Abstract
To determine the clinico-pathologic effects, safety and efficacy of diminazene aceturate repeat treatments, thirty adult albino rats were randomly assigned into six groups (A–F) of five rats each. Groups A–D were infected with 1.0 × 106 trypanosomes, while groups E and F served as uninfected controls. Groups A–E were treated once with 7 mg/kg Dinazene® on day 11 post-infection. Treatments were repeated once, twice and thrice in groups B–D respectively at 7 days interval. The serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, blood urea nitrogen, creatinine and conjugated bilirubin were assayed bi-weekly. Parasitaemia level was also monitored. An average pre-patent period of 7 days was recorded. Relapse infection was recorded on days 24, 31 and 24 following first, second and third treatments for groups A, B and C respectively. The serum levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, conjugated bilirubin and creatinine of the infected and repeatedly treated groups did not differ significantly from those of the control groups except for the groups in which relapse infection occurred. It was concluded that repeat treatments using 7 mg/kg diminazene aceturate was safe and protected against relapse after the fourth consecutive treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
African trypanosomosis has remained a serious challenge to human and livestock survival in sub-Saharan Africa (Obi et al. 2013), and the continual search for an effective preventive and chemotherapeutic action against the disease remains a mirage (Okochi et al. 2003). The disease is characterized by fluctuating parasitaemia, anaemia, pyrexia, subcutaneous oedema, dullness, production losses, lacrimation, corneal opacity, impaired immune response, reproductive disorders and death in untreated animals (Eke et al. 2017), thus impoverishing sub-Saharan Africa.
Chemotherapy using trypanocides is the most widely accepted means of controlling trypanosomosis either prophylactically or curatively (Shiferaw et al. 2015). However, no new drugs have been produced against the infection since the last five decades, leading to over-dependence on the few existing ones, thus precipitating resistance (Anene et al. 2006). In Nigeria, diminazene aceturate is the preferred drug for the treatment of animal trypanosomosis (Egbe-Nwiyi et al. 2003) because of its relatively high therapeutic index, rapid elimination and the low incidence of relapse (Holmes et al. 2004). However, the incidence of trypanocidal resistance and relapse following diminazene aceturate treatment is on the increase necessitating alternative strategies by most clinicians and veterinarians. One of such strategies commonly practised with some degree of success is the diminazene aceturate repeat treatment.
Diminazene aceturate is known to be toxic at high doses (Holmes et al. 2004) and has also been shown to accumulate in the excretory organs (liver and kidney) 48 h post administration (Onyelili and Anika 1989). It is therefore possible that since liver and kidney damage brings about the release of certain cellular enzymes and a subsequent upsurge in serum levels of these enzymes (Knoll 1998), diminazene aceturate–associated hepatotoxicity and nephrotoxicity may bring about changes in the serum levels of these enzymes following repeat treatments, despite its therapeutic merits.
This study therefore investigated the effect of diminazene aceturate (Dinazene®) repeat treatment in albino rats infected with Trypanosoma brucei with emphasis on efficacy, clinico-pathologic parameters and safety.
Materials and methods
Experimental animals, trypanosomes and drugs
Thirty (30) adult albino rats were obtained from the Laboratory Animal Unit of the Department of Veterinary Parasitology and Entomology, Faculty of Veterinary Medicine, University of Nigeria, Nsukka. The rats were kept in rat cages in the Laboratory Animal Unit of the Department of Veterinary Parasitology and Entomology, University of Nigeria, Nsukka. They were fed standard rat feed and given water ad libitum. They were acclimatized for 2 weeks before the commencement of the experiment. The Trypanosoma brucei used for this study was the Gboko strain obtained from the Nigerian Institute of Trypanosomosis Research (NITR) Zaria, Nigeria. The T. brucei was maintained by serial passage in mice prior to use.
Diminazene aceturate (Dinazene®; Vetindia Pharmaceuticals Limited) was the drug used at 7.0 mg/kg body weight.
