused by it remains unsolved till date. Several steroidal and non-steroidal anti-inflammatory drugs in current use to treat inflammatory diseases elicit side effects in longer run. Hence, in order to counteract these outcomes, a pharmacognistic study using a Lamiaceae plant, Plectranthus amboinicus has been focused in the present study. P. amboinicus commonly known as Indian borage has been used in folk medicine for years in treating numerous diseases. It is well known for the presence of major phytochemical constituents which possess anti-inflammatory properties. Assessment of biochemical constituents and molecular mechanisms behind the anti-inflammatory property of P. amboinicus leaves were carried out in in vivo and in vitro conditions. In vivo studies of formalin-induced nociception and paw edema in mice and for in vitro condition lipopolysaccharide-induced inflammation using IC-21 macrophage cells were performed. Aqueous and ethyl acetate leaf extracts of P. amboinicus were analyzed for its phytochemical constituents. Variations in phytochemical components were noticed in GC-MS analysis. The carvacrol containing ethyl acetate extract exhibited higher anti-nociception in terms of lower paw licking time in the later phase of nociception and lowered hind paw edema volume up to 35%. The biochemical analysis revealed that the oxidative stress markers such as malondialdehyde and antioxidant enzymes have been modulated upon pretreatment with both extracts and also modulated the expression of inducible nitric oxide synthase, cyclooxygenase 2, Interleukin-1β, histamine 1 receptor genes and nuclear factor kappa B protein. In addition, nitric oxide inhibitory effect was observed in P. amboinicus treated IC-21 macrophages. Therefore, the molecular mechanism put forth in this study provides insights that the presence of carvacrol in ethyl acetate extract enhances anti-inflammatory activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The complex multifactorial inflammatory reactions have evolved to tackle the deadly pathogenic organisms and also protect the host from harmful chemical stimulus. However, inappropriate and dysregulated immune responses cause major clinical consequences and thereby promote the development of various inflammation-mediated diseases (Gupta et al. 2018; Bäck et al. 2019). It ranges from arthritis to atherosclerosis and neurodegenerative diseases such Alzheimer’s disease and Parkinsons’s disease (Rea et al. 2018; Gupta et al. 2018). The disease progression and severity is correlated to the secretion of diverse array of inflammatory mediators such as cytokines and unregulated production of free radicals includes reactive oxygen and nitrogen intermediates (Rea et al. 2018). Further acute and chronic pain caused by many inflammation-mediated diseases further worsens the patient’s health conditions (Matsuda et al. 2019). Biphasic response is the result of acute inflammation, which manifests through pain and edema followed by infiltration of immune cells to the site of injury and besides, it involves in the destruction of tissue components that characterize the chronic phase of an inflammatory cascade (Posadas et al. 2004; Santos et al. 2004). It must also be emphasized that the resolution of ongoing inflammation be tightly regulated to avoid an excessive or deregulated immune response that could result in tissue injury and fibrosis or scarring (Lee and Jeong 2002).
The immune system is a central player in both elicitation and dampening inflammatory responses. Several mediators that intersect numerous cellular pathways have been reported (Di Meglio et al. 2005; Korhonen et al. 2005; Tanimoto et al. 2007; Jain et al. 2008; Farivar et al. 2014). However, the context in which these mediators affect pro- and anti-inflammatory responses need to be carefully studied. Another important concern is that there are various side effects of non-steroidal anti-inflammatory drugs (NSAIDs) such as opioids and corticosteroids, used presently for management of pain and are often associated with mild to serious side effects in a longer run. Hence, the need to develop safer alternatives and plant-based therapeutics are increasingly gaining importance which promises better medicines with lower costs without the risk of side effects. It is also relevant to discuss the consequence of oxidative stress mechanisms that exacerbate ongoing inflammatory signaling cascades. The generation of reactive oxygen species (ROS) is regulated under normal conditions by cellular antioxidant defense systems. However, conditions like prolonged or excessive inflammation can disturb the balance in favor of oxidants eventually leading to cell damage and death (Fukai and Ushio-Fukai 2011; Deponte 2013; Ayala et al. 2014). Several reports emphasize the reduction in oxidant overload concomitant by restoring cellular anti-oxidants levels via treatments with potentially bioactive compounds especially derived from natural sources (Yatoo et al. 2018).
Lamiaceae includes commercially important genera such as Plectranthus, Salvia, Ocimum, Thymus, and Mentha that have been attributed with properties which bestow a rich diversity of ethnobotanical benefits (Milica et al. 2016; Marco et al. 2017; Kolac et al. 2017; Habashy et al. 2018). The genus Plectranthus has a wide distribution in tropical and warmer places with more than 300 species inhabiting all over the world with high abundance in Asia, Africa, and Australia (Lukhoba et al. 2006). Among them, Plectranthus amboinicus has widely used for the treatment of various inflammatory ailments and many of the biomedical properties including rheumatoid arthritis (Chang et al. 2010) antioxidant (Praveena and Pradeep 2012), anti-hyperglycemic and anti-hyperlipidemic (Viswanathaswamy et al. 2011) anti-inflammatory (Gurgel et al. 2009; Chiu et al. 2012), nephroprotective (Palani et al. 2010) and anti-tumor (Gurgel et al. 2009) activities. The observed biomedical effects have been attributed to the presence of various phytochemical constituents mainly carvacrol a monoterpenoid phenol (Feng and Jia 2014; Kuo et al. 2017). However, wide variations in the phytochemical constituents were noticed in the leaf extracts of P. amboinicus even with similar regional presence (Arumugam et al. 2016). Chiu et al. (2012) noticed that carvacrol was present in the aqueous leaf extract of P. amboinicus collected from Taiwan. Swamy et al. (2017) reported that carvacrol was obtained from the hexane extract of Malaysian P. amboinicus but, not in acetone and methanol. The literature revealed that P. amboinicus is unique to its origin place and must be tested for its biomedical applications separately for the particular geographical location. In this connection, molecular mechanism behind the anti-inflammatory activity of Indian borage plant, P. amboinicus is limited and need to be investigated thoroughly for getting potential bio-therapeutic safer anti-inflammatory agents. Therefore, in the present investigation, we have attempted to study the anti-inflammatory activity of both aqueous and ethyl acetate-derived extracts of P. amboinicus against formalin-induced inflammatory conditions in mice paw edema and LPS-induced IC-21 macrophage cell line.
Materials and methods
Fine chemicals and reagents
Formalin, superoxide dismutase, epinephrine, 3,3′-diaminobenzidine tetrahydrochloride (DAB), thiobarbituric acid (TBA), 1-chloro-2,4-dinitrobenzene (CDNB), reduced glutathione, 5,5'-dithiobis-2-nitrobenzoic acid (DTNB), sulfanilamide, N- (1-Napthyl) ethylenediamine dihydrochloride, and diacetylmonoxime (DAM) were purchased from Sigma Chemical Company (St. Louis, USA). Primary antibody p65/NF-κB polyclonal rabbit antibodies were purchased from BD Bioscience, USA. Secondary antibody goat anti-rabbit IgG-HRP conjugated, trizol, diethylpyrocarbonate, and primers were obtained from Genei, Bangalore, India.
Plant material and preparation of crude extracts
Plectranthus amboinicus leaves were collected from surrounding areas of Chennai, Tamil Nadu. The plant was identified and authenticated at the herbarium facility, Department of Botany in Alagappa University. A voucher specimen (No.201903) was deposited in the Department of Botany in Alagappa University. About 10 g of P. amboinicus dried leaf powder was immersed in 100 ml of ethyl acetate at room temperature with constant stirring for 48 h and filtered to obtain crude ethyl acetate extract. Ten grams of P. amboinicus leaves were cleaned air-dried and powdered which was boiled in 100 ml of double-distilled water with constant stirring for 1 h. The resulting extract was filtered, concentrated, and freeze-dried to obtain the crude aqueous extract.
