Abstract
Anthropogenic impact and environmental threats can cause diseases to marine turtles and in severe cases death, contributing to population decline worldwide. The Gulf of Ulloa (GU) represents an important foraging habitat for loggerhead sea turtles and olive ridley turtles; it is also considered a highly productive area for fisheries and is known for the mortality of marine turtles, which has been associated to bycatch. However, there is little information in the area regarding the health status of marine turtles; thus, the aims of this study were to (1) assess the health of marine turtles via physical examination, (2) generate their vital signs, (3) determine their hematological values, and (4) describe blood cell characteristics and its relationship with functional damage which can affect the organism’s systems. Clinical examinations were performed; complete blood count, clinical biochemistry, and clinicopathological evaluations were made, and their relationships with the functioning of the organism’s systems were described. With the sequential integration of medical and clinicopathological analysis, 56 loggerhead sea turtles and 16 olive ridley turtles from the GU were diagnosed as healthy. These pathological analyses are essential to evaluate marine turtles’ health, as well as to evaluate the health status of free-ranging populations, and have important applications for treatment and rehabilitation of sick and injured marine turtles. The baseline generated in this research provides information that can be taken as a reference for future research and used to generate management plans and conservation strategies for the organisms and for the ecosystem, together with the authorities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the different stages of marine turtles’ life cycle, these species can face a wide range of factors specific to their environment and anthropogenic origin that can cause them disease and in severe cases death (Reséndiz et al. 2018). Based on their life history, size, longevity, and interaction between the water-air interfaces, these organisms represent a growing interest group as a potential bioindicator of marine ecosystems; thus, they can provide useful information on environmental changes at spatial, temporal, and trophic scales since they are especially vulnerable to anthropogenic degradation of the environment (Aguirre and Lutz 2004). Thus, the health status and productivity of the habitat where they inhabit and develop can be inferred. Internationally, several clinicopathological studies have been carried out on marine turtles (Casal and Orós 2007; Stacy and Innis 2017); some authors have reported their values and propose these analyses as a diagnostic orientation tool to complement the health studies of these species (Casal and Orós 2009; Delgado et al. 2011; Fazio et al. 2012a).
The Pacific coast of Baja California Sur (BCS) and particularly the Gulf of Ulloa (GU) waters represent an important foraging habitat for juvenile and adult loggerhead sea turtles (Caretta caretta) and olive ridley turtles (Lepidochelys olivacea) (Peckham et al. 2008; Seminoff et al. 2014) which have also presented courtship and mating behavior (Merino-Zavala et al. 2018). In the GU, which is considered a highly productive area, fisheries represent the main economic activity and the interaction of fishing gear with non-target species, such as marine turtles, resulted in the establishment of the GU as a fishing refugee (Cepeda et al. 2012; DOF 2016). The GU is also known for the high mortality of marine turtles previously reported, which has been associated mainly to bycatch (Peckham et al. 2008); however, disease, anthropogenic impact, and environment changes are also contributing to population decline. Thus, the effect and extent of disease have been examined in several populations (Ward and Lafferty 2004). Despite these, there is little information regarding the health status of loggerhead sea turtles and olive ridley turtles in the GU; the knowledge of normal vital signs, hematologic and biochemical values, and cell morphology for marine turtles from this region would allow investigations of their condition and status. In this manuscript, the objectives were to (1) assess the health of marine turtles via physical examination and document clinical signs, (2) generate their vital sign values, (3) determine hematological values of loggerhead sea turtles and olive ridley turtles from the GU, and (4) describe blood cell structural characteristics, morphology, and its relationship with functional damage which can affect the organism’s systems. This baseline information will be a reference for future research and will provide supporting information to evaluate the health status of loggerhead and olive ridley turtles’ population from BCS in an integral and sequential way. In addition, this regional data will be comparable with values reported at the national and international levels.
Material and methods
Study site
The GU is located in the continental shelf of BCS, between 25 and 27° North latitudes and between 112 and 114° West longitudes, including the coast from Punta Abreojos south to Cabo San Lázaro (Funes-Rodríguez et al. 2000) with depths from 0 to 400 m (Ramírez Rodríguez et al. 2010). The GU is considered a biological active center (BAC), where the interaction of different oceanographic processes such as the upwelling and convergence of masses of water from the North, Central, and Eastern Tropical Pacific, as well as the California Current and the Mexican Current, leads to high and recurrent biological activity (Lluch Belda 2000); thus, this region is a hotspot for a wide variety of species, including marine turtles (Seminoff et al. 2014).
Sample size and capture technique
The study populations were loggerhead and olive ridley turtles caught daily in the GU during daylight hours from September to December 2016. All turtles were captured by hand modifying rodeo-jumping technique proposed by Limpus (1978).
Morphometric data
For each turtle, curved carapace length (CCL) (centimeters) and weight (kilograms) were obtained following the methodology proposed by Bolten (1999); the turtles were classified into age classes according to Peckham et al. (2008) and Márquez (2002) and by sex according to the sexual dimorphism criteria indicated by Wyneken (2001).
Health assessment
Clinical examination
The clinical evaluation and the generation of the vital signs, including heart rate (HR) and corporal temperature (carapace, plastron, groin, and cloacae), was carried out following the methodology described by Reséndiz and Lara-Uc (2018) and Reséndiz et al. (2018). To calculate the individual pulse rate in relation to their body weight, the following equation was used: X (·) (Y − 0.25), where X = beats per minute (beats/min) and Y = weight in kilograms (kg) (Martínez 1994).
Blood collection and preparation
Blood samples were collected from each turtle using methods previously described (Owens and Ruiz 1980) and were stored in two 6-ml vacutainer© tubes. The first tube included lithium heparin (He/Li) as an anticoagulant to determine complete blood count (Work and Balazs 1999). The second tube lacked anticoagulant in order to later recover serum and perform hepatic and renal function tests by clinical biochemistry (Reséndiz et al. 2018). The hematologic analytes and measurements were as follows: hemoglobin (HGB), hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), erythrocytes (E), thrombocytes (TH), leukocytes (LEU), lymphocytes (LYM), monocytes (MOs), eosinophils (EOs), heterophils (HET), and basophils (BA) as described previously by Flint et al. (2010) and Reséndiz et al. (2018). HGB was determined by a commercial kit Labtest®; HCT was manually measured filling a capillary of the microhematocrit with the routine technique (Stacy et al. 2011). The total E and LEU cell count was made in a Neubauer chamber, using Natt and Herrick methodology (Stacy et al. 2011). The E indices (MCV, MCH, and MCHC) were calculated from the total E count, HCT, and HGB. The number of TH per 1000 E was counted. Differential white cell count (LEU) was carried out through the blood smears made at the time of blood sample collection (two blood smears were prepared, air-dried, and then fixed with methanol). Later on, they were stained with a rapid blood staining kit Hemacolor® (Merck-Millipore®) at the Marine Botany laboratory at the UABCS. Blood smear slides were reviewed for LEU and identified and classified based on the morphological features (Casal and Orós 2007; Sykes and Klaphake 2008; Casal et al. 2009; Ramírez-Acevedo et al. 2012) using a microscope Olympus® CX31 with × 100 lens. Plasma was separated by an automatic centrifuge model Ecospin III® (Medical Econet®) at 3000 rpm for 15 min, pipetted into 2-ml sterile vials, and stored in a freezer at − 4° until transferred for further hepatic and renal function tests by clinical biochemical analysis. Plasma biochemical analytes were urea, blood urea nitrogen (BUN), creatinine, aspartate aminotransaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total protein (TP), lactate dehydrogenase (LDH), gamma-glutamyl transferase (GGT), albumin, globulin, and values of albumin/globulin according to Aguirre et al. (1995) and were assessed using a chemistry analyzer and commercial kits with the routine technique at Fidelis labs®.
