Abstract
Goiter and other iodine deficiency disorders (IDD) are a worldwide problem. IDD prevalence rate of 52.7% has been reported among school children aged 9–12 years in Dhofar region, (WHO 2006) which resides adjacent to the Arabian Sea in the Sultanate of Oman. In a preliminary survey, 39.5 and 44.7% of the dromedary camels in the region exhibited low total T3 and total T4, respectively. An abattoir survey was carried out and the dromedary camels examined during the anti-mortem were clinically normal. Post-mortem examination of both thyroid lobes of slaughtered camel, revealed normal weight but 69.5% of these showed nodules of various sizes, numbers, shapes, colors and consistency in the capsular region and or deeply embedded in thyroid tissue. A wide range of histopathological changes were seen and categorized according to the dominant histological pattern into Hurthle cell nodules, papillary hyperplastic nodules, adenomatoid hyperplastic nodules and nodular colloid goiter only. The Hurthle cell nodules were either solid encapsulated cords or follicular variants of well-encapsulated macrofollicles and microfollicles of different sizes and shapes, and the Hurthle cells were seen lining the colloid follicles or forming the solid cords. In the papillary hyperplastic nodules, majority of cases lack capsule, the fronds were multiple and variable, and usually do not compress the surrounding parenchyma. Adenomatoid hyperplastic nodules formed of encapsulated follicular nodules with various sizes and shapes and containing little or no colloid. The nodular colloid goiter along with the papillary hyperplastic nodules had psammoma-like bodies without lamellation. No evidence was found of capsular or vascular invasion apart from one case which showed limited vascular and capsular invasion. The histopathology and immunohistochemistry using specific thyroid tumor markers did not reveal malignancy in all suspected cases. Total and free serum T3 and T4 and serum selenium were comparatively low, and CK enzyme was high in affected camels. The causes of the subclinical nodular goiter, probably multifactorial, were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Goiter and iodine deficiency disorders (IDD) are a worldwide problem and maps of areas showing such disorders have been developed around the globe (WHO 2006). The distribution of goiter and other IDD has been related to iodine content of the environment where iodine decreases in areas with increased distances from the sea (Saikat et al. 2004). Also, iodine deficiency is more common in sandy soils, water bleached, mountainous and volcanic areas in the world (Karmarkar et al. 1974; Harijoko et al. 2002; Vargas-Uricoechea et al. 2016).
Iodine deficiency is not the only cause of goiter in humans and animals; selenium deficiency in areas of marginally deficient iodine is also implicated and drugs such as thiouracil and sulphonamides are goitrogenic (Capen 1978). Also, more than 2000 plant species contain high levels of cyanogenic glycosides and may have a potential goitrogenic effect, including families Brassicaceae (e.g., turnips, rape, soybeans), Poaceae (e.g., sorghums, Zea maize, pearl millet) and Euphorbiaceae (e.g., cassava). These plants affect the animals when grazed for long time in areas with marginally deficient iodine (Bourdoux et al. 1978; Abdel Gadir and Adam 1999). On the other hand, there are areas where the cause of goiter is not elucidated such as Derbyshire in the UK; however, this may have been attributed to high carboniferous lime stone (Saikat et al. 2004). Other causes of goiter include thyroid tumors, thyroiditis, and inborn defects in thyroid hormogenesis.
Previous studies indicated that goiter is a common finding in the dromedary camel (Decker et al. 1979; Tageldin et al. 1985; Abu Damir et al. 1990; Rejeb et al. 2012). To the best of our knowledge, subclinical nodular goiter associated with Hurthle cell, adenomatoid hyperplasia, and papillary hyperplastic nodules has not been reported in the camel. The purpose of the present study was to document and shed more light on the gross, histopathological, histochemical and biochemical changes associated with subclinical nodular goiter in dromedary camels raised in mountainous and plain areas of calcareous soil close to the sea where iodine is expected to be adequate in the environment.
Materials and methods
Animals
For the study of the thyroid pathology, a total of 97 apparently healthy male and female dromedary camels from Dhofar region, Sultanate of Oman were used. Thirty-eight of these were randomly selected from apparently healthy camel herds to assess the thyroid hormone levels in the area. Thyroid and blood samples were randomly collected from a total of 59 male and female dromedary camels, majority1–3 years of age, brought to the Salalah, abattoir (Dhofar region) for slaughter during the period May–August. All the camels were apparently healthy, did not show thyroid enlargement and passed for human consumption. Blood was taken from 19 apparently healthy male adult camels from UAE to obtain baseline data for free T3 and free T4 for comparison.
