Abstract
The maintenance of the pro-oxidant–antioxidant equilibrium between ROS production and antioxidant protection systems is an important element of systemic defence and requires efficient control. The aim of the study was to monitor the dynamics of antioxidants and lipid peroxidation in mice challenged intraperitoneally with Escherichia coli (O111:B4) lipopolysaccharide (LPS) and to evaluate the antioxidant potential of the non-steroidal anti-inflammatory drug nimesulide. Albino mice were divided into three groups (n = 36). Group I received a single intraperitoneal (i.p.) injection with 25 μg/0.5 mL LPS. Thirty minutes before LPS, group II received orally (p.o.) 100 mg/kg nimesulide. The preparation was administered for 4 days. Group III received only nimesulide at the indicated dose for 4 days. The blood parameters were analysed at hour 0 (prior to treatment applied to each group), post treatment hours 6 and 24, and days 3, 5 and 9. Assayed parameters included catalase, reduced glutathione, albumin, glucose, ferric reducing ability of plasma (FRAP), malondialdehyde and oxidative stress index. LPS induced continuous hypoglycaemia, decreased catalase activity and reduced glutathione, but FRAP and albumin were preserved. The application of nimesulide alone did not alter oxidative stress index and enhanced FRAP. Its co-administration with LPS normalised reduced glutathione, decreased catalase and increased malondialdehyde concentrations and oxidative stress index. The application of nimesulide as antioxidant requires objective evaluation of associated benefits and risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intra- and extracellular antioxidant systems are an essential part of systemic defence, helping to eliminate the endogenous and exogenous free radicals (Noori 2012). Reactive oxygen species (ROS), produced during redox reactions, and phagocytic oxidative killing mechanisms at high concentrations react with various organic compounds inducing cell destruction, and structural modifications of albumin and serum proteins (Ahmad et al. 2016; Choudhary et al. 2016). Consequently, the metabolism, energy exchange and systemic regulatory systems are impaired, and cell functions and intracellular signalling pathways are altered (Fonseca et al. 2016). Therefore, the equilibrium between ROS production and antioxidant protection systems is important, albeit vulnerable (Mishra et al. 2005).
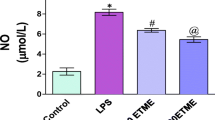
Numerous studies have shown that lipopolysaccharide (LPS) of Gram-negative bacteria induces mitochondrial dysfunction and oxidative stress (Israelachvili 2010; Birben et al. 2012). Liu and Bing (2011) provide evidence that this virulence factor activates apoptosis, production of inflammatory mediators, cytokines (IL-1, TNF-α, IL-6) and nitric oxide (NO). Despite the detailed investigations on the pathogenetic nature of LPS, therapeutical approaches to its inhibition through polymyxin B haemoperfusion (Hayashi 2012) and through synthetic peptides (Arana et al. 2003) did not yield the expected results (Buttenschoen et al. 2010). Also, more than 90% of new drugs tested on mice and rats (biological species with low sensitivity to LPS) failed in tests with humans (highly sensitive to LPS) but there is still no consistent explanation for the relevant mechanism responsible for the different sensitivity of biological species to LPS (Ghosn et al. 2010; Rosenfeld et al. 2010; Wimley 2010; Kaconis et al. 2011; Kosovrasti et al. 2016). Not only species-related LPS sensitivity but also breed- and age-related features are important to characterise clinical manifestations of the challenge (Deitschel et al. 2012; Holowaychuk et al. 2012). In research on LPS effects, the selection of experimental model is highly dependent on the dose, route of application and bacterial strain that produced LPS, as different bacteria and their strains contain variable amounts of endotoxin. Among the options for control of LPS-induced systemic damage is the efficient management of inflammation allowing it to restore its role of a vital non-specific mechanism of tissue protection and repair (Callahan et al. 2014).