Phytochemical and biophysical analyses
Qualitative analyses of various phytochemical compounds were performed for both the aqueous and ethyl acetate-derived P. amboinicus extracts. The presence of tannins and phenols were determined by the ferric chloride test, and the presence of carbohydrates, glycosides, saponins, and flavonoids was tested according to Yusuf et al. (2014) and Negi et al. (2018). Fourier-transform infrared (FTIR) spectrophotometer was utilized to identify possible biomolecules, and the spectrum was recorded by employing KBr pellet technique using a PerkinElmer model-983/G detector double-beam spectrophotometer. Furthermore, we employed the gas chromatography-mass spectrometer (GC-MS) QP2010 ultra Shimadzu to identify secondary metabolites of potential bio-therapeutic significance in our extracts. An HP5 column (30 m × 0.25 mm × 0.25 μm) was used with a 30 min temperature program of 80–280 °C at 10 °C/min followed by a 10 min hold at 280 °C. The injector temperature was 220 °C, and the flow rate of carrier gas helium was 0.8 ml/min. Identification of the compound was performed using NIST version 2 (National Institute of Standards and Technology) mass spectral library.
Maintenance of experimental animals
Healthy Swiss albino mice weighing about 20 g were procured from the Tamil Nadu Veterinary and Animal Sciences University, Veterinary Hospital, Madhavaram, Chennai. Mice were hygienically maintained in polypropylene cages with free access to drinking water and standard rat feed pellets (Hindustan Lever Ltd). The experiments were conducted according to the ethical norms approved by the Government of India and Institutional Animal Ethics Committee guidelines (IAEC. No. 02/06/17).
Experimental design for anti-inflammatory study
To test the anti-inflammatory activity of P. amboinicus, mice were divided into four groups each containing six animals, and the experiments were carried out in two separate batches for 24 and 48 h time point in order to observe morphological changes and pro-inflammatory markers. The dose concentration of aqueous or ethyl acetate extract administered was selected based on studies conducted by Asiimwe et al. (2014) and Chiu et al. (2012). Based on study reports, sub-lethal dose concentration (LD50) was fixed as 100 mg/kg body weight for aqueous extract and 50 mg/kg body weight for ethyl acetate extract, and they were administered orally.
-
Group I:
Mice were injected with phosphate-buffered saline (PBS) subcutaneously in hind paws and administered orally twice with physiological saline
-
Group II:
Mice were injected with formalin (2%) subcutaneously in hind paws and orally administered twice with physiological saline
-
Group III:
Mice were orally administered with aqueous leaf extract of P. amboinicus (100 mg/kg b.w) for twice followed by subcutaneous injection of formalin (2%) in the hind paw
-
Group IV:
Mice were orally administered with ethyl acetate leaf extract of P. amboinicus (50 mg/kg b.w) for twice followed by subcutaneous injection of formalin (2%) in the hind paw
Formalin-induced paw edema test
The animals were orally administered with aqueous (100 mg/kg b.w) or ethyl acetate (50 mg/kg b.w) extracts for twice in the experimental period. Accordingly, the extract was given on the first day then again repeated after 24 h and followed by subcutaneous administration of 20 μl of 2% formalin in mice hind paws was performed. The degree of paw edema volume was measured in each mouse before and after 25 min of formalin injection using vernier caliper (Soyocak et al. 2019). After 24- and 48-h post formalin administration, the mice were decapitated, and the hind paw tissues were quickly harvested for biochemical analysis.
Anti-nociceptive pain experiment-Formalin test
The nociceptive behavioral response of mice such as jerking, flexing of injected hind paw, and followed by licking are the signs of inflammatory pain caused by formalin. Therefore, formalin test was conducted on mice to know the anti-nociceptive effect of aqueous and ethyl acetate extracts of P. amboinicus according to Hort et al. (2018). Twenty microliters of formalin (2% in saline) was injected subcutaneously in right hind paw of each mice belonging to the above four groups. Then the mice were placed in a transparent box for easy viewing. Licking of injected hind paw by mice indicates the pain as well as nociceptive behavior. In order to quantify the intensity of nociceptive pain, the total time spent on licking the injected hind paw of each mouse was calculated by two-time intervals (0–3 min for early-phase reaction and 20–40 min for late-phase reaction).
Assays for oxidant and enzymic anti-oxidant levels
The oxidant and enzymic anti-oxidant assays were carried out with mouse hind paw tissues from each of the experimental groups at 24 and 48 h which were weighed separately and processed in ice-cold conditions for the assays. The tissue homogenate was prepared in TBS (pH 7.5) using glass hand homogenizer. The homogenate was centrifuged at 10,000 rpm for 20 min at 4 °C. The clear supernatant was used for biochemical assays. The proteins present in the homogenates were estimated by Lowry et al. (1951) using BSA as standard.
Estimation of malondialdehyde (MDA)
The level of malondialdehyde was estimated by the method of Ohkawa et al. (1979). To 0.2 ml of tissue homogenates, 0.2 ml of 20% SDS, 1.5 ml of 20% acetic acid, and 1.5 ml of 0.8% thiobarbituric acid were added. The mixture was made up to 4 ml with double-distilled water and then heated in a water bath at 95 °C for 60 min using glass balls as a conductor. After cooling, 1 ml of double-distilled water and 5 ml of n-butanol/pyridine mixture (v/v) were added and shaken vigorously and centrifuged at 11,000 rpm, for 10 min at 4 °C. The absorbance was read at 532 nm in a UV spectrophotometer, and lipid peroxide levels were expressed in nanomoles of MDA/mg protein.
Estimation of superoxide dismutase (SOD)
Superoxide dismutase was estimated by the method of Misra and Fridovich (1972). 0.1 ml of the tissue homogenate from each group was added to tubes containing 0.75 ml of ethanol. Then, 0.15 ml of chloroform (chilled in ice) was then added mixed well and centrifuged at 4000 rpm for 5 min at 4 °C. After centrifugation, supernatant was collected. 0.5 ml of supernatant was mixed with 0.5 ml of EDTA (0.6 mM) solution followed by 1 ml of bicarbonate buffer (0.1 M, pH 10.2) were added. The enzymatic reaction was initiated by the addition of 0.5 ml of epinephrine (1.3 mM), and the increase in the absorbance was read at 480 nm in a UV spectrophotometer. The enzyme activity was expressed in units/min/mg protein.
Estimation of catalase (CAT)
Catalase was estimated by the method of Beers and Sizer (1952). To 0.1 ml of tissue homogenates, 1.2 ml of 50 mM phosphate buffer (pH 7.0) was added. The reaction was initiated by the addition of 1.0 ml of 30 mM hydrogen peroxide solution. The decrease in absorbance was read at 240 nm for 3 min at 15 seconds interval in a UV spectrophotometer. The enzyme activity was expressed in units/min/mg protein.
Estimation of glutathione peroxidase (GPx)
The glutathione peroxidase level was estimated by the method of Rotruck et al. (1973). To 0.2 ml of tissue homogenates, 0.2 ml of 0.8 mM EDTA, 0.1 ml of 10 mM sodium azide, 0.1 ml of 2.5 mM H2O2, 0.2 ml of 4 mM reduced glutathione, and 0.4 ml of phosphate buffer (0.4 M; pH 7.0) were added and incubated at 37 °C for 10 min. The reaction was arrested by the addition of 0.5 ml of 10 % TCA, and the tubes were centrifuged at 4000 rpm for 5 min at 4 °C. To the supernatant, 3 ml of 0.3 M disodium hydrogen orthophosphate was added, and the absorbance was read immediately at 420 nm in a UV spectrophotometer. The enzyme activity was expressed in units/min/mg protein.