Clinical pathology and morphometric analysis of blood cells
Blood smears used for these measurements were those used for differential counting. 50 E and 20 LEU from each turtle were measured using an image analysis program, Image-Pro Plus, version 7.0 (Media Cybernetics), obtaining the length, width, and perimeter of both cells and nucleus (Casal and Orós 2007; Orós et al. 2010; Zhang et al. 2011; Ramírez-Acevedo et al. 2012). The measurements were taken on the x-y axes of a Cartesian plane, with the largest measure being assigned as length (maximum length) and the smallest measure as width (minimum length) (Fei-Yan et al. 2011). The quotient of the division between the maximum length and minimum length is known as morphological index (MI), and values close to 1 represent rounded morphologies while values farther from 1 represent elliptical shapes (Ramírez-Acevedo et al. 2012). Due to the scarcity of basophils in the blood of both groups of turtles, it was not possible to determine their dimensions. Finally, the clinical pathological interpretation of the morphological characteristics of the cells was carried out based on the description proposed by Núñez and Bouda (2007) and its relationship with structural and functional systemic changes according to Trigo (2011), Núñez and Bouda (2007), Campbell (2012), Stacy and Innis (2017), and Reséndiz et al. (2018).
Data analysis
Descriptive statistics, mean, standard deviation (SD), and minimum and maximum values, were reported for CCL and weight; carapace, plastron, groin, and cloacae temperatures; hearth rate and pulse rate; and values for each blood elements.
Results
Morphometric data and vital signs
Among the 72 studied organisms, 56 loggerhead sea turtles were classified as juvenile and 16 olive ridley turtles were adults; loggerheads were registered as 32 females (F), 18 undefined (U), and 6 males (M), and olive ridleys were 11 F, 4 M, and 1 U. All the organisms were “clinically healthy”; bodyweight, CCL, and vital sign index for clinically healthy turtles are shown in Table 1. The clinical exams did not show evidence of clinical signs, lesions, or neoplasms that compromise the function of the organisms or threaten its health.
Blood values
Complete blood count (CBC) and hepatic and renal function tests by clinical biochemistry (CB) values are shown in Tables 2 and 3, respectively.
Clinical pathology
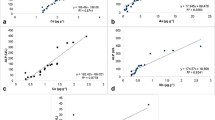
Blood cell description, morphology, and dimensions of blood cells are shown in Tables 4 and 5, respectively, and in Figs. 1, 2, 3, 4, and 5.
Discussion
Vital sign (corporal temperature, heart rate, and pulse rate according to the weight) results helped to determine immediate alterations in the basic functions of marine turtles, monitor health problems, and indicate the physiological state of core organs (Cunningham and Bradley 2009). These values indicate immediate functional changes in organisms that otherwise could not be qualified nor quantified (Barrett et al. 2010). Drastic changes in temperature can affect turtles’ immune system functions, making them vulnerable to threats such as infectious disease (Osborne et al. 2010) like fibropapillomatosis (FP) (Arthur et al. 2008; Van Houtan et al. 2014). Marine turtles’ core activities depend on environmental temperature; when it decreases, its metabolism and available energy decrease too, preventing them from developing their vital functions in low temperatures. Under 9 °C, they become lethargic and can be affected by cold stunning, resulting in death (Innis et al. 2007; Foley et al. 2007). In marine turtles, deep cloacal temperature is representative of the actual environmental temperature (Smith et al. 2000). According to the results for loggerhead sea turtles, there was evidence to affirm that deep cloacal and groin temperatures had the same average. For olive ridley turtles, carapace and plastron temperature had the same average temperature, as well as deep cloacal and groin temperatures. These values were similar to deep cloacal temperatures obtained by Sapsford and Hughes (1978), Southwood et al. (2003), and Norton (2005) from healthy marine turtles and are into the reference values for healthy Eastern Pacific green turtles (EPGT) in a nearby area (Reséndiz et al. 2018). In that context, based on the groin temperature values and with regard to the water average temperature in those months (22.5 °C), water temperature was discarded as a risk factor for cold stunning and FP, suggesting that marine turtles in the GU can carry out their physiological activities in a normal way during the sampling months. The results shown in this manuscript confirm the effectiveness of the digital infrared laser thermometer to monitor corporal temperature in the groin, being less invasive and stressful.
The HR matched with the values proposed by Southwood et al. (2003) and Norton (2005) for healthy marine turtles and are into the reference values for healthy EPGT in a nearby area (Reséndiz et al. 2018). As the HR obtained did not present considerable variations, physiological alterations that can led to tachycardia such as fatigue, excitation, digestive processes, or gravid females (Reséndiz et al. 2018) were discarded, and pathological processes like hyperthermia, hydremia, septicemia, and pericarditis in the case of the pathological disorders (Reséndiz et al. 2018) were eliminated. HR discarded bradycardia due physiological processes such as starvation or lethargy (Cunningham and Bradley 2009; Reséndiz et al. 2018) and due pathological processes such as cerebral compression, vagus nerve stimulation, and intoxication (Trigo 2011; Reséndiz et al. 2018). In marine turtles, the autonomic nervous system is the main determinant of pulse rate (Wyneken 2001); thus, in response to vagotonic stimuli, a bradysfixia will be presented and to sympatheticotonic stimuli, a tachysphyxia (Cunningham and Bradley 2009; Barrett et al. 2010). Pulse rate was within the reference values for healthy EPGT in a nearby area (Reséndiz et al. 2018) and it was considered regular. The data suggested that the succession of diastolic pauses was stable and continuous, and the duration between them was similar. Therefore, pathological causes such as arrhythmias due to variable blocks, polyextrasyses, or atrial fibrillation (Trigo 2011) were dismissed. Also, true intermittent pulse rates due to sinoatrial or atrioventricular blockages, false intermittent pulse rate by premature ventricular systoles, and/or hemodynamically inefficient extrasystoles (Trigo 2011; Reséndiz et al. 2018) were eliminated as pathological changes.