The selective СОХ-2 inhibitor nimesulide has marked anti-inflammatory properties (Bennet and Villa 2000; Bennet 2001; Rainsford et al. 2005), attributed to effect on key inflammatory signalling pathways (Bennet 1999; Rainsford 2006)—inhibition of prostaglandin synthesis (especially that of PGE2); pro-inflammatory cytokines (TNF-α, IL-1, IL-6); inhibition of release of platelet-activating factor, and of histamine produced by basophils and tissue mast cells (Rainsford et al. 2005); impairment of chemotaxis and transendothelial migration of blood cells. Some authors reported high hepatotoxicity of nimesulide (Krishanappa 2010; Das and Roy 2011; Sozer et al. 2011), while others recommended its use in lung inflammation and osteoarthritis (Zheng et al. 2000; Sohi and Khanduja 2003; Al-Abd et al. 2014). Antioxidant activity of nimesulide is also reported (Khalil et al. 2012). The existing controversies require detailed in vivo studies in animal models to evaluate the properties of the drug and its potential for optimisation and control of LPS-induced oxidative stress.
The aim of the present study was to monitor the dynamics of blood antioxidants and lipid peroxidation in mice challenged intraperitoneally with Escherichia coli (O111:B4) lipopolysaccharide and to evaluate the antioxidant potential of the non-steroidal anti-inflammatory drug nimesulide.
Materials and methods
Chemicals
Lipopolysaccharide from E. coli, Serotype O111:B4 (Sigma Chemical, USA Co, L-3024, Lot 11H4087) and nimesulide (Heisinn Birex Phamaceutical Ltd., Damastown, Mulhuddart, Dublin 15, Ireland) were used in the experiments.
Animals
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
One hundred and eight albino mice, weighing 23–32 g, about 3 months of age, from both sexes were obtained from the Experimental Laboratory Animals Breeding Base, Slivnitsa, Bulgaria. Mice were housed indoor at a temperature of 21 ± 2 °C, humidity (60 ± 10%), and light regimen, and had free access to water and pelleted feed suitable for the species. All animals underwent a 2-week period of adaptation before the start of the trial.
Еxperimental design
Mice were allotted into 3 groups with 36 mice each. Twelve hours before the experiment’s start, food was withheld. Mice from experimental group I received a single intraperitoneal (i.p.) injection with 25 μg/0.5 mL LPS. Thirty minutes prior to LPS injection, animals from experimental group II received orally (p.o.) via an oesophageal probe 100 mg/kg nimesulide. The preparation was administered for 4 consecutive days, once daily at 8.30 am before feeding. The mice from experimental group III received only nimesulide at the indicated dose via an oesophageal probe for 4 consecutive days. The monitoring of blood parameters included hour 0 (prior to treatment applied to each group), post treatment hours 6 and 24, and post treatment days 3, 5 and 9.
Sample collection and laboratory parameters
Blood samples were collected after sedation and euthanasia of mice. Parameters were assayed in erythrocyte lysate, serum and plasma. The following parameters were determined: catalase (CAT, kU/L)—by the method of Goth (1991); reduced glutathione (rGSH, mmol/L)—by the method of Beutler et al. (1963); albumin (g/L)—with a commercial bromocresol green assay kit (Giesse Diagnostics, Italy); glucose (mmol/L)—with a commercial enzymatic assay kit (Human Diagnostica, GmbH Germany). The ferric reducing ability of plasma (FRAP, mmol/L) was determined by the method of Benzie and Strain (1996), and serum malondialdehyde (MDA, μmol/L)—by the thiobarbituric acid method (Uchiyama and Michara 1978) modified by Andreeva et al. (1988). The oxidative stress index (OSI) was calculated as
Statistical analysis
Results are presented as mean ± SD. Comparisons were made both within each group as well as among the three groups at each time interval by means of one-way ANOVA test and Tukey’s post hoc test (Graph Pad InStat3). Differences were considered statistically significant at the p < 0.05 level.
Results
Changes in blood antioxidants
During the entire period of the study, catalase activity in blood remained lower than baseline in all groups (Fig. 1). In group I, its levels remained lower both at day 5 (p < 0.01) and 9 (p < 0.01) compared to hour 0. Nimesulide applied 30 min prior to LPS injection (group ІІ) did not succeed to change the trend to catalase reduction with statistically significantly lower concentration at day 3 (p < 0.01) and lowest levels by day 5 compared to baseline (p < 0.001) as well as vs hour 6 (p < 0.01) and hour 24 (p < 0.05). By the 9th day, blood catalase began to increase in this group but initial levels were not attained (p < 0.01). In mice from group III, catalase decreased gradually, even after nimesulide intake was interrupted with substantial differences vs baseline by post treatment days 5 (p < 0.05) and 9 (p < 0.01). No statistically significant inter-group differences were found out.