Estimation of gluthathione-S-transferase (GST)
The activity of gluthathione-S-transferase was estimated by the method of Habig et al. (1974). To 0.1 ml of tissue homogenates, 1.0 ml of 0.3 M phosphate buffer (pH 6.5), 1.7 ml of double-distilled water, and 0.1 ml of 30 mM 1-chloro-2-4-dinitrobenzene were added. After incubation at room temperature for 15 min, 0.1 ml of 30 mM GSH was added, and change in absorbance was read at 340 nm for 3 min at an interval of 30 s. The enzyme activity was expressed in units/min/mg protein.
Reverse-transcription PCR analysis
Mouse hind paw tissues from each of the experimental groups at 24 and 48 h were weighed separately and processed for isolation of total cellular RNA. The hind paw tissue was pulverized in a mortar and pestle using liquid nitrogen until it became powdery. Subsequently, the total cellular RNA was extracted using Trizol reagent, and 1 μg of this isolated RNA from each experimental group was reverse transcribed to cDNA using Thermo Scientific first-strand cDNA synthesis kit. This was followed by amplification with primers designed to facilitate target gene amplification. The primer sequences for these selected genes were designed using primer-BLAST (Supplementary Table 1).
Western blot analysis
Mouse hind paw tissues from each of the experimental groups at 24 and 48 h exposure period were weighed separately and snap frozen immediately in liquid nitrogen for western blot analysis. Prior to homogenization, frozen tissue was thawed and ground in a mortar pestle with approximately 2 ½ times the weight of tissue in a mild detergent buffer (50 mM Tris base, pH 7.4; 100 mM NaCl, 10% glycerol and 1% Triton X 100) on ice supplemented with freshly prepared 1 mM phenyl methyl sulfonyl fluoride (PMSF). Homogenates were centrifuged, and resulting clear supernatants were designated as the cytosolic fraction. This pellet was further treated in a solubilization buffer (100 mM Tris base, pH 7.4; 150 mM NaCl, 10% glycerol and 1% SDS with PMSF) at half the volume of mild detergent buffer used in preceding step. The resulting supernatant from the second centrifugation step was considered as the nuclear fraction. One hundred micrograms of each fraction from all experimental groups was electrophoresed under reducing denaturing conditions.
After electrophoretic separation, the gel was blotted onto a nitrocellulose membrane, blocked, and then incubated with the primary antibody, i.e., anti-IL-1β and p65/NF-κB polyclonal rabbit antibodies from (Santa Cruz, USA) at a dilution of 1:500, respectively and loading control beta-actin at a dilution of 1:10,000 followed by incubation with secondary antibody, goat anti-rabbit IgG-HRP conjugate at a dilution of 1:2000.
Cell culture
The IC-21 peritoneal macrophage was purchased from NCCS, Pune. The transformed cell line was maintained in RPMI-1640 medium containing 10% heat inactivated fetal bovine serum and 2 mM L-glutamine at 37 °C, 5% CO2. The cells were cultured in 25-cm2 culture flasks at 37 °C in a humidified atmosphere of 5% CO2 and were subcultured when they reached 90–100% confluence using PBS.
Alamar blue cytotoxicity assay
Cytotoxicity of aqueous and ethyl acetate leaf extracts of P. amboinicus was tested in IC-21 macrophage cell line using alamar blue dye method. The macrophage cells were seeded in a flat bottomed 96-well plate at a density of 1 × 104 cells per well and allowed to reach confluence provided with 10% FBS containing RPMI-1640 medium. Once confluence is reached, the 10% FBS containing medium is removed, and the cells were maintained in 0.5% containing RPMI-1640 medium for overnight. Two different concentrations (125 and 250 μg/ml) of aqueous or ethyl acetate extract of P. amboinicus were added to the wells and incubated for 24 h. After incubation 0.1% 10 μl of alamar blue was added to the treated and control wells and incubated for 5 h in the incubator. Cells with regular culture medium only are considered as positive control, and wells with culture medium without cells is considered as a negative control. Absorbance was measured at 570 nm and in 600 nm using (Power waves XS). Alamar blue reduction was calculated, and the percentage viability was obtained after normalizing the data by untreated control cells. Untreated control cells served as 100% cell viability.
In vitro assessment of anti-inflammatory activity in LPS-induced IC-21 macrophage
The nitric oxide inhibition activity of P. amboinicus was performed using Griess reagent according to Hsieh et al. (2007). To determine the in vitro anti-inflammatory effect of aqueous and ethyl acetate extracts of P. amboinicus, the IC-21 macrophage cells were induced by adding with 1.0 μg/ml of LPS for 24 h. Before starting the experiment, it was ensured that each well in the 96-well flat-bottom plates contains 2 × 105 cells. LPS was added and incubated for 24 h in control well or wells containing 125 μg/ml aqueous extract or 125 μg/ml ethyl acetate extract pretreated macrophages for 12 h. Control well consists of PBS instead of extracts. After exposure, the released nitric oxide present in the cell culture medium was quantified as nitrite using Griess reagent. One hundred microliters of cell culture supernatant from a well was mixed with 100 μl of Griess reagent (1% sulfonilamide and 0.1% N- (1-Napthyl) ethylenediamine dihydrochloride) and incubated for 20 min in dark. The absorbance was measured at 540 nm in ELISA microplate reader against suitable reagent blank. The concentration of NO was calculated using the standard graph derived from various concentrations of sodium nitrite, and the NO was expressed as μM nitrite released.
Statistical analysis
The statistical significance was assessed by using SPSS/10.0 software. The comparisons between different groups were performed with the Student’s t test. The minimum accepted level of statistical significance was p < 0.05 in all cases.
Results
Analysis of essential bioactive compounds
Presence of various bioactive compounds in different plant parts is essential for successful usage in folk medicine. Results from our qualitative phytochemical analysis revealed that the presence of carbohydrates, flavonoids, and saponins in both aqueous as well as ethyl acetate-derived extracts from leaves of P. amboinicus (Table 1). However, certain phytochemical compounds like glycosides, terpenoids, and steroids were detected only in the ethyl acetate-derived leaf extract of P. amboinicus. Furthermore, GC-MS chromatogram revealed peaks that correspond to 9-octadecenoic acid methyl ester and methyl stearate in the aqueous-derived fraction (Fig. 1a). Moreover, the important terpenoid compound carvacrol was identified only in ethyl acetate-derived leaves extract of P. amboinicus (Fig. 1b). In addition, FTIR analysis of the aqueous extract revealed a broad peak at 3355/cm which indicated the presence of -NH and represents secondary amine group. The peak at 1583/cm indicate the presence of a carbonyl group. Further, peaks at 1080/cm and 1405/cm show the presence of the amide group. The FTIR results of ethyl acetate extract are as follows: peak at 3432/cm indicates the presence of a secondary amine or amide group, peaks at 2929, 2937, 1466, 1375, 1264, and 1022/cm indicate the presence of aliphatic compounds, and peak at 1714/cm suggests the presence of ketone group (Supplementary file. 1 a & b).
Anti-nociceptive effect of P. amboinicus extracts
The nociceptive pain first originates when the nerve endings are damaged at first 0–3 min of exposure to toxic stimuli followed by the release of inflammatory mediators at 20–40 min duration. The aqueous and ethyl acetate extracts of leaves of P. amboinicus have reduced the noxious pain caused by the formalin injection in mice. The aqueous extract pretreated mice showed 30.98% reduction in paw licking time of formalin-injected hind paw (p < 0.05; Fig. 2) whereas the ethyl acetate extract pretreatment reduced to 48.42% over formalin alone-injected mice (p < 0.01; Fig. 2). However, anti-nociceptive effect of P. amboinicus was observed only during later phase of inflammation (20–40 min) but it had no effect on initial phase of reaction (0–3 min; p ˃ 0.05).