The CBC evaluation begins with the revision of the relationship between the HCT and the TP (Núñez-Ochoa 2007a; Stacy and Innis 2017), since several variants indicative of disease may be present, such as high HTC (erythrocytosis) with high TP (hyperproteinemia), normal range TP (normoproteinemia) or with low TP (hypoproteinemia); normal range HTC with high TP (hyperproteinemia), normal range TP (normoproteinemia) or with low TP (hypoproteinemia); and lastly, low HTC (anemia) with high TP (hyperproteinemia), normal range TP (normoproteinemia) or with low TP (hypoproteinemia) (Núñez-Ochoa 2007a). In these cases, the relationship of HCT and TP did not show any of the variants mentioned above. Blood biometrics from loggerhead sea turtles were similar and within the reference values reported internationally by Kakizoe et al. (2007), Casal and Orós (2009), Casal et al. (2009), Deem et al. (2009), Gelli et al. (2009), Basile et al. (2012), Fazio et al. (2012a), Alberghina et al. (2015), and Stacy et al. (2018) and to those previously reported in the area by Cordero-Tapia and Reséndiz Morales (2014) for healthy loggerhead sea turtles. Blood biometrics from olive ridley turtles were within the international reference values by Santaro and Meneses (2008), Zhang et al. (2011), and Ramakrishnan et al. (2018) and nationally by Ramírez-Acevedo et al. (2012) for healthy olive ridleys. However, blood values can vary according to different intrinsic (Pinto et al. 2015) and extrinsic factors (Stacy et al. 2018), as well as diseases, stress, and venipuncture site (Campbell 2012; Jenkins-Perez 2012). These numeric values will only be drastically increased or decreased by specific conditions such as diseases (Núñez and Bouda 2007), its relation to the status of the organism (Stacy and Innis 2017), and the presence of an etiological agent (Campbell 2012) among others.
Blood cell morphology was similar to previous reports (Work et al. 1998; Sykes and Klaphake 2008) in reptiles and other marine turtle species, for healthy loggerhead sea turtles (Casal and Orós 2007; Orós et al. 2010) and for healthy olive ridley turtles (Zhang et al. 2011; Ramírez-Acevedo et al. 2012; Ramakrishnan et al. 2018). The perimeter of both cell and nucleus were into the reference values for loggerhead sea turtles (Casal and Orós 2007; Orós et al. 2010); however, length and width measurements of E and TH varied from 1 to 4 μm, respectively, and although they were within the reference values, this only indicated that they had more elliptical or rounded shapes, respectively. Cell and nucleus morphology of olive ridley turtles coincided with previous reports for this species (Zhang et al. 2011; Ramírez-Acevedo et al. 2012). In these cases, the MI was similar to the data reported by Ramírez-Acevedo et al. (2012).
In the review of the different cells, immature E (in less quantity) were observed with characteristics similar to those previously reported by Ramírez-Acevedo et al. (2012). No intraerythrocyte inclusions were observed such as cytoplasmic spots and Howell-Jolley bodies; thus, pernicious anemia and splenic problems were dismissed. No Pappenheimer bodies were observed and excess of iron was discarded. No basophilic stippling was detected, and chronic anemia and intoxication were discarded (mainly by lead) (Mondragón-Vargas and Robles de la Torre 2007; Campbell 2012). No Cabot rings were spotted, and the possibility of exposure to hemolytic agents was eliminated (Mondragón-Vargas and Robles de la Torre 2007). There were no parasitic inclusions, so the presence of Theilera sp. was dismissed (Cordero-Tapia and Reséndiz Morales 2014); Heinz bodies were not perceived, and the presence of intraerythrocytic masses of denatured HGB by the action of oxidizing agents was discarded, as well as hemolytic anemia due to denaturation and precipitation of HGB (Mondragón-Vargas and Robles de la Torre 2007). Neither hemosiderin nor hemosiderosis was observed, so spleen, liver, bone marrow, and lymph node problems were eliminated (Mondragón-Vargas and Robles de la Torre 2007; Stacy and Innis 2017). In these reviews, no echinocytes (E with projections and cytoplasmic prolongations, irregular size, and shape) were observed, so glomerulonephritis and chronic toxicosis were dismissed (Mondragón-Vargas and Robles de la Torre 2007). There were no acanthocytes (E with prolongations of star-shaped membrane), and excessive cholesterol content was discarded in relation to the amount of phospholipids; with this, problems in the spleen and diffuse liver disease were dismissed, as well as portosystemic communications (Mondragón-Vargas and Robles de la Torre 2007). There was no presence of codocytes (abnormal E), and anemia due to iron deficiency was discarded as well as liver disease with cholestasis due to spleen problems and in hypothyroidism (Mondragón-Vargas and Robles de la Torre 2007). No oxidative damage was observed, and the possibility of damage to the E in at least three ways was eliminated: (1) by the oxidation of iron in the heme group, (2) by denaturing the protein part (globin) of HGB (Heinz bodies), and (3) by the oxidation of proteins that cross or are attached to the membrane (Mondragón-Vargas and Robles de la Torre 2007). There were no elliptocytes or ovalocytes (E with abnormal oval shape), and macrocytic anemia was discarded (Mondragón-Vargas and Robles de la Torre 2007). No schistosomes were found (fragmented E), distinctive in disseminated intravascular coagulation (DIC), and therefore problems such as uremia and malignant neoplasms were eliminated (Mondragón-Vargas and Robles de la Torre 2007). Stomatocytes were not spotted (E that in its clear central region have a mouth-shaped cleft), and hepatic problems and hemolytic anemia by autoantibodies were dismissed (Mondragón-Vargas and Robles de la Torre 2007; Stacy and Innis 2017). There was no presence of dacryocytes (abnormal E characterized by an elongated projection in a pole (tear form)); therefore, serious anemia and myelofibrosis with myeloid metaplasia, bone marrow problems, and extramedullary hematopoiesis were eliminated (Mondragón-Vargas and Robles de la Torre 2007). Anisochromia with hypochromia did not occur either, that is, when red blood cells have less color than normal, in consequence, it was inferred that there was enough pigment that carried oxygen (HGB) (Mondragón-Vargas and Robles de la Torre 2007). There were no cases of spherocytes (E that lost their biconcave shape), and immune-mediated hemolytic anemia of variable severity with the presence of peripheral blood spherocytes was eliminated (Mondragón-Vargas and Robles de la Torre 2007). No eccentrocytes (E with condensed HGB at its ends as a result of oxidative damage) were observed (Mondragón-Vargas and Robles de la Torre 2007; Campbell 2012).
Macrocytes (E larger than normal) were not observed, and deficiency in the ingestion or inadequate absorption of minerals and vitamins, as well as macrocytic, normal hypochromic anemia, problems in the synthesis of HGB, and regenerative anemia due to erythropoiesis, was dismissed (Jardón-Herrera 2007). Microcytes (E smaller than normal) were not present, and the deficiency of minerals such as copper, iron, and vitamins (pyridoxine and riboflavin) was discarded (Mondragón-Vargas and Robles de la Torre 2007). There were no cases of normocytes; therefore, the possibility of chronic protein deficiency was eliminated, which could lead to normocytic normochromic anemia, that is, when the E size and the amount of HGB are normal but the total number of E is diminished (Mondragón-Vargas and Robles de la Torre 2007; Ordóñez-Badillo 2007a). There was no megalocytosis and the possibility of megaloblastic anemia was eliminated. There was no poikilocytosis, and the presence of E in different shapes was dismissed, which is indicative of iron deficiency anemia and megaloblastic anemia due to vitamin deficiency (Ramírez-Díaz 2007; García-Escamilla 2007).