Time course of changes in blood catalase activity (kU/L) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant differences: *p < 0.05, **p < 0.01, ***p < 0.001 vs hour 0; ++p < 0.01 vs hour 6; v p < 0.05 vs hour 24 within each group
Erythrocyte content of reduced glutathione (rGSH) in group I was lower than initial one as early as the 6th hour after LPS challenge (p < 0.05) and persisted low until the end of the study (Fig. 2). In group II, no statistically significant deviations were recorded. On the contrary, mice from group III showed strongly reduced rGSH levels on hours 6 and 24 (p < 0.001 vs hour 0). The subsequent trend towards rGSH elevation was however constant and by the 5th and 9th days of the experiment, the levels even exceeded those in groups I and II (Fig. 2).
Time course of changes in blood reduced glutathione (mmol/L) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant within-group differences: *p < 0.05, ***p < 0.001 vs hour 0; ++p < 0.01, +++p < 0.001 vs hour 6. Statistically significant between-group differences: c1p < 0.05 vs group III
In group III, nimesulide administration did not lead to relevant changes in blood albumin concentrations which varied around baseline ones (Fig. 3). The LPS injected to mice from group I however resulted in statistically significant decrease in albumin on hour 6 (p < 0.05 vs hour 0; p < 0.001 vs group III). Afterwards, the parameter increased considerably by the 24th hour (p < 0.001 vs hour 6), 3rd day (p < 0.001 vs hour 6) and 5th day (p < 0.001 vs hour 6) to attain levels higher than baseline ones by the 9th day (p < 0.05). In mice from group II, time course of albumin was similar to that in group I. Its levels were considerably lower than initial ones on hour 6 (p < 0.01), hour 24 (p < 0.01) and day 5 (p < 0.05) (Fig. 3).
Time course of changes in blood albumin (g/L) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant within-group differences: *p < 0.05 vs hour 0; +++p < 0.001 vs hour 6; v p < 0.05 vs hour 24. Statistically significant between-group differences: c1p < 0.05, c2p < 0.01, c3p < 0.001
Blood glucose in group I was low as early as the 6th post treatment hour (p < 0.001 vs hour 0; p < 0.01 vs group ІІІ). It remained low also on day 5 with statistically significant differences vs hour 0 (p < 0.01), and group III (p < 0.05). Then, blood glucose rose to final concentrations (9th day) significantly higher compared to those on hour 6 (p < 0.001) and day 5 (p < 0.05) (Fig. 4). In group II, blood glucose also decreased on hour 6 (p < 0.05 compared to hour 0; p < 0.001 compared to group III), hour 24 and day 5 (p < 0.05). However, on days 3 and 9, its concentrations exceeded those at hours 6 and 24 (p < 0.01). Nimesulide, applied independently to group III did not result in any statistically significant deviations.
Time course of changes in blood glucose (mmol/L) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant within-group differences: *p < 0.05, **p < 0.01, ***p < 0.001 vs hour 0; +p < 0.05, ++p < 0.01, +++p < 0.001 vs hour 6; v p < 0.05, vv p < 0.01, vvv p < 0.001 vs hour 24; $ vs day 5. Statistically significant between-group differences: a1p < 0.05, a2p < 0.01, a3p < 0.001 vs group I; b1p < 0.05, b2p < 0.01, b3p < 0.001 vs Group II; c1p < 0.05, c2p < 0.01, c3p < 0.001 vs group III
The total plasma antioxidant capacity as seen from FRAP levels was increased on hour in all groups (Fig. 5). The greatest increase was noted in group III (p < 0.001 vs baseline) followed by group II (p < 0.01 vs group ІІІ), and finally by group І (p < 0.001 vs group III). In LPS-treated mice however, FRAP were significantly higher than initial ones on day 3 (p < 0.01), day 5 (p < 0.001) and day 9 (p < 0.001), prevailing over those in groups II and III at the same time intervals. In the group treated with nimesulide + LPS, FRAP varied around initial levels except for hour 24 when it was substantially lower than levels by hour 6 (p < 0.05).