The Dhofari camels graze on sparse desert steppe vegetation for most of the year or on weeds, shrubs, trees, and green grass that grow during the monsoon season from late June to early September in the mountains along the coastal area (ANON 1992; El-Sheikh 2013). Fodders such as dry Rhodes grass, sorghum, and lucerne, and dry crop residues such as wheat, barley, sorghum, millet, maize and vegetable residues of Brassica species are offered during the dry season in variable quantities (Al-Mishakhi and Koll 2007; MOA 2008). The soil of the area is highly calcareous and deficient in most micronutrients (ANON 1992).
Specimen collection
Blood samples were collected by venipuncture into plain vacutainer tubes and allowed to clot and the serum was separated by centrifugation at 3000 rpm and stored at − 80 °C until analyzed.
Chemical methods
Total tri-iodothyronine (TT3) and total thyroxine (TT4) were determined in serum by chemiluminescent assay using Cobas e411 (Roche Diagnostic, Mannheim, Germany) and kits from the same company. Free tri-iodothyronine (fT3) and free thyroxine (fT4) were determined in serum by radio-immunoassay (RIA, Multiskan R Spectrum, Thermo Electron Corporation, Finland) using standard kits (DRG International Inc., USA). Activity of enzymes AST and CK was estimated by Reflovet Plus (Roche, Germany) using kits manufactured by the company for in vitro diagnostic use (CHEK Reflotron, Roche, Germany). Serum for Se determination was digested by nitric acid and perchloric acid using microwave digestive system (Milestone, MLS-1200 MEGA, Italy). Selenium was determined in the prepared specimen by inductively coupled Argon plasma atomic emission spectrometer (ICP, Variant, Australia). Serum Vitamin E was measured by high performance liquid chromatography (HPLC, Waters, USA).
Histopathological methods
Both thyroid lobes were carefully dissected from the trachea of 59 dromedary camels checked and weighed. Representative pieces from each thyroid lobe were promptly fixed in 10% neutral buffered formalin, processed in paraffin and stained with hematoxylin-eosin (H&E) and Masson’s trichrome stain for connective tissue. Representative sections were fixed in coated slides and stained for CK19, GAL3 (Novo Costra, USA), calcitonin, CD56, and HBME1 (Cell Mark, USA) using Dako Autostainer (Dako Universal Staining System, Denmark) as tumor tissue markers. Positive and negative control slides were included in the staining. The marker pattern and intensity were recorded. Few slides were sent to the Department of Defense Armed Forces Institute of Pathology, Washington DC 20306–6000 for second opinion. These slides were stained for thyroglobulin, calcitonin and thyroid transcription factor-1 (TTF) using their standard AFIP protocol and incorporating external positive canine and normal camel tissue controls.
Statistical analysis
All statistical analyses were performed by analysis of variance (ANOVA) test using SPSS (Statistical Package for Social Sciences, version 17: SPSS, Chicago, USA). T test was used to determine significant differences between groups. Data were expressed as mean ± SEM, and P < 0.05 was considered statistically significant.
Results
All animals were apparently clinically normal, without any abnormality seen or palpated externally in thyroids and were passed for human consumption. Grossly, all thyroid glands were not enlarged, with an average weight of 20.6 and 21.4 g in normal camels and camels with subclinical nodular goiter, respectively (Table 1). The affected thyroids (69.5%) were generally pear-shaped but some were blunted, irregular or distorted (Fig. 1a). The thyroids color ranged from pale to dark brown and mottled compared to the normal ones (Fig. 1b). Mostly, those thyroids had rough surface which may contain single or multiple small or large nodules ranging in size from 3 to 15 mm in diameter. The cut surface may also show deeply embedded nodules in the thyroid tissue which may contain gelatinous, watery or hemorrhagic material but the nodules did not seem to harbor solid components (Fig. 1c). A honeycomb-like appearance was noted in gross section of some thyroids.