No disorders were observed in the cellular arrangements of the E such as agglutination (cluster). Therefore, the presence of antibodies against the erythrocytic membrane and immune-mediated anemia was discarded (Mondragón-Vargas and Robles de la Torre 2007; Stacy and Innis 2017). The accommodation of the E in the form of batteries or coins (rouleaux) was not observed, and an increase in inflammatory proteins was dismissed as well as lesions causing inflammation (Mondragón-Vargas and Robles de la Torre 2007; Campbell 2012). There was no natural destruction of E, which can be due to the lack of organelles or to the loss of their capacity to synthesize new membrane components; when they go through the circulation (especially in the spleen), E usually lose part of the plasmalemma, their enzymatic reserves are used up, and, over time, they adopt the spherical shape (Campbell 2012). Consequently, E do not tolerate the great deformation necessary to perform its function and they become more fragile, or after a half-life (which varies according to the species), the E modified by age are eliminated from the bloodstream and are degraded by macrophages from the liver and bone marrow but mainly from those of the spleen (Mondragón-Vargas and Robles de la Torre 2007). The iron released from the HGB is used again and, together with the iron in the diet, enters the production of new HGB for the new E. The non-iron part of the heme is transformed into the bile pigment called bilirubin. The globin portion of HGB is degraded to free amino acids, which become part of the organisms’ stock of amino acids (Mondragón-Vargas and Robles de la Torre 2007; Campbell 2012). There was also no E destruction by biochemical pathways such as (1) “Embden-Meyerhof pathway,” (2) “hexose monophosphate pathway,” (3) “methemoglobin-reductase pathway,” and (4)“Luebering-Rapoport pathway” (Mondragón-Vargas and Robles de la Torre 2007; Ramírez-Díaz 2007; Campbell 2012).
The morphology of the E observed in relation to the HCT suggests that there was no anemia, dehydration, hemorrhages, and deficiency of vitamins and minerals (Núñez-Ochoa 2007a; Stacy et al. 2011; Campbell 2012; Stacy and Innis 2017). The relationship between the morphological characteristics of the E and the numerical values obtained allowed to discard erythrocytosis and may confirm that there were no renal and heart diseases, drowning threats, and polycythemia (Mondragón-Vargas and Robles de la Torre 2007; Ordóñez-Badillo 2007a). These values and descriptions suggested there were no erythropenia, autoimmune diseases, infections, and neoplasms (Jardón-Herrera 2007; Mondragón-Vargas and Robles de la Torre 2007).
The values of HGB obtained, in regard to the morphology and numerical values of the E, indicated that there were no problems in the transport of oxygen from the lungs to the tissues and carbon dioxide in the opposite direction; this indicates that there were no faults in the regulation of the acid-base balance by the elimination of carbon dioxide from the lungs and by the buffering action of the globin imidazole and histidine groups (Ordóñez-Badillo 2007a; Stacy and Innis 2017). These elements together indicate that there were no hemoglobinopathies or dyshemoglobinemias, such as methemoglobin by the presence of oxidizing substances or carboxyhemoglobin by the combination of hemoglobin with carbon monoxide. HGB values indicated that there was no bleeding in the digestive tract (Mondragón-Vargas and Robles de la Torre 2007; Ordóñez-Badillo 2007a), for example, by the presence of hooks, neither hypoxia nor pulmonary lesions (Campbell 2012; Stacy and Innis 2017).
The E indices obtained (the relationship between HCT, HGB, and E) dismissed the presence of anemia, and related to the size, morphology, and characteristics of the E and the VCM, indicate that there was no microcytic and macrocytic anemia (Mondragón-Vargas and Robles de la Torre 2007; Ramírez-Díaz 2007). The characteristics and morphology of the E in relation to the numerical values of MCH indicated that the turtles evaluated did not present hypochromic and hyperchromic anemia (Ramírez-Díaz 2007; Fazio et al. 2012a). These characteristics together with the morphology in relation to the MCHC numeric values obtained suggested that there were no iron anemia deficiency, blood loss, neoplasms in the digestive tract, or hepatic problems (Ramírez-Díaz 2007; Núñez-Ochoa 2007a). The observed characteristics, morphology, MI, and normal disposition of E, in relation to the morphology and MI of the TH as well as the numerical values obtained from both, discarded hemorrhagic and thrombotic events, thrombocytopenia, and thrombocytosis (Ramírez-Díaz 2007; García-Escamilla 2007). These also dismissed autoimmune disorders, serious injuries (like blows by propellers) in addition to marrow defects that limit adequate thrombocytopoiesis, immune-mediated thrombocytosis, TH consumption during DIC, and deficiency of some of the coagulation factors (García-Escamilla 2007).
The characteristics and morphology described, as well as the number of LEU obtained, indicate that there was no leukocytosis; therefore, there was no presence of infectious and inflammatory diseases, severe physical stress, or tissue damage (Núñez-Ochoa 2007b; Stacy and Innis 2017); leukopenia was dismissed indicating that there were no autoimmune diseases (Martínez-Silvestre et al. 2011), nor hepatic, renal, and spleen pathological changes (Quiroz-Rocha and Bouda 2007; Campbell 2012; Jenkins-Perez 2012). LYM presented similar characteristics and morphology to those described in other species of healthy marine turtles and reptiles (Sykes and Klaphake 2008); these characteristics, numerical values, and MI were into the reference intervals according to Work et al. (1998), Casal et al. (2009), Fei-Yan et al. (2011), and Ramírez-Acevedo et al. (2012) and dismissed lymphocytosis; therefore, struggle and lymphocytosis in young animals by physiological reasons (Núñez-Ochoa 2007b; Campbell 2012) were discarded. There was no lymphopenia, indicating that there were no severe stress, endocrine diseases, viral infections (Martínez-Silvestre et al. 2011; Campbell 2012), lymphangiectasia, and quilotorax (Núñez-Ochoa 2007b). According to MO characteristics, morphology, MI, and numerical values, chronic and granulomatous inflammations, tissue degradation, and stress were dismissed (Núñez-Ochoa 2007b; Reséndiz et al. 2018). EO characteristics, morphology, and MI were similar to those previously reported (Casal et al. 2009; Fei-Yan et al. 2011; Ramírez-Acevedo et al. 2012). It should be noted that it was the only cell type that had notable differences in the size of intracytoplasmic granules, although it was considered a normal characteristic (Jardón-Herrera 2007). These integrated with its numerical values and the other analyses allowed to discard eosinophilia, indicating no stress, no cases of parasitosis, no tissue degradation, and no hypoadrenocorticism (Campbell 2012; Stacy and Innis 2017). HET morphology was consistent with previous reports for healthy marine turtles (Casal et al. 2009; Fei-Yan et al. 2011). No toxic HET were observed, which indicated that there were not inflammatory processes such as (1) local basophilia (Döhle bodies), (2) diffuse basophilia, (3) vacuolation with diffuse basophilia, (4) toxic granulation, and (5) giant HET (Jardón-Herrera 2007). In relation to its MI and together with its numerical values, it was confirmed that there were no inflammatory processes either (Jardón-Herrera 2007; Ramírez-Acevedo et al. 2012). The “left shift” was not observed in the HET evaluation, which indicated that there was no increase in the absolute values or in the percentage of immature HET. Therefore, there was no presence of inflammation due to an increase in the demand for HET, with the bone marrow being unable to cover it, which results in the release of immature cells into the circulation (Jardón-Herrera 2007). The “right shift” was also dismissed, that is, the increase in nuclear lobes in HET; therefore, there was no lengthy presence of the HET in the circulation by hyperadrenocorticism, or destabilization of the cell membranes, which inhibits the HET migration towards the tissues (Jardón-Herrera 2007). In these cases, there were no pathological changes caused by bacteria and fungi (Ramírez-Díaz 2007; Stacy and Innis 2017). During this study, BA were not observed; thus, there was no response to diseases nor antigen-specific production or mastocytemia (Núñez-Ochoa 2007b).