Time course of changes in ferric reducing ability of plasma (mmol/L) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant within-group differences: **p < 0.01, ***p < 0.001 vs hour 0; +p < 0.05, +++p < 0.001 vs hour 6; v p < 0.05, vv p < 0.01, vvv p < 0.001 vs hour 24; Statistically significant between-group differences: b2p < 0.01 vs group II; c2p < 0.01, c3p < 0.001 vs Group III
Oxidative stress markers
Serum MDA concentrations on hour 6 were significantly higher than initial values in all groups, with the highest elevation in LPS-challenged group (p < 0.001) and nimesulide-treated group (p < 0.01) (Fig. 6). At the end of the experiment, MDA levels in these groups remained higher than baseline ones. No significant changes were observed in mice treated with LPS + nimesulide except for day 9 when concentrations of the marker were lower than those in group III (p < 0.01).
Time course of changes in serum malondialdehyde (MDA) (μmol/L) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant within-group differences: *p < 0.05, **p < 0.01, ***p < 0.001 vs hour 0; ++p < 0.01, +++p < 0.001 vs hour 6. Statistically significant between-group differences: b1p < 0.05, vs group II; c2p < 0.01 vs group III
Oxidative stress index (OSI) values between hour 24 and day 3 in mice from group II were the highest (Fig. 7). The peak on hour 24 corresponded to that on hour 6 in Group I. Then, OSI in LPS-challenged animals decreased considerably compared to baseline on hour 24 (p < 0.01), days 3, 5 and 9 (p < 0.001). In the group that received nimesulide only, OSI values did not change remarkably with slight variations except for day 9 (p < 0.05 vs day 5).
Time course of changes in oxidative stress index (OSI) in mice, treated i.p. with pure lipopolysaccharide (LPS) (group I), with the combination p.o. nimesulide + i.p. LPS (group II) and with p.o. nimesulide only (group III) at 100 mg/kg for 4 days. Statistically significant within-group differences: **p < 0.01, vs hour 0; ++p < 0.01, +++p < 0.001 vs hour 6; v p < 0.05, vv p < 0.01, vvv p < 0.001 vs hour 24; $ vs day 5. Statistically significant between-group differences: b1p < 0.05, b3p < 0.001 vs group II; c1p < 0.05, c3p < 0.001 vs group III
Discussion
The used experimental model allowed revealing disturbance in antioxidant status, interactions between enzyme and non-enzyme antioxidant systems in mice, treated either with LPS or nimesulide alone, or with both substances. Data for group I showed a very early (hour 6) reduction of blood catalase activity, attributed to the fast destructive effect on LPS on cell detoxication potential. It could be suggested that changes in CAT antioxidant activity resulted from accumulation of considerable amounts of hydrogen peroxide after LPS challenge as the enzyme catalyses its degradation to water and oxygen (Held 2015). A large number of small non-enzyme molecules with detoxication function are also part of the antioxidant potential. Glutathione is an antioxidant tripeptide scavenging hydroxyl and peroxyl radicals (Held 2015) and it is indirectly involved as a co-factor of glutathione peroxidase in Н2О2 elimination (Arthur 2000). rGSH levels in mice from group I remained lower than baseline throughout the experiment (Fig. 2). Watson et al. (2003) affirmed that disturbances in rGSH had an effect on extracellular antioxidants—vitamins Е and С, as glutathione regenerates their active forms. According to Navarro et al. (1997) and Jones (2000), the ratio of oxidised (GSSG) to reduced (rGSH) glutathione is a dynamic marker of oxidative stress which is not commonly determined due to laborious GSSH assay. At the background of low concentrations of САТ and rGSH in mice from group I, the kinetics of non-enzymatic blood antioxidants, albumin and glucose, deserves to be discussed. By its thiol groups, albumin binds to erythrocytes and inhibits iron-dependent production of free radicals (Roche et al. 2008). Its concentrations on hour 6 after the LPS challenge were reduced but thereafter increased significantly and remained higher than baseline during the rest of the study period (Fig. 3). Considering the hepatotoxic effect of LPS (Buttenschoen et al. 2010; Ronco et al. 2010), changes could not be attributed to enhanced liver synthesis of albumin. It could be therefore suggested that high blood albumin levels either resulted from LPS-induced damage of renal nephrons or it originated from depots, e.g. from the skin—an important reserve of extravascular albumin which could pass in blood vessels if necessary. Albumin has an important role in hydrogen peroxide inactivation and ROS damage prevention (Zhu and Crouch 