Experimental design
The rats were arbitrarily allocated into six groups (A–F) consisting of five rats each. Groups A–D rats were inoculated intraperitoneally with 1.0 × 106 trypanosomes, while group E rats were the uninfected and treated control. Group F rats were neither infected nor treated. Pre-infection parameters—serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) and serum levels of blood urea nitrogen (BUN), creatinine and conjugated bilirubin (CB)—were determined. Blood samples were screened daily for parasitaemia following infection of rats in groups A–D using the rapid matching method (Herbert and Lumsden 1976). Serum samples were also collected bi-weekly and assayed for ALT, AST, ALP, creatinine, BUN and CB levels until 10 weeks (70 days) post-treatment using standard methods (Fawcett and Scott 1960; Klein et al. 1960; Reitman and Frankel 1975; Garber 1981; Fossati et al. 1983). At the peak of parasitaemia, i.e. day 11 post-inoculation (PI), groups A–E were treated once on day 11 post-infection (PI). Groups B, C and D rats were further treated on day 18, days 18 and 25, and days 18, 25 and 32 PI respectively. All treatments were done at 7.0 mg/kg body weight using Dinazene®.
Statistical analysis
Data generated were computed into means and standard error of means and then subjected to one-way analysis of variance (ANOVA). The least significant difference was used to separate the means, and probability values of < 0.05 were considered significant.
Results
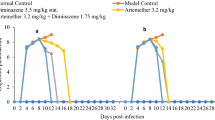
The parasites were first detected in groups A and D 4 days post-infection and became obvious in all the groups by day 7 post-infection. The parasitaemia reached a peak on the 11th day post-infection, and the rats were treated. Following treatment, a substantial drop (p < 0.05) in parasitaemia 24 h post treatment and total parasite clearance at 120 h (5 days) post-treatment were observed in all the treated groups (Fig. 1). However, relapse of infection occurred in 80% of the rats of group A 24 days post-treatment while relapse was recorded in 40% of rats in group B 31 days after the second treatment. Relapse was recorded in only one rat (20%) in group C 24 days after the third treatment. No relapse infection was recorded in group D.
Mean log parasitaemia of rats experimentally infected with Trypanosoma brucei and treated with repeated doses of diminazene aceturate (Dinazene®). Group A: infected with Trypanosoma brucei and treated once with 7 mg/kg DA. Group B: infected with Trypanosoma brucei and treated twice with 7 mg/kg DA. Group C: infected with Trypanosoma brucei and treated thrice with 7 mg/kg DA. Group D: infected with Trypanosoma brucei and treated four times 7 mg/kg DA
At week 11 PI, a considerably higher (p < 0.05) activity of ALT in groups B and C (Fig. 2) was detected compared with the control. By week 7 PI, the activities of serum AST of rats of groups A and B were appreciably higher (p ˂ 0.05) than those of group C, while at week 11 PI, the activities of serum AST of group B rats were considerably higher (p ˂ 0.05) than those of groups D, E and F (Fig. 3). The activities of serum ALP of rats did not vary statistically (p ˃ 0.05) across the groups over weeks 1, 3, 5, 9 and 11 PI (Fig. 4). Nevertheless, group A rats had a lower (p < 0.05) activity than groups B, C, D and F on week 7 PI.
By week 1 PI, the BUN level of rats in group E was higher (p ˂ 0.05) compared with that of groups C and F, while that of Group F was significantly lower (p ˂ 0.05) compared with the other groups at week 3 PI (Fig. 5). However, by week 11 PI, the BUN level of group B rats was appreciably higher (p ˂ 0.05) than that of the others.
The serum creatinine levels of groups B, C and E were notably lower (p < 0.05) than those of the others at week 3 PI (Fig. 6). At week 5 PI, despite a general decrease in the serum creatinine levels, group A rats had considerably higher creatinine levels (p ˂ 0.05) compared with group C. By week 7 and 11 PI, the serum creatinine levels of rats in group B were higher (p ˂ 0.05) than those of groups C, D and E. The serum CB levels did not vary substantially across the groups except at week 3 PI, when rats in group A had significantly lower serum CB levels compared with the other groups (Fig. 7).