Numerical values of hepatic and renal function tests were also similar and within the reference values to those reported internationally by Flint et al. (2010), Fazio et al. (2012b), and Söbílen and Kaska (2018) and in the area by Cordero-Tapia and Reséndiz Morales (2014), Ley-Quiñones et al. (2017), and Espinoza-Romo et al. (2018) for healthy loggerhead and olive ridley turtles. The concentration of proteins in the plasma indicates functions of the hormonal, nutritional, water balance, and other factors that affect the health status, as well as the status of renewal of the different proteins. Therefore, the interpretation of protein concentrations in blood should consider the general data of the organisms, clinical history data, and information about the current problem (Mondragón-Vargas 2007).
The measurement of the chemical elements that compound the blood with other lab procedures and clinical exams helps to diagnose, to emit a prognosis, and to evaluate the efficiency of a treatment (Stacy et al. 2011). Collectively, plasma proteins perform a nutritive function, exert colloid osmotic pressure, and help maintain acid-base balance. Individually, they work as enzymes, coagulation factors, hormones, and transport substances (Latimer et al. 2005; Osborne et al. 2010). In these cases, hyperproteinemia was not observed, neither hyperglobulinemias associated with chronic inflammatory diseases, hyperalbuminemias, nor hemolysis (Campbell 2012; Stacy and Innis 2017). Hypoproteinemia was not observed; thus, possible cases of chronic malnutrition, protein malabsorption, poor digestion, protein-losing enteropathies (parasitism), blood loss, chronic hepatitis, or renal diseases (Mondragón-Vargas 2007; Quiroz-Rocha and Bouda 2007) were all dismissed.
The values of albumin were within the reference values according to Casal et al. (2009) and Söbílen and Kaska (2018), indicating that there were no dehydration, no alterations in protein synthesis (starvation, bad absorption of the small intestine, hepatic processes, and severe trauma), nor a decrease in protein synthesis from the kidney, intestine, hemorrhage, and sepsis (Mondragón-Vargas 2007; Ordóñez-Badillo 2007a, b). Globulin values were within the reference values at international level proposed by Kelly et al. (2015) and Söbílen and Kaska (2018) in juvenile loggerhead sea turtles, Ley-Quiñones et al. (2017) in BCS for loggerhead sea turtles, and for Espinoza-Romo et al. (2018) in olive ridley turtles from Sinaloa. Therefore, possible cases of hyperglobulinemia and hypoglobulinemia were dismissed (Mondragón-Vargas 2007; Núñez-Ochoa 2007a, b). The A/G ratio obtained did not show a difference with Kelly et al. (2015), Söbílen and Kaska (2018), Ley-Quiñones et al. (2017), and Espinoza-Romo et al. (2018) in a nearby area; thus, possible cases of renal proteinuria or production of immunoglobulins by antigenic stimulation were dismissed (Mondragón-Vargas 2007; Ramírez-Díaz 2007). Urea values rise due to increased protein degradation, which is caused by intestinal hemorrhage, necrosis, hyperthyroidism, and others. These values can also be increased by a reduction of renal perfusion (shock and hypoalbuminemia), for acute or chronic renal insufficiency and for obstruction of urinary flow, while values decrease if there is abnormal hepatic function or by reduced protein intake (Quiroz-Rocha and Bouda 2007; Campbell 2012; Stacy and Innis 2017). In this study, urea levels were similar to those reported by Kelly et al. (2015) and Söbílen and Kaska (2018) internationally, in the same place for Cordero-Tapia and Reséndiz Morales (2014) and Ley-Quiñones et al. (2017) in loggerhead sea turtles and for Espinoza-Romo et al. (2018) in olive ridley turtles in a nearby zone. The results of creatinine did not show difference to the ones proposed by Deem et al. (2009), Kelly et al. (2015), and Söbílen and Kaska (2018) for healthy loggerhead sea turtles; therefore, acute and chronic renal failure, obstruction of the primary urinary flow, and rupture of the bladder (Bouda and Quiróz 2007; Campbell 2012) were dismissed. AST values observed were within the limits reported by Cordero-Tapia and Reséndiz Morales (2014), Ley-Quiñones et al. (2017), and Espinoza-Romo et al. (2018) for healthy loggerhead sea turtles and olive ridley turtles in similar conditions and age class, so hepatic damage, skeletal and cardiac muscle damage (ischemia), septicemia, and toxemia (Ordóñez-Badillo 2007b) were dismissed. The results of ALT were within the reference values proposed internationally by Deem et al. (2009) and Söbílen and Kaska (2018) for healthy loggerhead sea turtles and for Ley-Quiñones et al. (2017) and Espinoza-Romo et al. (2018) in Mexico; therefore, hepatic failures (Ordóñez-Badillo 2007b; Quiroz-Rocha and Bouda 2007) were dismissed. ALP values were similar to those obtained by Deem et al. (2009) and Fazio et al. (2012b) and to those described by Cordero-Tapia and Reséndiz Morales (2014) and Ley-Quiñones et al. (2017) in BCS and Espinoza-Romo et al. (2018) in Sinaloa, so it was inferred that there was no biliary obstruction, extensive or generalized bone disease, neoplasms, or septicemia (Ordóñez-Badillo 2007b; Stacy and Innis 2017). BUN levels did not show noticeable changes with the data of Deem et al. (2009), Basile et al. (2012), and Söbílen and Kaska (2018), and locally with Ley-Quiñones et al. (2017) and Espinoza-Romo et al. (2018); thus, heart failure, excessive levels of protein in the digestive tract, and renal diseases, as well as malnutrition and hepatic failure (Quiroz-Rocha and Bouda 2007), were dismissed. Similar values of LDH were observed to those reported by Kelly et al. (2015), Delgado et al. (2011), and Söbílen and Kaska (2018) at international level, and Cordero-Tapia and Reséndiz Morales (2014), Ley-Quiñones et al. (2017), and Espinoza-Romo et al. (2018) in Mexico. Thus, blood flow deficiency, hepatic diseases, muscle injury, or muscle weakness (Ordóñez-Badillo 2007b; Campbell 2012) and abnormal formation of new tissues (neoplasms) (Reséndiz et al. 2018) were dismissed.