Anti-nociceptive activity of aqueous and ethyl acetate extracts of leaves from P. amboinicus was quantified by counting the licking of mice on formalin injected hind paw at two different time intervals (0–3 min) and (20–40 min). Development of neurogenic pain in the formalin-injected mice was indirectly measured by counting the time spent for licking their hind paw of mice. Control contains mice that were injected with 20 μl of formalin (2%) in hind paw and orally gavaged twice with physiological saline. The treated group consists of aqueous leaf extract (100 mg/kg b.w) or ethyl acetate leaf extract (50 mg/kg b.w) pretreated mice twice followed by formalin (2%) injection in the hind paw. Mean ± SD values were represented here. The differences in mean paw licking time (sec) in two different time intervals of control group was compared with extract treated groups with Student’s t test. Statistical significance *indicates p < 0.001 and NS indicates no statistical difference between the mean values
In vivo anti-inflammatory effect of P. amboinicus in formalin-induced paw edema model
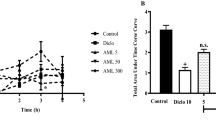
Reduction of swelling in the injured tissue caused by the inflammatory agent is the beneficial sign of recovery from the inflammation. In the present study, injection of 20 μl of 2% of formalin, in the dorsal plantar region of the hind paw region, caused edema in this region (Fig. 3). However, mice pretreated with aqueous and ethyl acetate extracts showed protection from formalin-induced inflammatory edema (Fig. 3). 25.21% reduction of paw edema volume within 30 min was noticed in aqueous extract pretreated mice (p < 0.05). However, higher anti-inflammatory activity was observed in ethyl acetate extracts of P. amboinicus leaves as it reduced 34.76% paw edema volume at 25 min (p < 0.01). Hind paw edema volume measurements revealed a significant reduction in paw edema volume in mice which was correlated with a visible reduction in edema and tissue redness of groups III and IV compared to group II (Supplementary file 2). The reduction in paw edema volume at an earlier stage such as 30 min after the formalin insults revealed that the components present in the extracts might inhibit the action of inflammatory markers that caused the inflammatory pain.
Effects anti-inflammatory activity of aqueous and ethyl acetate extracts of leaves from P. amboinicusin formalin-induced paw edema in mice before 30 min duration. At 25th minute after the injection of formalin, the hind paw edema volume was measured in all four groups of mice by using vernier caliper. Group I: mice were injected with PBS subcutaneously in hind paws and gavaged orally twice with physiological saline; group II: mice were injected with 20 μl of formalin (2%) in hind paw and orally gavaged twice with physiological saline; group III: aqueous leaf extract (100 mg/kg b.w) pretreated mice followed by formalin (2%) injection in the hind paw; group IV: Ethyl acetate leaf extract (50 mg/kg b.w) pretreated mice followed by formalin (2%) injection in the hind paw. Mean ± SD values were represented here. The differences in mean paw edema volume of Group II mice were compared to Groups III and IV mice by student’s t test. Statistical significance * indicates p < 0.05 and ** indicates p < 0.01
Effects of P. amboinicus extract on the restoration of cellular antioxidant defences
Formalin injection in the mice hind paw is known to induce inflammatory conditions in mice. Initiation of neuropathic pain followed by inflammatory pain in the injured tissue caused huge alteration in the normal antioxidant enzymatic system. In order to identify the protective effects of leaf extract from P. amboinicus in formalin-induced paw edema model, the inflammatory tissues recovered after 24 and 48 h period was evaluated for its antoxidation potential. In line with this, we observed nearly a twofold increase in the level of MDA in group II mice which were considerably reduced in groups III and IV animals that were orally administered with aqueous and ethyl acetate extract respectively (Table 2). This was statistically significant (**p < 0.01) when compared to group II animals at 24 and 48 h time points. By contrast, a decrease in SOD level in group II mice when compared to control animals (group I). However, animal administered with aqueous or ethyl acetate extract (groups III and IV) were significantly increased when compared to group II animals (Table 2). The enzymic antioxidant levels of other major cellular mediators such as catalase, glutathione system-associated enzymes like glutathione peroxidase (GPx), and glutathione S-transferase (GST) were all found to be decreased in formalin alone-injected mice (group II) at both 24 and 48 h. Oral administration of either extract, in groups III and IV animals, restored antioxidant levels similar to control mice (group I). In all the aforementioned assays, we unambiguously report that levels of cellular antioxidants from group IV to be statistically significant at (**p < 0.01) while those from group III to be statistically significant (*p < 0.05) compared to group I treated animals (Table 2).
Effects of aqueous leaf extract from P. amboinicus on pro-inflammatory genes
The relative percentage of expression of candidate genes in formalin-induced paw edema was analyzed using the expression of GAPDH in control animal as 100%. iNOS expression showed no significant modulation in formalin injected (group II; lane ii) and aqueous treated (group III; lane iii) animals at 24 h compared to group I animals (Fig. 4a; lane i). On the other hand, treatment of mice with aqueous extract and formalin for 48 h showed a significant reduction of inducible nitric oxide synthase (iNOS) expression (Fig. 4b; group III; lane iii). Interleukin-1beta (IL-1β) was markedly elevated in aqueous extract treated (Fig. 4a; group III; lane iii) at 24 h time point but moderately decreased (*p < 0.05) at 48 h time point in (Fig. 4b; group III; lane iii). In addition, minimal changes were observed in H1R gene expression at both 24 and 48 h (Fig. 4a, b group III; lane iii). As shown in Fig. 5, there was no observable difference of expression in pro-form of IL-1β cytokine at 24 h, whereas a noticeable reduction was observed at 48 h in (group II; lane ii) mice. Furthermore, we probed for the active form of cytokine at 24 h revealed an intriguing feature with respect to the processing of pro-IL-1β to the mature active cytokine. Aqueous-derived leaf extract administered mice in (group III; lane iii) demonstrated a reduction (*p < 0.05) in the mature form of inflammatory cytokine as opposed to group II treated mice. In the extended period of 48 h, group III treated mice exhibited a comparable level in pro-IL-1β to that of group I (*p < 0.05) and relatively higher than mice hind paw homogenates of group II mice (Fig. 5). Analysis of inflammatory marker genes revealed that the aqueous extract prepared from leaves of P. amboinicus had modulatory activity on immune genes thereby it reduces the inflammation.
Effects of aqueous leaf extract of P. amboinicus on inducible nitric oxide synthase, histamine-1 receptor and interleukin-1 beta gene expression in formalin induced paw edema mice at 24 and 48 h. lane i: group I; lane ii: group II; lane iii: group III. The data are considered to be statistically significant at (*p < 0.05)
Western blot analysis of IL-1β protein expression in control and P. amboinicus aqueous leaf extract pretreated conditions in formalin induced paw edema in mice at 24- and 48-h period. a 24 h: lane i, group I; lane ii, group II; lane iii, group III. b 48 h: lane i, group I; lane ii, group II; lane iii, group III. The SDS-PAGE under non reducing condition was processed for western blot analysis and blots were pre-incubated was pre-incubated with anti- IL-1β polyclonal rabbit antibody (1:500 dilution) and then with goat anti-rabbit IgG-HRP conjugate (1:2000 dilution). The data is statistically significant (*p < 0.05)
Effects of ethyl acetate leaf extract from P. amboinicus on pro-inflammatory genes
The relative expression of pro-inflammatory genes like iNOS, IL-1β, and cyclooxygenase-2 (COX-2) was assayed in all experimental mice. As shown in Fig. 6a, b, pro-inflammatory genes were increased significantly in group II animals which were injected with 2% formalin alone. In contrast, oral gavaging the mice with ethyl acetate derived leaf extract from P. amboinicus (group IV; lane iii) led to a significant reduction (*p < 0.05) of pro-inflammatory genes such as iNOS, IL-1β, and COX-2 at both 24 and 48 h time point (Fig. 6a, b).