1992). The precise control of albumin content is important as, being a primary serum protein, it maintains a number of homeostatic parameters, oncotic pressure, and is involved in transport of electrolytes and hormones (Infusino and Panteghini 2013). LPS impaired glucose homeostasis inducing long-term hypoglycaemia in mice from group I, well pronounced by hour 6 and day 5 (Fig. 4). Our data are in line with those of Raetzsch et al. (2009). Possibly, LPS-mediated changes in glucose homeostasis are complex but their presence as early as the 6th hour suggests a hormonal control. The research of Nguyen et al. (2014) and Nohr et al. (2016) using gene expression analysis proves without doubt cytokine-mediated activation of insulin secretion by LPS. Vogel et al. (1991) discussed the role of IL-1 and TNF in LPS-induced hypoglycaemia and showed decrease liver glucose production and food intake associated with the effect of these cytokines on the appetite centre. In rats with endotoxaemia, Cornell (1989) found out increase in IL-1, while Ellingsgaard et al. (2011) demonstrated that IL-6 increased insulin secretion through glucagon-like peptide 1 (GLP-1) by L-cells and alpha cells. LPS-induced changes in antioxidant status influence oxidative stress markers. On post treatment hour 6, OSI values in Group I exceeded those in the other two groups (Fig. 7). This corresponded with established high serum MDA concentrations at that time (Fig. 6). Then, oxidative stress parameters MDA and OSI in this group decreased, although Ronco et al. (2010) affirmed that LPS-induced oxidative stress was among the leading pathogenetic mechanisms of tissue damage in endotoxaemia.
The possibility for ROS control by antioxidants is a reasonable approach used in the present experiment. The selection of nimesulide as a drug in LPS-challenged animals is motivated both by its anti-inflammatory properties and its antioxidant effects (Khalil et al. 2012). Our studies showed that when nimesulide was applied independently (group III), FRAP levels increased substantially and until the 3rd day they remained higher than those in groups I and II and compared to baseline levels (Fig. 5). Furthermore, mice from group III did not exhibit any statistically significant deviations in OSI values. This confirms the antioxidant properties of the drug. Bennet (1999) discussed the ability of nimesulide to inhibit the production of superoxide anions from neutrophils, whereas Bevilacqua et al. (1994) indicated that its effects are achieved through suppression of type IV phosphodiesterase. This enzyme increased intracellular cAMP levels and exerted a negative feedback on synthesis of pro-inflammatory cytokines, leukotrienes and histamine (Rainsford 2006). Al-Abd et al. (2014) established that applied independently nimesulide altered myeloperoxidase activity of neutrophils and reduced considerably their infiltration. Our studies showed that it did not affect blood non-enzymatic antioxidants (albumin, glucose) as seen from the lack of significant changes in group III. Despite the beneficial effects of nimesulide on extracellular antioxidants, the analysis of blood CAT and rGSH showed lower levels than baseline throughout the study which agrees with the results of Singh et al. (2012). The authors focused their attention on mitochondria and demonstrated that nimesulide increased nitric oxide concentrations. The result is mitochondrial dysfunction (Aamir et al. 2014). Possibly, the mechanisms of action of nimesulide on antioxidant and redox enzyme systems are rather complex and varying, displaying the modulating potential of the drug. This is confirmed by changes in lipid peroxidation (Fig. 6) in group III with MDA levels higher than initial ones. Compared to other non-steroid anti-inflammatory drugs, nimesulide has a different chemical structure, and pharmacological and pharmacokinetic properties (Bennet 1999; Rainsford 2006; Krishnappa 2010). Its anti-inflammatory effects are well investigated and rely on blockade of key signalling pathways of inflammation (Krishnappa 2010). Several studies however have documented its adverse effects (Bessone 2010; Modi et al. 2012; Aamir et al. 2014; Al-Abd et al. 2014; Bhattacharya et al. 2015). According to Rainsford (2006), the bioconversion of nimesulide in the liver by cytochrome Р450 (CYP-2C9, CYP-2C19 and CYP-1A2) yielded a reactive metabolite. Bhattacharya et al. (2015) reported vasculitis and hepatitis, while Singh et al. (2012) provided evidence for reduced mitochondrial activity, increased membrane permeability and activation of caspase-9 and caspase-3, indicating triggering of apoptosis.