With the integration of the results obtained, 56 juvenile loggerhead sea turtles and 16 adult olive ridley turtles from the GU were diagnosed as “healthy.” The analysis of CBC and hepatic and renal function tests, when related to the clinical examinations, vital signs, cell morphology, characteristics, and MI, indicated an absence of systemic, autoimmune and metabolic diseases, nutritional problems, neoplasms, and traumatisms.
Conclusion
The values of vital signs, hematological reference values, and the characteristics and morphology of blood cells for loggerhead sea turtles and olive ridley turtles in the GU (baseline) were generated and can be used as a reference for future research.
Based on the physical examination, the vital signs, and the hematological results (numerical values, characteristics, morphology, and morphological index), it was concluded that the condition of the stock of turtles evaluated was healthy.
Numerical values of CBC and hepatic and renal function tests are an orientation tool for diagnosis; it is not an effective method to correctly or completely diagnose the health status of marine turtles; sequential integration of more clinical studies is needed.
Numerical values of CBC and hepatic and renal function tests will only be drastically increased or decreased by specific conditions such as diseases, and according to their progression and chronicity, and in relation to the status of the organism, the presence of a damage or an etiological agent, and its pathogenic capacity, among others; these values depend and are related to the morphological changes of the blood cells.
These analyses are essential to evaluate marine turtles’ health and have important applications for treatment and rehabilitation of sick and injured marine turtles.
This research provides information that can be taken as a reference to generate management plans and conservation strategies for the organisms and for the ecosystem, together with the corresponding authorities.
References
Aguirre A, Lutz P (2004) Marine turtles as sentinels of ecosystem health: is fibropapillomatosis an indicator? EcoHealth 1:275–283
Aguirre AA, Balazs GH, Spraker T, Gross T (1995) Adrenal and hema- tological responses to stress in juveniles green turtles (Chelonia mydas) with and without fibropapillomas. Physiol Zool 68:831–854
Alberghina D, Panzera M, Maccarrone M, Spadola F, Insacco G, Piccione G (2015) Study of some blood parameters in Caretta Caretta during a recovery period. Comp Clin Path 24:193–195. https://doi.org/10.1007/s00580-014-1923-9
Arthur K, Limpus C, Balazs GH, Capper A, Udy J, Shaw G, Keuper- Bennett U, Bennett P (2008) The exposure of green turtles (Chelonia mydas) to tumour promoting compounds produced by the cyanobacterium Lyngbya majuscula and their potential role in the aetiology of fibropapillomatosis. Harmful Algae 7:114–125. https://doi.org/10.1016/j.hal.2007.06.001
Barrett K, Barman S, Boitano S, Brooks H (2010) Fisiología Médica. McGraw-Hill Interamericana, México
Basile F, Di Santi A, Ferretti L, Bentivegna F, Pica A (2012) Hematology of the Mediterranean population of sea turtle (Caretta caretta): comparison of blood values in wild and captive, juvenile and adult animals. Comp Clin Path 21:1401–1406
Bolten A (1999) Techniques for measuring sea turtles. In: Eckert K, Bjorndal K, Abreu-Grobois A, Donnelly M (eds) Research and management techniques for the conservation of sea turtles, IUCN/ SSC marine turtle specialist group publication no. 4 pp. 126–131
Campbell TW (2012) Clinical pathology of reptiles. In: Thrall MA, Weiser G, Allison R, Campbell T (eds) Veterinary hematology and clinical chemistry, 2nd edn. John Wiley & Sons Inc, Ames, pp 601–604
Casal AB, Orós J (2007) Morphologic and cytochemical characteristics of blood cells of juvenile loggerhead sea turtles (Caretta caretta). Res Vet Sci 82(2):158–165
Casal AB, Orós J (2009) Plasma biochemistry and haematology values in juvenile loggerhead sea turtles undergoing rehabilitation. Vet Rec 164(21):663–665
Casal AB, Camacho M, López-Jurado LF, Juste C, Orós J (2009) Comparative study of hematologic and plasma biochemical variables in Eastern Atlantic juvenile and adult nesting loggerhead sea turtles (Caretta caretta). Vet Clin Pathol 38:213–218
Cepeda MF, Crespo D, Sanjurjo E (2012) Planeación para la conservación de la tortuga amarilla o caguama (Caretta caretta) en el Golfo de Ulloa, Baja California Sur. World Wildlife Fund, México
Cordero-Tapia A, Reséndiz Morales E (2014) Reporte Médico y Forense de la Tortuga Amarilla (Caretta caretta) en Bahía de Ulloa B.C.S. México. In: Informe final de investigación. Proyecto: Estudio Sobre las Causas de Muerte de la Tortuga Amarilla (Caretta caretta) en la Costa Occidental de Baja California Sur (Golfo De Ulloa). CONACYT, UABCS, CICIMAR, CIB-Nor pp. 115, 122–195. DOI: 10.13140/ RG.2.1.4091.3523: http://entorno.conanp.gob.mx/documentos/INFORME_FINAL_PROYECTO_MORT_T_AMARILLA_GOLFO_ULLOA1_CONANP_UABCS_CIBNOR_CICIMAR-JUNIO_2014.pdf
Cunningham JG, Bradley GK (2009) Fisiología Veterinaria, 4a edn. Elsevier, Barcelona
Deem SL, Norton TM, Mitchell M, Segars A, Alleman AR, Cray C, Poppenga RH, Dodd M, Karesh WB (2009) Comparison of blood values in foraging, nesting, and stranded loggerhead turtles (Caretta caretta) along the coast of Georgia, USA. J Wildl Dis 45:41–56
Delgado C, Valente A, Quaresma I, Costa M, Dellinger T (2011) Blood biochemistry reference values for wild juvenile loggerhead sea turtles (Caretta caretta) from Madeira Archipelago. J Wildl Dis 47:523–529
Diario oficial de la federación DOF (2016) Acuerdo por el que se establece la zona de refugio pesquero y nuevas medidas para reducir la posible interacción de la pesca con tortugas marinas en la costa occidental de Baja California Sur. Diario Oficial de la Federación de México. http://dof.gob.mx/nota_detalle.php?codigo. Accessed 22 Nov 2017
Espinoza-Romo BA, Sainz-Hernández JC, Ley-Quiñónez CP, Hart CE, Leal-Moreno R, Aguirre AA et al (2018) Blood biochemistry of olive ridley (Lepidochelys olivacea) sea turtles foraging in northern Sinaloa, Mexico. PLoS One 13(7):e0199825. https://doi.org/10.1371/journal.pone.0199825