a, b Effects of ethyl acetate leaf extract of P. amboinicus on inducible nitric oxide synthase, interleukin-1 beta, and cyclooxygenase-2 gene expression in formalin induced paw edema mice at 24 and 48 h. Lane i, group I; lane ii, group II; lane iii, group IV. The data are considered to be statistically significant at (*p < 0.05)
Effects of leaf extracts from P. amboinicus regulates on p65/NF-κB nuclear translocation
The role of NF-κB as a major regulator during inflammation was characterized via assaying for cytosolic p65/NF-κB in all the experimental groups at both 24 and 48 h, respectively. Animals injected with formalin alone revealed a decreased level in p65/NF-κB (Fig.7A, B; group II; lane ii) compared to control (group I; lane i). However, p65/NF-κB expression increased in animals administered with aqueous or ethyl acetate extracts from P.amboinicus (Fig. 7A, B; groups III and IV; lane iii) which was similar to that of control.
A Effects of a aqueous leaf extract and B ethyl acetate leaf extract of P. amboinicus on p65/NF-κB protein expression in formalin-induced paw edema mice at 24 and 48 h. A (a) Lane i, group I; lane ii, group II; lane iii, group III. A (b) Lane i, group II; lane ii, group I; lane iii, group III. B (c) Lane i, group II; lane ii, group I; lane iii, group IV. B (d) Lane i, group I; lane ii, group IV; lane iii, group II. The data is statistically significant (*p < 0.05)
Cytotoxicity and in vitro anti-inflammatory activity of extracts
Before assessing the anti-inflammatory activity, the aqueous and ethyl acetate leaf extracts from P. amboinicus were tested for its cytotoxicity in IC-21 macrophages. Twenty four hour exposure of extracts at two different concentrations (125 and 250 μg/ml) to IC-21 macrophages did not produce any significant changes in the viability of IC-21 macrophages (p ˃ 0.05; data not shown). LPS at 1 μg/ml for 24 h exposure elicited the inflammatory condition in IC-21 macrophages by releasing a large amount of nitric oxide. The generation of nitric oxide by macrophages upon exposure indicates the inflammation in LPS alone IC-21 macrophages. As shown in Fig. 8, the maximal tested concentration of aqueous extract (250 μg/ml) inhibited the 37.31% of nitric oxide generation (p < 0.001). However, ethyl acetate extract pretreated IC-21 macrophages effectively curtail 52.24% of LPS-mediated generation of nitric oxide at 250 μg/ml concentration level (p < 0.001).
In vitro anti-inflammatory activity of aqueous and ethyl acetate extracts of leaves from P. amboinicus on LPS-induced inflammation in IC-21 macrophages. The anti-inflammatory activity of P. amboinicus was quantified by the degree of inhibition of nitric oxide generation in macrophages upon LPS stimulation (1 μg/ml). The nitric oxide generation by macrophages was quantified with Griess reagent by measuring the stable end product nitrite present in the cell culture medium. Control consists of macrophages alone without LPS elicitor; LPS alone group consists of macrophages were elicited by LPS for 24 h; the treated groups consists of either P. amboinicus leaves aqueous extract or ethyl acetate extract pretreated macrophages for 12 h period followed by LPS induction for 24 h. Mean ± SD values were represented here. The mean difference between LPS alone group versus extract pretreated groups was compared by student’s t test. * indicates p < 0.01; ** indicates p < 0.001 statistical significant differences observed between the mean values
Discussion and conclusion
Leaves of Plectranthus amboinicus are consumed since ancient times due to its strong aroma and medicinal properties. P. amboinicus is an edible herb widely used in food supplements in India, Mexico, and South-East Asian countries. It is one of the major natural sources for treating conditions like inflammation, skin allergies, indigestion, asthma, heart failure, and snakebite (Lukhoba et al. 2006). However, wide variations in the presence of biochemical constituents were noticed in the leaves of P. amboinicus collected from different regions of the world (Arumugam et al. 2016; Swamy et al. 2017). Therefore, in the present study, two different extracts were prepared from P. amboinicus and tested for their efficacy in formalin-induced paw inflammation in mice. Formerly to this, we sought to identify the different phytochemical components present in both the extracts by analyzing qualitatively. The aqueous and ethyl acetate leaf extracts of P. amboinicus were observed to possess flavonoids as well as saponins, and it has been previously reported that their presence in the aqueous and ethyl acetate extract showed antioxidant and anti-inflammatory properties (Nile et al. 2018). Variations in the phytochemical components in the leaf extract of P. amboinicus have been noticed previously (Arumugam et al. 2016; Uma et al. 2011; Swamy et al. 2017). GC-MS analysis revealed that the carvacrol (C10H14O; CAS: 499-75-2) was present in the ethyl acetate-derived leaf extract from P.amboinicus but, not in the aqueous extract. The volatile terpenoid has been reported to be a major constituent in essential oils obtained from leaf extracts (Govindaraju and Arulselvi 2017). Further to this, several studies have noted the biological significance of this compound in eliciting potent anti-inflammatory, antioxidant, and anti-apoptotic activities (Arigesavan and Sudhandiran 2015). Interestingly, Cui et al. (2015) reported that carvacrol induced suppression of NF-κB signaling in vitro study. In a related study utilizing the Freund’s complete adjuvant-induced paw edema, inflammation in mice was decreased while administration of 50 and 100 mg/kg of carvacrol as well as reduced IL-1β, COX-2 expression in addition, upregulated anti-inflammatory IL-10 production (Lima et al. 2013). FTIR spectrum is a reliable and sensitive method to detect the biomolecular composition of plant extracts. Our results revealed that the aqueous extract predominantly contains amide groups due to the presence of polar proteins. The ethyl acetate extracts, in addition to secondary amines, also possess ketones, amides, and aliphatic compounds. Interestingly, clustering of peaks highlighted the overlap of constituents present in an extract with closely related functional groups.
Management of pain in an inflammatory disease condition is still a challenging task, whereas 80% of older people suffer worldwide (Sjöling et al. 2005). Nociception is the physiological ability of the nervous system to detect and counteract against noxious tissue-damaging agents. Chronic inflammatory disease like rheumatoid arthritis has been majorly linked to nociceptor neurons and inflammatory cells (Pinho-Ribeiro et al. 2017). Therefore, the use of anti-nociceptive agents would minimize the inflammatory reaction that would curtail pain. Reduction in nociception stimuli is achieved at the initial and later phase of formalin-induced nociception test. In the present study, both the extracts of P. amboinicus significantly reduced the paw licking time thereby protecting the mice from the later phase of nociception process. Ethyl acetate extract showed potent anti-nociceptive property as it reduces 48% of paw licking time in formalin-injected mice. Zakaria et al. (2018) reported that methanolic leaf extract of Clinacanthus nutans reduces the capsevicin-induced nociception in mice. Similarly, Eugenol extracted from Eugenia cariophyllata reduced the number of flinches and shakes in formalin-induced nociception in rat (Lugo-Lugo et al. 2019). Meantime, the aqueous and ethyl acetate extracts of P. amboinicus significantly reduced the paw edema swelling in formalin-injected mice. However, the highest percentage of edema inhibition was noticed in ethyl acetate extract pretreated mice. Similarly, Ghildiyal et al. (2013) and Madhuri et al. (2016) reported that Laghupanchamoola herbal formulation and aqueous extract of Mangifera indica reduced the formalin-induced hind paw edema in mice and rat respectively. Therefore, in the present study, the leaf extracts prepared from Indian borage P. amboinicus exhibited anti-nociceptive and anti-inflammatory activities in the formalin-induced mice model. Increased anti-inflammatory activity was observed in ethyl acetate extract of P. amboinicus due to the presence of carvacrol and its compounding effect with other phytochemical components present in the extract.
