Abstract
Study of mallard blood cell can reveal the physiological status and condition of this livestock. This study was based upon biochemical, cytomorphometrical and haematological analysis of different blood cells of the duck with respect to sex. Blood of the bird was collected from its metatarsal vein, kept in EDTA for haematological test and the smear was prepared immediately after the blood was obtained for morphometrical analysis. For biochemical test, blood was kept in EDTA containing fluoride and left it for 2–3 h and centrifuged. Plasma chemistry for the glucose, cholesterol, TPP, albumin and globulin was determined. The result of biochemical test differs significantly at P ≤ 0.05. The cell length, cell breadth, cell area, nuclear area and nuclear breadth of erythrocyte differ significantly at P < 0.001 and N/C ratio and nuclear length reflect nonsignificant value. In case of lymphocyte, the cell length, cell breadth, cell area, nuclear area and nuclear length show significance at P < 0.001 and nuclear breadth is significant at P < 0.05 and N/C ratio does not show significant difference. Monocyte cell length and cell area are significant at P < 0.001 and cell breadth shows nonsignificant values. In case of granulocytes, eosinophil of male and female is significant at P < 0.05, basophil of male and female is significant at P < 0.001 and heterophil of male and female does not differ significantly. Haematological parameters like PCV and RBC differ significantly at P ≤ 0.05, leukocyte differs significantly at P ≤ 0.01, MCH, MCV and MCHC do not show any significant values and parameters of DLC differ significantly at P < 0.05 and P < 0.01. This study concluded that the difference in blood cell parameters in male and female is due to various factors like increase in hormonal level, basal metabolic rate, stress, courtship and egg laying stage and some infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Study of biochemical parameters mainly deals with the understanding how biological molecules give rise to the processes that occur within living cells. Biochemical parameters mainly deals with the structures, functions and interaction of biological molecules like proteins, nucleic acids, carbohydrates and lipids. That provides the structure of cells and it performs many functions which are associated with life. The process by which cells harness energy from their environment through chemical reactions is known as metabolism. Biochemical can be seen as a study of the component and composition of living things and how they come together to become life. Biochemical parameters include estimation of glucose, cholesterol, protein, albumin, globulin and A/G ratio.
Cytomorphometry is the quantitative process of measurement of cellular characteristics, inputting the data points into a computer via a graphic interface, with the aim of standardizing image analysis. Image analysis was performed to obtain cellular diameter, cytoplasmic area, nuclear area and N/C ratio. It is the measurement of both cellular and nuclear length and breadth of erythrocytes and leukocytes (Nivia et al. 2015). Cytomorphometry of blood cells can reveal the physiological condition of the livestock.
The branch of medicine that deals with the study of the cause, treatment and prevention of different types of disorders all related to blood is referred as haematology. Haematology helps to analyse and detect blood-related issues and also helps to treat discovered conditions. It includes the treatment of blood disorders and malignancies, including the types of haemophilia, leukaemia, lymphoma and sickle cell anaemia. Haematological parameters include haemoglobin, TEC, WBC, DLC, PCV, ESR, MCH, MCHC and MCV.
Materials and method
Experimental site
The study was conducted at Centurion University of Technology and Management, BBSR where the ducks are kept under captive condition (Fig. 1). For this study, blood was collected from 10 male and 10 female duck and which were considered as unhealthy by physical examination were not included.
Blood sampling
Blood samples were collected by veterinary doctor from metatarsal vein of left leg using sterile 26 gauge needle and these were collected during morning hour to avoid diurnal variation. Blood samples were kept in EDTA to avoid clotting and for biochemical test, samples were kept in EDTA containing fluoride and left it for 2–3 h. Blood smears were prepared immediately after the collection of blood.
Plasma biochemistry
Plasma biochemistry was measured by using an A25 Biosystems Spectrophotometer autoanalyser. The plasma constituents analysed were glucose, cholesterol, protein and albumin. The concentration of glucose was determined by glucose oxidase peroxidise (GOD/POD) method (Trinder 1969). The intensity of the colour which formed was measured by spectrophotometer at wavelength of 505 nm. The amount of cholesterol was determined by the cholesterol oxidase and phenol4-aminoantipyrine (CHOD/PAP) (Meiattini et al. 1978). The concentration of cholesterol is determined spectrophotometrically at wavelength of 505 nm. The concentration of protein was determined by biuret reagent (Gornall et al. 1949). The intensity of colour which formed is directly proportional to the amount of protein present in the sample. The concentration of albumin was analysed spectrophotometrically at 630 nm wavelength by using Bromocresol green solution (Doumas et al. 1971). The globulin concentration was determined by deduction of albumin from total plasma protein.