Applied to mice challenged intraperitoneally with LPS, nimesulide succeeded to normalise rGSH concentrations but blood catalase activity remained lower than baseline one. In this group, OSI was high until the 5th day consequently both to low FRAP and high serum MDA levels during that period. Albumin values in the co-administered group remained lower than baseline ones during the entire period of observation unlike those in group I when albumin was higher between post treatment days 5 and 9. Although nimesulide alone (group III) did not influence this parameter, its toxicity increased when combined with LPS evidenced by commented changes in albumin, synthesised in the liver. In mice from group II, nimesulide corrected LPS-induced hypoglycaemia (between the 3rd and 9th days) (Fig. 4).
In conclusion, the supplementation of LPS-challenged mice with nimesulide had a beneficial effect expressed with normalisation of blood rGSH concentration and regulation of LPS-induced hypoglycaemia. The benefit/risk ratio should be, however, thoroughly evaluated to differentiate also adverse effects, low catalase activity and total antioxidant capacity of plasma, high oxidative stress index and serum MDA concentration.
References
Aamir M, Mahmood A, Qaiser J, Anum S, Muhammad W, Muhammad AA (2014) Hepatoprotective investigations of Cyminum dried seeds in nimesulide intoxicated albino rats by phytochemical and biochemical methods. Int J Pharm Pharm Sci 6(4):506–510
Ahmad A, Manjrekar P, Yadav C, Agarwal A, Srikantiah RM, Hegde A (2016) Evaluation of ischemia-modified albumin, malondialdehyde, and advanced oxidative protein products as markers of vascular injury in diabetic nephropathy. Biomark Insights 11:63–68
Al-Abd AM, Al-Abbasi FA, Nofal SM, Khalifa AE, Williams RO, El-Eraky WI, Nagy AA, Abdel-Naim AB (2014) Nimesulide improves the symptomatic and disease modifying effects of leflunomide in collagen induced arthritis. PLoS One 9(11):e111843
Andreeva LI, Kozhemyakin LA, Kishkun AA (1988) A modified thiobarbituric acid test for measuring lipid peroxidation. Lab Delo 11:41–43
Arana MJ, Vallespi G, Chinea (2003) Inhibition of LPS-responses by synthetic peptides derived from LBP associates with the ability of the peptides to block LBP-LPS interaction. J Endotoxin Res 9(5):281–291
Arthur JR (2000) The glutathione peroxidases. Cell Mol Life Sci 57(13–14):1825–1835
Bennet A (1999) Overview of nimesulide. Rheumatology 38(suppl.1):1–3
Bennet A (2001) Nimesulide: a well-established cyclo-oxygenase-2 inhibitor with many other pharmacological properties relevant to inflammatory disease. In: Vale JR, Botting RM (eds) Therapeutic roles of selective COX-2 inhibitors. William Harvey Press, London, pp 524–540
Bennet A, Villa G (2000) Nimesulide: an NSAID that preferentially inhibits COX-2, and has various unique pharmacological activities. Exp Opin Pharmacother 1:277–286
Benzie FF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Bessone F (2010) Non-steroidal anti-inflammatory drugs: what is the actual risk of liver damage? World J Gastroenterol 16(45):5651–5661
Beutler E, Duron O, Kelly BM (1963) Improved method for determination of blood glutathione. J Lab Med 61:882–888
Bevilacqua M, Vago T, Baldi G, Renesto E, Dallegri F (1994) Nimesulide decreases superoxide production by inhibiting phosphodiesterase type IV. Eur J Pharmacol 268:415–423
Bhattacharya PK, Burman B, Roy A, Jamil M, Lyngdoh M, Mishra J (2015) Nimesulide induced leukocytoclastic vasculitis and hepatitis: a case report. SpringerPlus 4:302. https://doi.org/10.1186/s40064-015-1081-9
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5(1):9–19
Buttenschoen K, Radermacher P, Bracht H (2010) Endotoxin elimination in sepsis: physiology and therapeutic application. Langenbeck's Arch Surg 395(6):597–605