Discussion
Seven (7) days pre-patent period was observed in this study, and it was in consonance with the results of other studies conducted using rats (Egbe-Nwiyi et al. 2003; Ezeokonkwo et al. 2007). Treatment with 7.0 mg/kg diminazene aceturate cleared the parasites within 5 days post-treatment. Similar findings have also been reported by Ezeh et al. (2016). This reduction in parasitaemia following treatment, which dragged over a period of 5 days, suggested that the trypanosomes may be resistant to the trypanocide or may be due to drug insufficiency leading to the relapse infections observed after 24 days of single treatment in group A, 31 days and 24 days following double and triple treatments in groups B and C respectively. Relapse infections in trypanosomosis have been reported to occur when parasites invade tissues which are not accessible to the trypanocidal drugs, i.e. inability of drugs to cross the blood-brain barrier (Ezeh et al. 2009; Holmes et al. 2004). It could also be that the parasite is no longer very susceptible to treatment using this compound as evidences abound to show incidences of trypanocidal resistance (Mungube et al. 2012; Shiferaw et al. 2015).
Diminazene aceturate achieves therapeutic concentration within 24–48 h of administration within which parasites in the blood are cleared (Peregrine 1994). Failure to achieve this may imply drug resistance or drug insufficiency. This resistance was obviously manifested in the fact that only the group that received four repeat treatments gained protection against relapse within the experimental observation period. On the other hand, the test drug (Dinazene®) may contain a sub-optimal concentration of the active principle (diminazene aceturate), which may be responsible for the perceived resistance. This is plausible as studies have also shown that 42–70% of veterinary drugs, principally trypanocides circulating in sub-Saharan Africa, have quality issues (Teko-Agbo et al. 2008) contributing to the menace and spread of drug-resistant trypanosomes in Nigeria and Africa in general.
Increases in activities of serum enzymes follow cellular damage and leakage of cellular contents into the extracellular environment in quantities above the thresholds (Knoll 1998). This increase may arise due to infections or to the introduction of toxins into the body. Though the trypanocide diminazene aceturate is known to produce toxicity in animals when used in high doses, to the best of our knowledge, information on their toxic effects following repeated use at normal dose is not available. From this study, the ALP, ALT, AST, CB, BUN and creatinine levels of the infected and repeatedly treated groups were comparable with the control groups. This implies that the repeat treatment with 7.0 mg/kg diminazene aceturate was probably well tolerated by the rats and may not have caused any marked organ damage.
It is also interesting to note the apparent elevations in the activities and levels of some of the serum enzymes in the single or double treatment groups as against the treatment groups that received higher doses. This could be attributed to relapse of the infection in those treatment groups, associated with high parasitaemia, which therefore rules out the possibility of toxicity due to repeat treatment using this brand of diminazene aceturate. Elevations of creatinine, ALP, BUN, AST, ALT and CB levels have also been widely reported in Trypanosoma brucei infected animals (Ezeokonkwo et al. 2010).
In conclusion, diminazene aceturate repeat treatments at 7.0 mg/kg protected against relapse infection following the fourth consecutive treatments, and even so, it was safe.
References
Anene BM, Brock JM, Barrett MP, Ezeokonkwo RC, Koning HP, Mmesirionye TI, Tettey JNA (2006) A diminazene-resistant strain of Trypanosoma brucei brucei isolated from a dog is cross-resistant to pentamidine in experimentally infected albino rats. Parasitol 132:127–133
Egbe-Nwiyi TN, Igbokwe IO, Onyelili PA (2003) The pathogenicity of Diminazene- resistant Trypanosoma brucei in rats after treatment with the drug. J Comp Pathol 128:188–191
Eke IG, Eze IO, Ezeudu TA, Eze UU, Anaga AO, Onyeyili PA (2017) Anti-trypanosomal activity of secnidazole in vitro and in vivo. Trop J Pharm Res 16:535–541