Fazio E, Liotta A, Medica P, Giacoppo E, Ferlazzo A (2012a) Effects of different health status on blood haematochemical values of loggerhead sea turtles (Caretta caretta). Comp Clin Path 21:105–109
Fazio E, Liotta A, Medica P, Bruschetta G, Ferlazzo A (2012b) Serum and plasma biochemical values of health loggerhead sea turtles (Caretta caretta). Comp Clin Path 21:905–909
Fei-Yan A, Pi–Peng L, He-Xiang G, Ming-Bin Y (2011) Hematology, morphology, and ultrastructure of blood cells of juvenile olive ridley sea turtles (Lepidochelys olivacea). Chelonian Conserv Bi 10(2):250–256
Flint M, Morton JM, Limpus CJ, Patterson-Kane JC, Mills PC (2010) Reference intervals for plasma biochemical and hematologic measures in loggerhead sea turtles (Caretta caretta) from Moreton Bay, Australia. J Wildl Dis 46:731–741
Foley A, Singel K, Dutton P, Summers T, Redlow A, Lessman J (2007) Characteristics of a green turtle Chelonia mydas assemblage in northwestern Florida determined during a hypotermic stunning event. Gulf Mex Sci (2):131–143
Funes-Rodríguez R, Hernández-Rivas ME, Saldierna-Martínez RJ, Hinojosa-Medina AT, Avendaño-Ibarra R, Jiménez-Rosenberg SPA (2000) Composición y abundancia del ictioplancton del Golfo de Ulloa, Baja California Sur, un Centro de Actividad Biológica. In: Lluch Belda D, Elourdy GJ, Lluch-Cota S, Ponce DG (eds) BAC, Centros de Actividad Biológica del Pacífico Mexicano. CIB–Nor, CICIMAR, CONACYT, pp. 185–197
García-Escamilla RM (2007) Hemostasia. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 68–75
Gelli D, Ferrari V, Zanella A, Arena P, Pozzi L, Nannarelli S, Vaccaro C, Bernardini D, Romagnoli S (2009) Establishing physiological blood parameters in the loggerhead sea turtle (Caretta caretta). Eur J Wildlife Res 55:59–63
Innis C, Tlusty M, Merigo C, Weber ES (2007) Metabolic and respiratory status of cold-stunned Kemp’s ridley sea turtles (Lepidochelys kempii). J Comp Physiol B 177:623–630
Jardón-Herrera G (2007) Hematopoyesis. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 27–34
Jenkins-Perez J (2012) Hematologic evaluation of reptiles: a diagnostic mainstay. Vet Tech 33(8)
Kakizoe Y, Sakaoka K, Kakizoe F, Yoshii M, Nakamura H, Kanou Y, Uchida I (2007) Successive changes of hematologic characteristics and plasma chemistry values of juvenile loggerhead turtles (Caretta caretta). J Zoo Wildl Med 38(1):77–84
Kelly TR, McNeill JB, Avens L, Hall AG, Goshe LR, Hohn AA, Godfrey MH, Mihnovets AN, Cluse WM, Harms CA (2015) Clinical pathology reference intervals for an in-water population of juvenile loggerhead sea turtles (Caretta caretta) in Core Sound, North Carolina, USA. PLoS One 10:e0115739. https://doi.org/10.1371/journal.pone.0115739
Latimer K, Mahaffey E, Prasse K (2005) Patología Clínica Veterinaria, 4th edn. Duncan & Prasse’s, Oxford
Ley-Quiñones CP, Rossi-Laferriere N, Espinoza-Carreon TL, Hart C, Peckham H, Aguirre AA, Zavala-Norzagaray AA (2017) Associations between trace elements and clinical health parameters in the North Pacific loggerhead sea turtle (Caretta caretta) from Baja California Sur, Mexico. Environ Sci Pollut Res 24(10):9530–9537
Limpus CJ (1978) The reef: uncertain land of plenty. In: Lavery HJ (ed) Exploration north—a natural history of Queensland. Lloyd O’Neill Pty Ltd, Sydney, p 243
Lluch Belda D (2000) Centros de Actividad Biológica en la Costa Occidental de Baja California. In: Lluch Belda D, Elourdy GJ, Lluch-Cota S, Ponce DG (eds) BAC. Centros de Actividad Biológica del Pacífico Mexicano, CIB-Nor, CICIMAR, CONACYT p 367
Márquez R (2002) La vida de las tortugas marinas. In: Las tortugas marinas y nuestro tiempo. 3a Ed. La ciencia para todos, México 32–92
Martínez A (1994) Manual Clínico de Reptiles. Grass Iatros Ediciones, Madrid
Martínez-Silvestre A, Lavín S, Cuenca R (2011) Hematology and blood cytology in reptiles. Clin Vet Peq Anim 31(3):131–141
Merino-Zavala AS, Reséndiz E, Hernández-Gil Y, Lara-Uc MM (2018) First report of courtship and mating behavior by loggerhead sea turtle (caretta caretta) in Gulf of Ulloa, Baja California Sur, México. Lat Am J Aquat Res 46(1):237–239
Mondragón-Vargas RL (2007) Disproteinemias. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 89–100
Mondragón-Vargas RL, Robles de la Torre P (2007) Eritrocitos. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 35–41
Norton T (2005) Chelonian emergency and critical care. Topics in medicine and surgery. Semin Avian Exot Pet Med 14(2):106–130
Núñez L, Bouda J (2007) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México
Núñez-Ochoa L (2007a) Relación del hematocrito y las proteínas totales. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 42–44
Núñez-Ochoa L (2007b) Leucocitos. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 55–62
Ordóñez-Badillo ML (2007a) Eritrocitosis. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 45–48
Ordóñez-Badillo ML (2007b) Enzimas. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 96–100
Orós J, Casal A, Arencibia A (2010) Microscopic studies on characterization of blood cells of endangered sea turtles. In: Méndez-Vilas A, Díaz J (eds) Microscopy: science, technology, applications and education 1(4):75–84
Osborne AG, Jacobson ER, Bresette MJ, Singewald D, Scarpino R, Bolten AB (2010) Reference intervals and relationships between health status, carapace length, body mass, and water temperature, and concentrations of plasma protein and protein fractions of the Atlantic loggerhead sea turtle (Caretta caretta) and the green turtle (Chelonia mydas). J Am Vet Med Assoc 237:561–567
Owens DW, Ruiz GJ (1980) New methods of obtaining blood and cerebrospinal fluid from marine turtles. Herpetologica 38:17–20
Peckham S, Maldonado-Díaz D, Koch V, Mancini A, Gaos A, Tinker M (2008) High mortality of loggerhead turtles due to bycatch, human consumption and strandings at Baja California Sur, Mexico, 2003 to 2007. Endanger Species Res 5(2): 171–183. http://www.int-res.com/articles/esr2008/5/n005p171.pdf.