Callahan GN, Yates RM, Warren AL (2014) Basic veterinary immunology. University Press of Colorado, Boulder, p 80303
Choudhary S, Boldogh I, Brasier AR (2016) Inside-out signaling pathways from nuclear reactive oxygen species control pulmonary innate immunity. J Innate Immun 8:143–155
Cornell RP (1989) Hyperinsulinemia elicited by interleukin-1 and nonlethal endotoxemia in rats. Circ Shock 28:121–130
Das SK, Roy C (2011) The protective role of Benincasa hispida on nimesulide-induced hepatotoxicity in albino rat model. Int J Green Pharm 5:192–197
Deitschel SJ, Kerl ME, Chang CH (2012) Age-associated changes to pathogen-associated molecular pattern-induced inflammatory mediator production in dogs. J Vet Emerg Crit Care 20(5):494–502
Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AMK, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY (2011) Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide 1 secretion from L cells and alpha cells. Nat Med 17:1481–1489
Fonseca LA, Goncalves RC, Filho JDR, Girardi FM, Filho WPC, Dias DCR, Bento LD (2016) Influence of selenium and vitamin E supplementation on energy metabolism in horses used in policing activity. Comp Clin Pathol 25:351–355
Ghosn EEB, Cassado AA, Govoni GR, Fukuhara T, Yang Y, Monack DM, Bortoluci KR, Almeida SR, Herzenberg LA, Herzenberg LA (2010) Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci USA 107:2568–2573
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Hayashi Y (2012) Polymixin B hemoperfusion (PMX-F) for severe sepsis and septic shock. Nihon Rinsho 70(2):311–314
Held P (2015) An introduction to reactive oxygen species. Measurement of ROS. in Cell BioTek Rev 1–26
Holowaychuk MK, Birkenheuer AJ, Li J (2012) Alterations in calcium homeostasis in dogs with induced endotoxemia. J Vet Intern Med 26(2):244–251
Infusino I, Panteghini M (2013) Serum albumin: accuracy and clinical use. Clin Chim Acta 419:15–18
Israelachvili JN (2010) Intermolecular and surface forces. Academic Press, London
Jones DP (2000) Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 348:93–112
Kaconis Y, Kowalski I, Howe J, Brauser A, Richter W, Razquin-Olazaran I, Inigo-Pestana M, Garidel P, Rossle M, Martinez de Tejada G, Gutsmann T, Brandenburg K (2011) Biophysical mechanisms of endotoxin neutralization by cationic amphiphilic peptides. Biophys J 100:2652–2661
Khalil NA, Ahmed EM, El-Nassan HB, Ahmed OK, Al-Abd AM (2012) Synthesis and biological evaluation of novel pyrazoline derivatives as anti-inflammatory and antioxidant agents. Arch Pharm Res 35:995–1002
Kosovrasti VY, Nechev L, Amiji MM (2016) Peritoneal macrophage-specific TNF-α gene silencing in LPS-induced acute inflammation model using CD44 targeting hyaluronic acid nanoparticles. Mol Pharm 13(10):3404–3416
Krishanappa H (2010) Investigations of toxicologic and immunotoxicologic potential of nimesulide. A thesis, Department of Industrial Biotechnology Dr. MGR Educational and Research Institute University Chennai
Liu M, Bing G (2011) Lipopolysaccharide animal models for Parkinson’s disease. Parkinson’s Dis. https://doi.org/10.4061/2011/327089
Mishra V, Baines M, Wenstone R, Shenkin A (2005) Markers of oxidative damage, antioxidant status and clinical outcome in critically ill patients. Ann Clin Biochem 42:269–276
Modi CM, Mody SK, Patel HB, Dudhatra GB, Kumar A, Avale M (2012) Toxicopathological overview of analgesic and anti-inflammatory drugs in animals. J Appl Pharm Sci 02(01):149–157
Navarro J, Obrador E, Pellicer JA, Asensi M, Vina J, Estrela JM (1997) Blood glutathione as an index of radiation-induced oxidative stress in mice and humans. Free Radic Biol Med 22(7):1203–1209