Ezeh IO, Agbo LI, Emehelu CO, Nweze EN, Ezeokonkwo RC, Onah DN (2009) Berenil-resistant Trypanosoma brucei brucei infection in a hunting dog in Nsukka area, Enugu state, Nigeria. Niger Vet J 29:34–42
Ezeh IO, Ugwu EN, Enemuo OV, Obi CF, Iheagwam CN, Ezeokonkwo RC, Onah DN (2016) Efficacy of repeated doses of diminazene aceturate (Dinazene) in the treatment of experimental Trypanosoma brucei infection of albino rats. Iran J Vet Res 17:124–129
Ezeokonkwo RC, Okoro FC, Ezeh IO (2007) The efficacy of increasing doses of Samorenil® in the treatment of Trypanosoma brucei infected albino rats. Niger Vet J 28:24–32
Ezeokonkwo RC, Ezeh IO, Onukwo JI, Obi PO, Onyenwe IW, Agu WE (2010) Comparative haematological study of single and mixed infections of mongrel dogs Trypanosoma congolense and Trypanosoma brucei brucei. Vet Parasitol 173:48–54
Fawcett JK, Scott JE (1960) A rapid and precise method for the determination of urea. J Clin Pathol 13:156–159
Fossati P, Prencipe L, Berti G (1983) Enzymatic creatinine assay: a new colorimetric method based on hydrogen peroxide measurement. Clin Chem 29:1494–1496
Garber CC (1981) Jendrassik-Grof analysis for total and direct bilirubin in serum with a centrifugal analyzer. Clin Chem 27:1410–1416
Herbert WJ, Lumsden WH (1976) Trypanosoma brucei: a rapid “matching” method for estimating the host’s parasitaemia. Exp Parasitol 40:427–431
Holmes PH, Eisler MC, Geerts S (2004) Current chemotherapy of animal trypanosomiasis. In: Maudin I, Holmes PH, Miles MA (eds) The trypanosomiasis. CAB International, Wallingford, pp 369–402
Klein B, Read PA, Babson LA (1960) Phenolphthalein monophosphate method for the evaluation of serum levels of alkaline phosphatase. Clin Chem 6:269–275
Knoll JS (1998) Collection and submission of laboratory samples, diagnostic procedures for the private practice laboratory. In: Aijello S (ed) The Merck veterinary manual. Merck and Co Inc., New Jersey
Mungube EO, Vitouley HS, Allegye-Cudjoe E, Diall O, Boucoum Z, Diarra B, Sanogo Y, Randolph T, Bauer B, Zessin K, Clausen PH (2012) Detection of multiple drug resistant Trypanosoma congolense populations in village cattle of south-east Mali. Parasit Vectors 5:155
Obi CF, Obidike IR, Ezeh IO, Omoja VU, Iheagwam CN, Idika IK, Ezeokonkwo RC (2013) Effects of Trypanosoma brucei infection and diminazene aceturate therapy on testicular morphology and function of Nigerian local dogs. Vet Parasitol 196:283–288
Okochi VI, Okpuzor J, Okubena MO, Awoyemi AK (2003) The influence of African herbal formula on the haematological parameters of trypanosome infected rats. Afr J Biotechnol 2:312–316
Onyelili PO, Anika SM (1989) Chemotherapy of Trypanosoma brucei brucei infection. Use of DMFO, diminazene alone and in combination. J Small Anim Pract 30a:505–510
Peregrine AS (1994) Chemotherapy and delivery stystems: haemoparasites. Vet Parasitol 54:223–248
Reitman S, Frankel S (1975) A colorimetric method for determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–62
Shiferaw S, Muktar Y, Belina D (2015) A review on trypanocidal drug resistance in Ethiopia. J Parasitol Vector Biol 7:58–66
Teko-Agbo A, Ndjana FM, Walbadet L, Akoda K, Niang EH, Abiola FA (2008) Quality of veterinary medicinal products in circulation in Cameron and Senegal. OIE Conference on Veterinary Medicinal Products in Africa, Dakar
Author information
Authors and Affiliations
Contributions
IOE and RCE designed the study. NEU, VOE, CFO, MIO, CNI and IOE carried out the laboratory experiment. IOE performed the statistical analysis, while NEU, CFO and IOE drafted the manuscript. All authors read, critically revised the manuscript for intellectual content and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Valid approval and ethical clearance were obtained from the Ethics Committee for Medical and Scientific Research of the University of Nigeria, Nsukka before the commencement of this study. Also, all applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ezeh, I.O., Ugwu, N.E., Obi, C.F. et al. Diminazene aceturate experimental repeat treatments in albino rats: efficacy and clinico-pathologic considerations. Comp Clin Pathol 28, 1527–1532 (2019). https://doi.org/10.1007/s00580-019-03009-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-03009-7