Maintenance of cellular redox balance is crucial in normal physiology and perturbations were noticed in inflammation and disease conditions (Trachootham et al. 2008). The cellular insult was visualized by the generation of ROS through different mechanisms that dealt with a different combination of potent antioxidant mediators here forth eventually redresses an imbalance in cellular homeostasis (Aprioku 2013). The major ROS generated includes superoxide anion, hydroxyl radical, nitric oxide, and peroxides. It is reported that essential oil of P. amboinicus possesses significant antioxidant property both in in vitro and in vivo model which may be due to the presence of phytochemical constituents. In the present study, we assessed the cellular levels of different antioxidant enzymes in addition to lipid peroxidation product that is reliable as an oxidant stress marker. During edematous condition, lipid peroxidase seems to play an important role in producing oxidative stress (Goulart et al. 2005). It is known that formalin induces its pro-inflammatory activity via promoting oxidative stress, oxidant-mediated cell damage, and as a consequence depleting levels of cellular antioxidant enzymes. Treatment with either of the P. amboinicus-derived extracts attenuated such oxidant mediated detrimental effects against formalin-induced paw edema tissue.
The complex interplay of transcription factors like NF-κB and pro-inflammatory mediators like IL-1β, IL-6, and TNF-α and COX-2 produced during inflammation are of major concern. Histamine, a main mediator of allergy, produces inflammatory symptoms like vasodilation, swelling, as well as redness at the injured site, and its signaling is mainly regulated by H1 receptor (Fukui 2008) that plays a central role in acute inflammation (Benly 2015; Gilmore 2006). A search of plants with potential anti-inflammatory property is in limelight in current research studies, and plants belonging to Lamiaceae family have been explored widely for its anti-inflammatory properties in recent years. The oil and water extracts of Thymus vulgaris in synergism with ethanolic extract of Chlorella vulgaris has been reported to possess anti-inflammatory property by curing hydrocortisone-induced osteoporosis in rat through dampening major inflammatory genes expression like NF-кB, COX-2, and TNF-α in bone cells (Abu-Serie and Habashy 2018). Methanolic extract of Sideritis bilgeriana has been found to reduce the expression of pro-inflammatory cytokines like IL-1β, TNF-α, IL-6, NF-κB, and the extract was stated to possess anti-inflammatory as well as anti-neuropathic properties (Mariana et al. 2020). Therefore, we tested, the ability of aqueous and ethyl acetate leaf extracts of P. amboinicus was investigated, whether it can dampen the gene as well as protein expression of inflammatory mediators like iNOS, IL-1β, COX-2, and H1R. Our results from gene expression analysis, via amplification of cDNAs from different experimental groups at 24 and 48 h, revealed that aqueous derived extract seemed to dampen the pro-inflammatory cytokines like iNOS and IL-1β in formalin-induced tissues at 48 h, whereas a marginal decrease is observed in H1 receptor expression of group III aqueous treated mice at 24 h. However, ethyl acetate extract revealed a considerable downregulation of the expression of major pro-inflammatory genes like iNOS, IL-1β, and COX-2 in group IV mice at both 24 and 48 h time points.
The most interesting fact was that the results obtained for the protein expression levels and their modulations across different experimental groups were examined using the western blotting technique for samples at 24 and 48 h. The NF-κB has been known to regulate inflammatory signaling, and its subunits are sequestered in the cytosol as an inactive complex by binding to its inhibitory factor, IκB-α, (inhibitory kappa B kinase) in non-stimulated cells (Kuntzen et al. 2007). Upon stimulation with pro-inflammatory signals, IκB-α is phosphorylated by IκB-α kinase (IKK) through ubiquitin-mediated degradation, and the resulting free NF-κB is translocated to the nucleus and acts as a major transcription factor of inflammatory mediators (Mitchell et al. 2016). We observed the level of cytosolic inactive p65 subunit of NF-κB to be lowest in group II indicating translocation of p65 subunit at nucleus to activate NF-κB-mediated transcription. On the other hand, groups III and IV mice exhibited significant retention of p65 subunit comparable to that of group I. The p65 subunit of NF-κB is mainly involved in governing the molecular mechanism of SOD gene, which is induced by various stimuli like LPS, TNF-α and IFN-γ, and also induction of SOD has been reported to play an important role in inflammation as well as oxidative stress (Kayoko et al. 2000). In our result, we observed complete sequestration of p65 subunit in the aqueous treated group at 48 h, and increased level of SOD was also observed in a similar group at 48 h. It is a common understanding that NF-κB translocation to the nucleus results in the transcription of pro-inflammatory genes. However, it must be emphasized that the dual role of this transcription factor can modulate the expression of genes which also influence cell survival, growth, and response to cellular stress (Hazuda et al. 1990). Henceforth, the results obtained from other experiments undertaken so far indicate mitigation of cellular stress induced by formalin upon extract supplementation.
Furthermore, we probed the possible modulation in protein expression of a downstream mediator of NF-κB, a pro-inflammatory cytokine IL-1β to study its modulation among the three experimental groups. It is a well-known fact that precursor and inactive form of IL-1β protein (at approximately 37 kD molecular weight) is processed by inflammasome complex that includes caspase-1 to result in the release of mature cytokine that exerts its pro-inflammatory effects. It is reported that activation of NF-κB signaling pathway could, in turn, promote inflammasome mediated processing of pro-IL-1β to its active form. Western blot data revealed that pro-form of the cytokine was found to be expressed relatively the same in all groups at 24 h. However, this was not the case further at 48 h, in group II mice paw homogenates it exhibited a decrease in the level of inactive protein. It can be construed that an inactive precursor would have been proteolytically processed into a mature form of the protein which would trigger inflammation. We also attempted to probe for a mature form of IL-1β, but only at an earlier time point of 24 h, wherein we demonstrated that processing of the precursor to an active form of cytokine which was lowered in group III mice. This would indicate that aqueous derived leaf extract preferentially targets the post-translational modification of IL-1β and this certainly warrants further investigation. The possible mechanism of action of extracts from P. amboinicus on mice formalin-induced inflammation was summarized in Supplementary file 3.
The in vitro analysis of aqueous or ethyl acetate extract of P. amboinicus has a less toxic effect on IC-21macrophage cells. The previous study has reported that the extract of P. amboinicus has membrane-stabilizing property in in vitro condition (Devi and Periyanayagam 2010). Further, in vitro anti-inflammatory activity of P. amboinicus extracts was revealed and found to have a protective effect against LPS-stimulated inflammatory conditions by curtailing the NO generation by 52% when compared to untreated IC-21 cells. Pretreatment of aqueous and ethyl acetate extracts of P. amboinicus leaves protected the LPS-mediated cellular insult by scavenging the potent inflammatory mediator NO. Wang et al. (2008) reported that ethyl acetate extract of Lindera erythrocarpa fruits inhibited the NO production in LPS-induced inflammation in RAW 264.7 macrophages. Similarly, the aqueous leaf extract of Taiwanian P. amboinicus pretreatment significantly reduces the NO generation in LPS-induced RAW 264.7 macrophage cell line (Chiu et al. 2012). Excess production of NO in inflammatory condition causes tissue injury in rheumatism (Clancy et al. 1998) and several neurodegenerative disorders (Liu et al. 2002). Therefore, NO inhibition was considered as a potential therapeutic option for many inflammatory diseases. The potent NO inhibitory activity observed in the ethyl acetate extracts of Indian P. amboinicus leaves may act as a suitable candidate for alternative medicine.