Pinto FE, Buzin AR, Neto EP, Ferreira GB, Castheloge VD, Ferreira PD et al (2015) A hematologic and biochemical profile on 3-month old hatchlings of Lepidochelys olivacea. Comp Clin Path 24(6):1333–1337
Quiroz-Rocha, Bouda J (2007) Patología Clínica de Hígado. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 122–137
Ramakrishnan A, Palanivelrajan M, Thangapadiyan M, Sumathi D, Senthilkumar K (2018) Haematology analysis of rescued olive ridley sea turtles (Lepidochelys olivacea). J Entomol Zool Stud 6(3):1128–1131
Ramírez Rodríguez M, de la Cruz G, Marín E, Ojeda MA, Ponce G (2010) Estudio sobre la caracterización socioeconómica y pesquera del área del Golfo de Ulloa, Baja California Sur, Informe Técnico Final del Proyecto. CICIMAR, México.
Ramírez-Acevedo LM, Martinez Blas SS, Fuentes-Mascorro G (2012) Hemogram and morphological characteristics of blood cells in the olive ridley turtle (Lepidochelys olivacea) of Oaxaca. México Rev cient Fac Cienc Vet 22(5):468–476
Ramírez-Díaz G (2007) Anemia. In: Núñez L, Bouda J (eds) Patología Clínica Veterinaria, 2nd edn. Facultad de Medicina Veterinaria y Zootecnia UNAM, México, pp 49–54
Reséndiz E, Lara-Uc MM (2018) Health assessments in free-ranging sea turtles: perspective of animal welfare. In: Abubakar M, Manzoor S (eds) Wildlife, Animal Welfare, IntechOpen, pp 29–49. https://doi.org/10.5772/intechopen.76111 Available from: https://www.intechopen.com/books/animal-welfare/health-assessments-in-free-ranging-sea-turtles-perspective-of-animal-welfare-in-wildlife
Reséndiz E, Fernández-Sanz H, Lara-Uc MM (2018) Baseline health indicators of Eastern Pacific green turtles (Chelonia mydas) from Baja California Sur, Mexico. Comp Clin Path 27(5):1309–1320
Santaro M, Meneses A (2008) Haematology and plasma chemistry of breeding olive ridley sea turtles (Lepidochelys olivacea). Vet Rec 161(24):818–819
Sapsford C, Hughes G (1978) Body temperature of the loggerhead sea turtle Caretta Caretta and the leatherback sea turtle Dermochelys Coriacea during nesting. Zool Af 13(1):63–69. https://doi.org/10.1080/00445096.1978.11447606
Seminoff JA, Eguchi T, Carretta J, Allen CD, Prosperi D, Rangel R, Gilpatrick JW, Forney K, Peckham SH (2014) Loggerhead sea turtle abundance at a foraging hotspot in the eastern Pacific Ocean: implications for at-sea conservation. Endanger Species Res 24(3):207–220
Smith CR, Hancock AL, Turnbull BS (2000) Comparison of white blood cell counts in cold-stunned and subsequently rehabilitated loggerhead sea turtles (Caretta caretta). Proc am Assoc zoo vet Int Assoc Aquat Anim Med 50–53
Söbílen D, Kaska Y (2018) Biochemical blood parameters and hormone levels of foraging, nesting, and injured loggerhead sea turtles (Caretta caretta) in Turkey. Turk J Zool 42:287–296, ©TÜBİTAK. https://doi.org/10.3906/zoo-1801-19
Southwood A, Reina R, Jones V, Jones D (2003) Seasonal diving patterns and body temperatures of juvenile green turtles at Heron Island. Australia Can J Zool 81:1014–1024, https://doi.org/10.1139/Z03-081
Stacy NI, Innis CJ (2017) Clinical pathology. In: Manire CA, Norton TM, Stacy BA, Harms CA, Innis CJ (eds) Sea turtle health and rehabilitation. J. Ross Publishing, Plantation, pp 147–207
Stacy NI, Alleman AR, Sayler KA (2011) Diagnostic hematology of reptiles. Clin Lab Med 31(1):87–108
Stacy NI, Bjorndal KA, Perrault JR, Martins HR, Bolten AB (2018) Blood analytes of oceanic-juvenile loggerhead sea turtles (Caretta caretta) from Azorean waters: reference intervals, size-relevant correlations and comparisons to neritic loggerheads from western Atlantic coastal waters. Conserv Physiol 6(1):coy006. https://doi.org/10.1093/conphys/coy006
Sykes J, Klaphake E (2008) Reptile hematology. Vet Clin Exot Anim 11:481–500
Trigo TF (2011) Patología Sistémica Veterinaria, 5a edn. Interamericana, Mexico
Van Houtan KS, Smith CS, Dailer ML, Kawachi M (2014) Eutrophication and the dietary promotion of sea turtle tumors. PeerJ 2:e602. https://doi.org/10.7717/peerj.60
Ward JR, Lafferty KD (2004) The elusive baseline of marine disease: are diseases in ocean ecosystems increasing? PLoS Biol 2:542–547
Work T, Balazs GH (1999) Relating tumor score to hematology in green turtles with fibropapillomatosis in Hawaii. J Wildl Dis 35:804–807
Work TM, Raskin RE, Balazs GH, Whittaker SD (1998) Morphologic and cytochemical characteristics of blood cells from Hawaiian green turtles. Am J Vet Res 59(10):1252–1257
Wyneken J (2001) The anatomy of sea turtles. U.S. Department of Commerce NOAA Technical Memorandum NMFS-SEFS
Zhang F, Li P, Gu H, Ye M (2011) Hematology, morphology, and ultrastructure of blood cells of juvenile olive ridley sea turtles (Lepidochelys olivacea). Chelonian Conserv Biol 10(2):250–256
Acknowledgments
We thank Dr. Juan Manuel López-Vivas from the Marine Botany laboratory from the UABCS and Jorge Armando Vega-Bravo, Yoalli Hernández Gill, Ana Sofía Merino-Zavala, and Valeria Lucero Silva from the UABCS for their help during fieldwork. Finally, we also thank Captains Fernándo Romero Romero and Aarón Romero Romero from Puerto Adolfo López Mateos and Miguel Angel Ramírez Mercado, Daniel Porras, and Rogelio Ojeda Pimentel from Procuraduria Federal de Protección al Ambiente (PROFEPA - SEMARNAT) for their assistance and fieldwork support.
Funding
We thank Proyectos Procer 2016 “Condición y Distribución de las tortugas marinas del Golfo de Ulloa y Playa San Lázaro BCS” (OFICIO. E.No.F00.DRPBCPN/696/2016) from Programa de Conservación de Especies en Riesgo (PROCER) of the Comisión Nacional de Áreas Naturales Protegidas (CONANP) for funding this research and for their assistance during field work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This research was conducted with the permits SGPA/DGVS/05533/16 and SGPA/DGVS/07915/16.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reséndiz, E., Fernández-Sanz, H., Barrientos-Torres, D.S. et al. Clinical pathology and health reference values for loggerhead sea turtles (Caretta caretta) and olive ridley turtles (Lepidochelys olivacea) in the Gulf of Ulloa, Baja California Sur, Mexico. Comp Clin Pathol 28, 1637–1650 (2019). https://doi.org/10.1007/s00580-019-02985-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-019-02985-0