Nguyen AT, Mandard S, Dray C, Deckert V, Valet P, Besnard P, Drucker DJ, Lagrost L, Grober J (2014) Lipopolysaccharide-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes 63:471–482
Nohr MK, Dudele A, Poulsen MM, Ebbesen LH, Radko Y, Christensen LP, Jessen N, Richelsen B, Lund S, Pedersen SB (2016) LPS-enhanced glucose-stimulated insulin secretion is normalized by resveratrol. PLoS One 11:e0146840. https://doi.org/10.1371/journal.pone0146840
Noori S (2012) An overview of oxidative and antioxidant defensive system. Open Access Sci Rep 1(8):1–9
Raetzsch CF, Brooks NL, Alderman JM, Moore KS, Hosick PA, Klebanov S, Akira S, Bear JE, Baldwin AS, Mackman N, Combs T (2009) LPS inhibition of glucose production through the TLR4, MYD88, NF-kB pathway. Hepatology 50(2):592–600. https://doi.org/10.1002/hep.22999
Rainsford KD (2006) Nimesulide – a multifactorial approach to inflammation and pain: scientific and clinical consensus. Curr Med Res Opin 22(6):1161–1170
Rainsford KD, Bevilacqua M, Dallegri F, Gago F, Ottonello L, Sandrini G, Tavares IG (2005). In: Nimesulide - actions and uses, Birkhauser Verlag AG, pp 133–244 doi: https://doi.org/10.1007/3-7643-7410-1_4, Pharmacological properties of nimesulide
Roche MP, Rondeau P, Sing NR, Tarnus E, Bourdon E (2008) The antioxidant properties of serum albumin. FEBS Lett 582:1783–1787
Ronco C, Piccinni P, Rosner MH (2010) Endotoxemia and endotoxin shock: disease, diagnosis and therapy. Contrib Nephrol Basel, Karger 167:14–24
Rosenfeld Y, Lev N, Shai Y (2010) Effect of the hydrophobicity to net positive charge ratio on antibacterial and anti-endotoxin activities of structurally similar antimicrobial peptides. Biochemistry 49:853–861
Singh BK, Tripathi M, Chaudhari BP, Pandey PK, Kakkar P (2012) Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS One 7(4):e34200
Sohi KK, Khanduja KL (2003) Nimesulide affects antioxidant status during acute lung inflammation in rats. Indian J Biochem Biophys 40:238–245
Sozer S, Ortac R, Lermioglu F (2011) An investigation of toxicity potential of nimesulide in juvenile rats. Turk J Pharm 8(2):147–158
Uchiyama M, Michara M (1978) Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Biochemistry 86:271–278
Vogel SN, Henricson BE, Neta R (1991) Roles of interleukin-1 and tumor necrosis factor in lipopolysaccharide-induced hypoglycemia. Infect Immun 59:2494–2498
Watson WH, Chen Y, Jones DP (2003) Redox state of glutathione and thioredoxin in differentiation and apoptosis. Biofactors 17(1–4):307–314
Wimley WC (2010) Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem Biol 5:905–917
Zheng SX, Mouithys-Mickalad A, Deby-Dupont GP, Deby CMT, Maroulis AP, Labasse AH, Lamy ML, Crielaard JMR, Reginster JYL, Henrotin YE (2000) In vitro study of an antioxidant properties on nimesulide and 4-OH nimesulide: effects on HRP- and luminol-dependent chemiluminescence produced by human chondrocytes. Osteoarthr Cartil 8:419–425
Zhu L, Crouch RK (1992) Albumin in the cornea is oxidized by hydrogen peroxide. Cornea 11(6):567–572
Acknowledgments
Our heartiest thanks to Mrs. Daniela Ivanova, Faculty of Veterinary Medicine, for her help in assaying oxidative stress parameters and the technical assistance in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on animal rights
All institutional and national guidelines for the care and use of animals were followed.
Conflict of interest
The author declares that she has no conflict of interest.
Ethical approval
I, Maria Andonova, declare that in this study all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Andonova, M. Nimesulide effects on the blood pro-oxidant–antioxidant status in lipopolysaccharide-challenged mice. Comp Clin Pathol 28, 1003–1011 (2019). https://doi.org/10.1007/s00580-018-2877-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2877-0