Based on the above results, we may conclude that leaves of P. amboinicus possess anti-nociception activity thereby it acts as a pain-relieving agent and reduces the paw edema volume in formalin-induced inflammatory condition together proved that it is an ideal medicinal plant for treating inflammatory disease conditions. Furthermore, antioxidant property noticed in formalin-induced edematous conditions by reducing the lipid peroxidation and increasing the antioxidant enzymes proved to be ideal for routine usage. The molecular mechanism behind the Indian P. amboinicus revealed that the leaf extracts have inhibited the p65/NF-κB subunit nuclear translocation by blocking IκB-α degradation thereby downregulating the pro-inflammatory genes such as iNOS, IL-1β, COX- 2, and TNF-α in formalin-induced inflammatory condition. Moreover, NO inhibitory activity in LPS-stimulated IC-21 cells further warrants for their anti-inflammatory activity.
Data availability
Available upon specific request.
Code availability
Not applicable.
References
Abu-Serie MM, Habashy NH (2018) The ameliorating effect of the combined extract from Greek Thymus vulgaris and bee’s honey on the hydrocortisone-induced osteoporosis in rat bone cells via modulating the bone turnover, oxidative stress, and inflammation. RSC Adv 8:28341–28355
Aprioku JS (2013) Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil 14(4):158–172
Arigesavan K, Sudhandiran G (2015) Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1,2-dimethylhydrazine plus dextran sodium sulfate-induced inflammation associated carcinogenicity in the colon of Fischer 344 rats. Biochem Biophys Res Commun 461(2):314–320
Arumugam G, Swamy MK, Sinniah UR (2016) Plectranthus amboinicus (Lour.) Spreng: botanical, phytochemical, pharmacological and nutritional significance. Molecules 21(4):e369
Asiimwe S, Karlsson AKB, Azeem M, Mugisha KM, Namutebi A, Gakunga NJ (2014) Chemical composition and toxicological evaluation of the aqueous leaf extracts of Plectranthus amboinicus Lour. Spreng. Int J Pharm Sci Invent 3(2):19–27
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxiadtion: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med Cell Longev:1–31
Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT (2019) Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol 16(7):389–406
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Benly P (2015) Role of histamine in acute inflammation. J Pharm Sci Res 7:373–376
Cavalcanti, MRM, Passos, FRS, Monteiro, BS, Gandhi, SR, Heimfarth, L, Lima, BS, Nascimento, YM, Duarte, MC, Araujo, AAS, Menezes, IRA, Coutinho, HDM, Zengin, G, Ceylan, R, Aktumsek, A, Quintans-Júnior, LJ, Quintans, JSS, HPLC-DAD-UV analysis, anti-inflammatory and anti-neuropathic effects of methanolic extract of Sideritis bilgeriana (Lamiaceae) by NF-κB, TNF-α, IL-1β and IL-6 involvement, J Ethnopharmacol 2020.
Chang JM, Cheng CM, Hung LM, Chung YS, Wu RY (2010) Potential use of Plectranthus amboinicus in the treatment of rheumatoid arthritis. Evid Based Complement Alternat Med 7(1):115–120
Chiu Y, Huang T, Chiu C, Chen Y, Peng W, Chen C (2012) Analgesic and anti-inflammatory activities of the aqueous extract from Plectranthus amboinicus (Lour.) Spreng both in vitro and in vivo. Evid Based Complement Alternat Med:1–11
Clancy RM, Amin AR, Abramson SB (1998) The role of nitric oxide in inflammation and immunity. Arthritis Rheum 41(7):1141–1151
Cui Z, Xie Z, Wang Z, Zhong X, Chen Y, Sun Q et al (2015) Carvacrol protects neuroblastoma SHSY5Y cells against Fe2+-induced apoptosis by suppressing activation of MAPK/JNK-NF-κB signaling pathway. Acta Pharmacol Sin 36:1426–1436
Lima MS, Quintans-Junior LJ, de Santana WA, Kaneto CM, Soares MBP, Villarreal CF (2013) Anti-inflammatory effects of carvacrol: Evidence for a key role of interleukin-10. Eur J Pharmacol 699: 112-117.
Deponte M (2013) Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta 1830:3217–3266
Devi KN, Periyanayagam K (2010) In vitro anti-inflammatory activity of Plectranthus amboinicus (Lour) Spreng by HRBC membrane stabilization. Int J Pharm Sci Res 1(1):26–29
Di Meglio P, Ianaro A, Ghosh S (2005) Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-κB activation. Arthritis Rheum 52(3):951–958
Farivar S, Hassani M, Shiari R (2014) Interleukin-1 as a key factor in the development of inflammatory diseases. Arch Pediatr Infect Dis 2(4):e18177
Feng X, Jia A (2014) Protective effect of carvacrol on acute lung injury induced by lipopolysaccharide in mice. Inflammation 37(4):1091–1101
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15(6):1583–1606
Fukui H (2008) Progress in allergy signal research on mast cells, up-regulation of histamine signal-related gene expression in allergy model rats. J Pharmacol Sci 106:325–331
Ghildiyal S, Gautam MK, Joshi VK, Goel RK (2013) Anti-inflammatory activity of two classical formulations of Laghupanchamula in rats. J Ayurveda Integr Med 4(1):23–27
Gilmore TD (2006) Introduction to NF-κB: players, pathways, perspectives. Oncogene 25:6680–6684
Goulart AC, Dos Santos Correia FA, De Sousa SCOM, de Cerqueira Luz JG (2005) Study of the inflammatory process induced by injection of carrageenan or formalin in the rat temporo mandibular joint. Braz Oral Res 19(2):99–105
Govindaraju S, Arulselvi PI (2017) Characterization of Coleus aromaticus essential oil and its major constituent carvacrol for in vitro antidiabetic and antiproliferative activities. Int J Geogr Inf Syst 24(1):37–51
Gupta SC, Kunnumakkara AB, Aggarwal S, Aggarwal BB (2018) Inflammation, a double-edge sword for cancer and other age-related diseases. Front Immunol 9:2160
Gurgel AP, Da Silva JG, Grangeiro AR, Oliveira DC, Lima CM, da Silva AC et al (2009) In vivo study of the anti-inflammatory and antitumor activities of leaves from Plectranthus amboinicus (Lour.) Spreng (Lamiaceae). J Ethnopharamacol 125:361–363
Habashy NH, Abu Serie MM, Attia WE, Abdelgaleil SAM (2018) Chemical characterization, antioxidant and anti-inflammatory properties of Greek Thymus vulgaris extracts and their possible synergism with Egyptian Chlorella vulgaris. J Funct Foods 40:317–328
Habig WH, Pabst MJ, Jacoby BW (1974) Glutathione S-transferase-the first enzymatic step in mercapturic acid formation. J Biol Chem 249:1730–1737
Hazuda DJ, Stricklerll J, Kueppersll F, Simon PL, Young PR (1990) Processing of precursor interleukin 18 and inflammatory disease. J Biol Chem 265:6318–6322
Hort MA, Silva Júnior FMRD, Garcia EM, Peraza GG, Soares A, Lerner C, Muccillo-Baisch AL (2018) Antinociceptive and anti-inflammatory activities of marine sponges Aplysina caissara, Haliclona sp. and Dragmacidon reticulatum. Braz Arch Biol Technol 61:e18180104
Hsieh YH, Kuo PM, Chien SC, Shyur LF, Wang SY (2007) Effects of Chamaecyparis formosensis Matasumura extractives on lipopolysaccharide-induced release of nitric oxide. Phytomedicine 14(10):675–680
Jain NK, Ishikawa T, Spigelman I, Herschman HR (2008) COX-2 expression and function in the hyperalgesic response to paw inflammation in mice. Prostaglandins Leukot Essent Fat Acids 79(6):183–190