a Dromedary camel’s thyroid gland gross appearance. Several thyroids depicting mottled surfaces with a number of variable-sized cysts bulging from the thyroid tissue giving them an irregular shape. b Dromedary camel’s thyroids, fish-like, smooth surfaces, tapered at both ends. c Cross-section of right and left thyroid glands. They are not enlarged. The right lobe is more elongated. Both showed multi-nodular variable sized fluid filled cyst
Histopathological findings
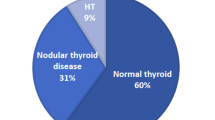
30.5% (18/59) of the thyroids collected did not show pathological changes. In these, the thyroid follicles contained colloid lined by cuboidal cells with normal nuclei resting on intact cellular membrane. The overall cases of subclinical nodular goiter constituted 69.5% (41/59) of cases. They were grouped according to the dominant histological pattern as Hurthle (oncocytic) cell nodules, papillary hyperplastic nodules, adenomatoid hyperplastic nodules and nodular colloid goiter only. The main histopathological features of these groups are summarized in (Table 2).
11.9% (7/59) of thyroids exhibited Hurthle (oncocytic) cell nodules raised in the background of multinodular goiter. The growth pattern of the Hurthle cell nodules were either solid cords or follicular variants, and the latter were characterized by well-encapsulated macrofollicles and microfollicles of different sizes and shapes. The solid cords were encapsulated by a thick capsule. An irregular aggregate of microfollicles of different sizes with scant colloid, marked hypercellularity haphazardly located and separated by a thin fibrous capsule was noted. The follicles with colloid were lined by a single layer of cuboidal epithelium. Empty vacuoles were also found at the basement membrane of the lining follicular epithelium (Fig. 2a). Several encapsulated follicles devoid of colloid had dense nuclei haphazardly distributed in the follicles. Some of them showed clear nuclei with prominent nucleoli (Fig. 2b). In some areas, nuclear pleomorphism characterized by different sizes and shapes were evident. Five cases showed clear ground-glass nuclei with bubbles. Free-floating island of Hurthle cells of various sizes and shapes were entrapped within the capsule but not connected to the main nodule. Few follicles had scant colloid with vacuolated or coarse chromatin (Fig. 2b). The capsule tends to be thicker and more irregular dividing the follicles into compartments (Fig. 2c). Five cases had in addition papillary hyperplasia. There was no evidence of vascular and/or capsular invasion. The papillary hyperplastic forms constituted the majority of cases 25.4% (15/59) and arise in the background of nodular goiter. The fronds have an overall papillary pattern and lacked evidence of capsular and vascular invasion. They were multiple and variable in histological appearance, arrangement and usually did not compress the surrounding parenchyma (Fig. 3a, b). Some cases were encapsulated and showed comparable growth patterns with the adjacent normal tissue. Others displayed marked papillary in-folding with psammoma-like bodies (Fig. 3a). Papillary projection into the follicular lumen was occasionally present. No mitotic figures were seen.
a Encapsulated cluster of Hurthle cells (solid cord) vary in size with dark nuclei and pale or clear cytoplasm. Capsule trapped several follicles that lacked colloid (left portion and upper left corner) (H&E ×20). b High-power Hurthle cells, several follicles devoid of colloid were encapsulated by a thick fibrous tissue. The nuclei were haphazardly distributed filling the capsule. Some follicles contained clear nuclei with prominent nucleoli. H&E ×40. c Free-floating island of follicles of various sizes and shapes trapped in a thick fibrous capsule. They were not connected to the main nodule. Few follicles contained scant colloid. H&E ×20. d Thyroid macrofollicles and microfollicles of variable sizes and shapes, containing finely granular, eosinophilic cytoplasm. They were surrounded by thick fibrous capsule. Some follicles contained scant colloid. The nuclei were large, oval with prominent nucleoli. Masson’s Trichrome stain ×20
Adenomatoid hyperplastic nodules had follicular pattern arising in the background of nodular goiter. This group constituted 13.6% (8/59) of cases. All this group comprised encapsulated follicular nodules varying in size and shape and containing little or no colloid, and every nodule was fairly uniform in histological pattern. Sometimes a group of follicles were fused forming a central lumen which contained colloid or were devoid of it (Fig. 4a). The follicular epithelial cells were cuboidal to low columnar. The nuclear appearance was divided into three categories; crowded overlapping nuclei with dense chromatin pattern (Fig. 4b), clear ground-glass nuclei, with margination of chromatin along nuclear membrane (Orphan Annie eye nucleus) with few nuclear bubbles (Fig. 4c), and nuclei showing focal pyknosis or charyolysis (Fig. 4d) confined to a microscopically identified area and not diffused throughout the nodule. It was remarkable that portion of the cells lining the follicles as well as the cells in some follicles did not show clear ground-glass nuclei (Fig. 4c).