Morphometrical analysis
For morphometrical analysis, blood smear was prepared at site on grease-free slides [Borosil Glass Works Ltd. 1101, Crescenzo, G-Block, MCA Club, Bandra Kurla Complex, Bandra(E), Mumbai-400 051, (India), Ground edges and lint free packing, measuring size 76 mm long, 26 mm wide and 1 mm thickness], air-dried for 10 min and fixed in methanol [Sisco Research Laboratories Pvt. Ltd. Plant site 1:D-88/2, MIDC, Turbhe – 400705, New Mumbai, India] for staining. The slides were stained with Giemsa stain prepared from Giemsa liquid [HiMedia Laboratories Pvt. Ltd. Works: B/4-6, M.I.D.C., Dindori, Nashik, MH. Reg.off: 23, Vadhani Ind. Est., LBS Marg, Mumbai-400086, India] and water, as per protocol in laboratory for cytomorphometrical analysis. Then, the slide was kept inside the coupling jar containing Giemsa stain and kept undisturbed for 40–45 min. Then, the slide was taken out from the staining rack and washed under a slow running tap water. From each specimen, 10 slides were prepared and kept ready for observation. The stained slide was then observed under compound microscope [BLISCO, INDIA, An ISO 9001:2008 Certified Co] using 40× objectives and photomicrographed after observation under the compound microscope. During observation well spread, clean cells were measured for metrical analysis by the help of stage micrometre and ocular micrometre.
Haematology
For determination of haematological parameters such as TLC, TEC, Hb, PCV, MCV, MCHC and MCH, the following procedure was assumed. TEC and TLC were counted by using haemocytometer, Hayem’s fluid and Turks fluid. By using Sahil’s haemometer, haemoglobin concentration was measured. Packed cell volume was determined using micro-haematocrit method. Erythrocyte indices like mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were calculated from the values obtained from the packed cell volume, haemoglobin and RBC.
Statistical analysis
Biochemical, cytomorphometrical and haematological parameters were expressed as mean ± SE in both male and female of mallard duck using the Microsoft Office Excel 2010. Comparison of morphometrical parameters of male and female duck was performed in excel sheet by using t test: two sample assuming equal variances. Differences were classified as significant at P < 0.05, P < 0.01 and P < 0.001 and hypothesis was tested.
Result and discussion
Plasma biochemical analysis
In biochemical analysis, the result shows that mean and range of whole blood biochemical parameters vary with respect to sex. Result shows significant difference in glucose, cholesterol, protein, albumin, globulin and A/G ratio. The mean value of glucose in female mallard duck is 102.71 ± 0.9 mg/dl (Table 1) in blood, where as in male, it was 105.66 ± 0.72 mg/dl (Fig. 2). The amount of glucose in blood remains more in male as compared to the female due to some environmental factors (Artacho et al. 2007). The difference was significant statistically P ≤ 0.05. The cholesterol concentration (mg/dl) of male duck was observed to be 299.62 ± 0.93 and in case of female, it was 294.9 ± 2.12 (Fig. 3) and it differs significantly at P ≤ 0.05. The protein, albumin, globulin and A/G ratio (Figs. 4, 5, 6 and 7) are found to be higher in male in comparision to females but significant variations is recoded only in protein at p < 0.05 (Rodriguez et al. 2005).
Cytomorphometrical analysis
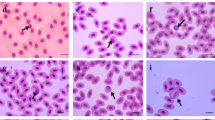
The effect of sex on cytomorphometry of different types of blood cells of mallard duck is recorded in micron metre (μm) and analysed. In erythrocyte, the male species have larger size as compared to the female species due to some hormonal effect and basal metabolic rate which is higher in male (Keçeci and Çöl 2011; Albokhadaim 2012; Dolka et al. 2014). The cell length, cell breadth, cell area, nuclear area and nuclear breadth of erythrocyte differ significantly at P < 0.001 (Fig. 8). The nuclear length and N/C ratio of erythrocyte were not having significant values (Table 2). The female lymphocytes were little larger as compared to male lymphocyte due to courtship, egg laying stage and early incubation stage which leads to rise of some hormone that may change the count and shape of female lymphocyte (Halie and Chanie 2014). The cell length, cell breadth, cell area, nuclear area and nuclear length of lymphocyte differ significantly at P < 0.001. The nuclear breadth of lymphocyte is significant at P ≤ 0.05. The N/C ratio does not show significance among male and female species as P > 0.05 (Fig. 9). The size of male monocyte was larger than the size of female monocyte due to some physiological activities and environmental effect (Orawan and Aengwanich 2007). The cell length and cell area of monocyte were significant at P < 0.001. The cell breadth of monocyte does not show any significant value (Fig. 10). All the cytomorphometrical parameters of eosinophil of male and female differ significantly at P < 0.05 (Fig. 11). The difference in size of eosinophil in male and female may due to some allergic or parasitic condition which resulted due to pollution and infection (Halie and Chanie 2014). The males had a lesser size of basophil which may be due to some parasitic disturbances or some diseases (Halie and Chanie 2014). Cytomorphometrical parameters of basophil differ significantly at P < 0.001 (Fig. 12). The heterophil shows nonsignificant cytomorphometrical parameters in both male and female and their size does not reveal a great difference due to some hormonal changes or may due to some environmental stress (Figs. 13 and 14) (Halie and Chanie 2014) (Table 3).