Kayoko M, Hasegawa T, Ken-ichi IA (2000) NF-kB p65 Subunit is indispensable for activating manganese superoxide dismutase gene transcription mediated by tumor necrosis factor-a. J Cell Biol 77:474–486
Kolac UK, Ustuner MC, Tekin N, Ustuner D, Colak E, Entok E (2017) The anti-inflammatory and antioxidant effects of Salvia officinalis on lipopolysaccharide-induced inflammation in rats. J Med Food 20(12):1–8
Korhonen R, Lahti A, Kankaanranta H, Moilanen E (2005) Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy 4:471–479
Kuntzen C, Zazzeroni F, Pham CG, Papa S, Bubici C, Knabb JR et al (2007) A method for isolating prosurvival targets of NF-kappaB/Rel transcription factors. Methods Mol Biol 399:99–124
Kuo PJ, Hung TF, Lin CY, Hsiao HY, Fu MW, Hong PD, Chiu HC, Fu E (2017) Carvacrol ameliorates ligation-induced periodontitis in rats. J Periodontol 88(7):e120–e128
Lee IO, Jeong YS (2002) Effects of different concentrations of formalin on paw edema and pain behaviours in rats. J Korean Med Sci 17:81–85
Liu BIN, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS (2002) Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci 962(1):318–331
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lugo-Lugo DE, Pozos-Guillén ADJ, Zapata-Morales JR, Rodríguez-Chong A, Rangel-López ADJ, Saavedra-Leos MZ, Vértiz-Hernández AA (2019) Antinociceptive local activity of 4-allyl-1-hydroxy-2-methoxybenzene (eugenol) by the formalin test: an anti-inflammatory effect. Braz J Pharm Sci 55:e18022
Lukhoba CW, Simmonds MSJ, Paton AJ (2006) Plectranthus: a review of ethnobotanical uses. J Ethnopharmacol 103:1–24
Madhuri AS, Mohanvelu R, Ramabhimaiah S (2016) Evaluation of anti-inflammatory activity of aqueous extract of Mangifera indica leaves in albino rats. Int J Basic Clin Pharmacol 5(3):635
Marco B, Loizzoa MR, Acquavivab R, Malfab GA, Aielloa F, Tundisa R (2017) Anti-inflammatory and antioxidant agents from Salvia Genus (Lamiaceae): an assessment of the current state of knowledge. Anti-inflamm Antiallergy Agents Med Chem 16(2):70–86
Matsuda M, Huh Y, Ji RR (2019) Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 33(1):131–139
Milica K, Dušanka K, Petrović MB, Jevtović-Stojmenov T, Jović M, Petrović A et al (2016) Anti-inflammatory effect of the Salvia sclarea L. ethanolic extract on lipopolysaccharide-induced periodontitis in rats. J Ethnopharmacol 199:52–59
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay of superoxide dismutase. J Biol Chem 247:3170–3175
Mitchell S, Vargas J, Hoffmann A (2016) Signaling via the NFκB system. WIREs Systems Biology and Medicine 8(3): 227-241.
Negi A, Dobhal K, Ghildiyal P (2018) Antioxidant potential and effect of extraction solvent on total phenol content, flavonoids content and tannin content of Ficus palmata Forssk. Int J Pharm Sci Rev Res 49(2):19–24
Nile SH, Keum YS, Nile AS, Jalde SS, Patel RV (2018) Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol 32(1):e22002
Ohkawa H, Ohishi N, Yogi T (1979) Assay for lipid peroxides in animal tissues by the thiobarbituric acid reaction. Anal Biochem 95:351–358
Palani S, Raja S, Naresh R, Senthil Kumar B (2010) Evaluation of nephroprotective, diuretic, and antioxidant activities of Plectranthus amboinicus on acetaminophen-induced nephrotoxic rats. Toxicol Mech and Methods 20(4): 213–221
Pinho-Ribeiro FA, VerriJr WA, Chiu IM (2017) Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol 38(1):5–19
Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin C (2004) Carrageenan-induced mouse paw edema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Brit J Pharmacol 142:331–338
Praveena B, Pradeep SN (2012) Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus). Food Nutr Sci 3:1–7
Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA (2018) Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol 9:586
Rotruck JT, Pope AL, Ganther HF, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science. 179:588–590
Santos JMM, Maria Aparecida Tatsuo KF, Turchetti-Maia RMM, Marcela CG, Lisboa de Francischi JN (2004) Leukocyte recruitment to peritoneal cavity of rats following formalin injection: role of tachykinin receptors. J Pharmacol Sci 94:384–392
Sjöling RM, Ågren RY, Olofsson N, Hellzén RO, Asplund RK (2005) Waiting for surgery; living a life on hold-a continuous struggle against a faceless system. Int J Nurs Stud 42(5):539–547
Soyocak A, Kurt H, Cosan DT, Saydam F, Calis IU, Kolac UK, Koroglu ZO, Degirmenci I, Mutlu FS, Gunes HV (2019) Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum Exp Toxicol 38(11):1296–1301
Swamy MK, Arumugam G, Kaur R, Ghasemzadeh A, Yusoff MM, Sinniah UR (2017) GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinicus leaves. Evid Based Complement Alternat Med:e1517683
Tanimoto A, Wang KY, Murata Y, Kimura S, Nomaguchi M, Sei N (2007) Histamine upregulates the expression of inducible nitric oxide synthase in human intimal smooth muscle cells via histamine H1 receptor and NF-κB signaling pathway. Arterioscler Thromb Vasc Biol 27:1556–1561
Trachootham D, Lu W, Ogasawara MA, Rivera-del Valle N, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10(8):1343–1374
Uma M, Jothinayaki S, Kumaravel S, Kalaiselvi P (2011) Determination of bioactive components of Plectranthus amboinicus Lour by GC-MS analysis. NY Sci J 4(8):66–69
Viswanathaswamy AHM, Koti BC, Gore A, Thippeswamy AHM, Kulkarni RV (2011) Antihyperglycemic and antihyperlipidemic activity of Plectranthus amboinicus on normal and alloxan-induced diabetic rats. Indian J Pharm Sci 73(2):139–145
Wang SY, Lan XY, Xiao JH, Yang JC, Kao YT, Chang ST (2008) Anti-inflammatory activity of Lindera erythrocarpa fruits. Phytother Res 22(2):213–216
Yatoo MI, Gopalakrishnan A, Saxena A, Parray OR, Tufani NA, Chakraborty S, Tiwari R, Dhama K, Iqbal HMN (2018) Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders - a review. Recent Patents Inflamm Allergy Drug Discov 12(1):39–58
Yusuf AZ, Zakir A, Shemau Z, Abdullahi M, Halima SA (2014) Phytochemical analysis of the methanol leaves extract of Paullinia pinnata Linn. J Pharmacogn Phytother 6(2):10–16
Zakaria ZA, Rahim A, Hafiz M, Roosli RAJ, Sani M, Hijaz M, Omar MH, Tohid M, Farah S, Othman F, Ching SM (2018) Antinociceptive activity of methanolic extract of Clinacanthus nutans leaves: Possible mechanisms of action involved. Pain Res Manag:9536406
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Yes.
Consent for publication
All the authors have read and approved the manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 157 kb)
Rights and permissions
About this article
Cite this article
Duraisamy, P., Manikandan, B., Koodalingam, A. et al. Anti-inflammatory, anti-nociceptive and anti-oxidant activities of carvacrol containing leaf extracts of edible Indian borage plant Plectranthus amboinicus: an in vivo and in vitro approach. Comp Clin Pathol 30, 397–413 (2021). https://doi.org/10.1007/s00580-021-03230-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-021-03230-3