Haematological analysis
The difference of male and female RBC was statistically significant at P < 0.05 (Fig. 15). Here, the count of RBC in male was greater as compared to female due to some hormonal changes and environmental factors (Gayathri and Hegde 1994). Haemoglobin concentration was higher in male as compared to female and was significant at P ≤ 0.05 (Fig. 16). The reason for the difference may be during specific reproductive stages. The packed cell volume of male and female differs significantly at P ≤ 0.05 (Fig. 17). Here, the PCV of female was lesser than 35% due to diseased condition like anaemia (Campbell 1988). The values of WBC (Fig. 18) vary between male and female significantly at P < 0.01 (Table 4). In this study, female had more WBC value due to egg laying stage. The ESR value of female was higher than male and significantly different at P < 0.01 (Fig. 19). The values of MCV, MCH and MCHC (Figs. 20, 21 and 22) were not significant as the value of male and female was similar (Table 5). In DLC count, lymphocyte, eosinophil and heterophil differ significantly at P < 0.01, monocyte differs significantly at P < 0.05 and basophil does not show any significant values (Fig. 23), due to egg laying stage or early incubation period (Halie and Chanie 2014) of the female duck (Table 6).
Conclusion
The study revealed the effect of sex on biochemical, cytomorphometry and haematology of blood of mallard duck (Anas platyrhynchos) reared in the campus of Centurion University of Technology and Management of BBSR. This study may be helpful in determining the health and physiological status of mallard duck and may be helpful in preventing high mortality rate and increasing their survival rate. Certain factors like courtship, egg laying stage, hormonal effect, stress and parasitic infection affect the blood cells. This study may be helpful in detecting early signs of clinical pathology as well as presence of disturbance in their habitat (aquatic environment).
References
Albokhadaim I (2012) Haematological and some biochemical values of indigenous chickens in Al-Ahsa, Saudi Arabia during summer season. Asian J Poultry Sci 6:138–145
Artacho P, Soto-Gamboa M, Verdugo C, Nespolo RF (2007) Blood biochemistry reveals malnutrition in black-necked swans (Cygnus melanocoryphus) living in a conservation priority area. Comp Biochem Physiol, Part A: Mol Integr Physiol 146:283–290
Campbell TW (1988) Avian haematology and cytology. Iowa state university press, Ames, p 101
Dolka B, Włodarczyk R, Żbikowski A, Dolka IP, Szeleszczuk P, Kluciński W (2014) Hematological parameters in relation to age, sex and biochemical values for mute swans (Cygnus olor). Vet Res Commun 38:93–100
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin bromocresol green. Clin Chem Acta 31:87–96
Gayathri KL, Hegde SN (1994) Sexual differences in blood values of the pigeon, Columba livia. Comp Biochem Physiol B 109:219–224
Gornall AG, Bardawill CS, David MM (1949) Determination of serum protein by means of biuret reaction. J Biol Chem 177:751–766
Halie Y, Chanie M (2014) Comparative aspects of the clinical haematology of birds. A review. Brit J Poultry Sci 3(3):88–95
Keçeci T, Çöl R (2011) Haematological and biochemical values of the blood of pheasants (Phasianus colchicus) of different ages. Turk J Vet Anim Sci 35:149–156
Meiattini F, Prencipe L, Bardelli F, Giannini G, Tarli P (1978) The 4-hydroxybenzoate/4aminophenazone chromogenic systems used in the enzymic determination of serum cholesterol. Clin Chem 24:2161–2165
Nivia M, Sunil SN, Rathy R, Anilkumar TV (2015) Comparative cytomorphometric analysis of oral mucosal cells in normal, tobacco users, oral leukoplakia and oral squamous cell carcinoma. J Cytol 32(4):253–260
Orawan C, Aengwanich W (2007) Blood cell characteristics, haematological values and average daily gained weight of Thai indigenous, Thai indigenous crossbreed and broiler chickens. Pak J Biol Sci 10(2):302–309
Rodriguez P, Tortosa FS, Villafuerte R (2005) The effects of fasting and refeeding on biochemical parameters in the red-legged partridge (Alectoris rufa). Comp Biochem Physiol A Mol Integr Physiol 140:157–164
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6:24–27
Acknowledgements
The authors would like to express their thanks to the School of Applied sciences, Department of Zoology, Centurion University of Technology and Management, BBSR, Odisha, for providing facilities for carrying of current research programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This investigation followed all the guidelines for the care and use of animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gayatri, A., Subhashree, R., Swayamprava, D. et al. Effect of sex on biochemical, cytomorphometrical and haematological parameters of mallard duck, Anas platyrhynchos (Linnaeus, 1758). Comp Clin Pathol 29, 201–211 (2020). https://doi.org/10.1007/s00580-019-03026-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03026-6