a Several follicles ruptured and coalesced forming one large pseudolumen containing colloid and separated from the adjacent tissue by a fibrous capsule. The colloid rest on vacuoles of various size. Other similar structures lacked colloid. H&E ×60. b Encapsulated adenomatoid nodules with scant colloid, nuclear crowding and overlapping were evident. The nuclei were large with dense coarse chromatin and distinct nucleoli in some of them. H&E ×60. c Encapsulated follicles, one with colloid. Follicles that lacked colloid had a clear ground-glass nuclei, few had bubbles. H&E ×100. d Encapsulated adenomatoid hyperplastic nodule, follicles varied in size and shape. Few follicles showed scant colloid. Presence of degenerative necrotic changes with the loss of nuclei in some follicles. Note the dark stained nuclei (pyknotic). H&E ×60. e Encapsulated follicular-patterned nodule. All follicles were embedded in a thick fibrous capsule and lacked colloid. Some follicles displayed reduced luminal diameter. An unequivocal minimal vascular (arrow) and capsular (asteroid) invasion were evident. H&E ×60
One case contained macrofollicles and microfollicles, devoid of colloid and with thickened interfollicular space. The follicles were lined by a single layer of cuboidal epithelium. This case demonstrated an unequivocal minimal focus of vascular and capsular invasion (Fig. 4e). In three cases, simultaneously adenomatoid hyperplastic nodules and papillary hyperplasia were evident.
18.6% (11/59) of samples had nodular colloid goiter only. All of them had psammoma-like bodies without lamellation (Fig. 5). This brought the total number of psammoma-like bodies to 37.3% (22/59) of the total cases. Brownish intracytoplasmic inclusions ranging from granules to globules were also noted in all psammoma-like cases (Fig. 6).
The thyroid tissues showing adenomatous hyperplasia were negative for all the five immunohistochemistry stains (CK19, GAL3, Calcitonin, CD56, and HBME1). The thyroid tissues showing adenomatous hyperplasia and sent for second opinion were negative for the three immune stains: calcitonin, thyroglobulin, and TTF-1.
Biochemical findings
The total T 3 and T 4 of 38 camels randomly selected from the region are presented in Table 3. 39.5 and 44.7% of serum samples were below 2.2 and 120 nmol/l total T 3 and total T 4, respectively.
The results of thyroid hormones analyses for slaughter camels in Dhofar region and basal data taken from apparently normal camels from UAE are presented in Table 4. The fT3 levels were low in camels slaughtered in Dhofar region; 42% of values were below 1.8 pmol/L while in UAE camels, only one case (5.2%) was reported to be below the threshold. However, the difference was not significant (P > 0.05) in the apparently normal and affected Dhofari camels. Also, the fT4 values were low in both groups of camels in Dhofar; 10.5% were below 10 pmol/L while in the normal group in UAE, the values were all above the threshold. The fT3 and fT4 values were significantly lower (P < 0.05) in both normal and affected camels raised in Dhofar region compared to those from UAE. Also, the affected camels had significantly lower (P < 0.05) fT4 values compared to the apparently normal Dhofari camels. The results of serum biochemical analysis are presented in Table 5: Se was lower in camels showing subclinical goiter. 25% of all specimens from both groups were between 10 and 64. CK and AST activities were higher in the affected camels. Vitamin E values were almost similar in both groups.
Discussion
This is the first report describing subclinical endemic nodular goiter with Hurthle cell nodules in dromedary camels. These camels were raised in the Dhofar region which borders the Arab sea, in the Sultanate of Oman. All the thyroids of the surveyed camels were within normal size and weight. However, 69.5% of them showed pathological lesions including discoloration, distortion, and nodules of various sizes, colors, and numbers located at the surface or embedded inside the tissue. Histopathologically, different hyperplastic lesions were seen and categorized into Hurthle, papillary, adenomatoid, and colloid nodules. In the colloid nodular goiter, the nodular follicles were fully distended, partially filled with or totally devoid of colloid, and mostly lined by cuboidal or tall columnar epithelium. These lesions differ from the previous reports of Decker et al. (1979), Tageldin et al. (1985), and Abu Damir et al. (1990) where the thyroids of camels in this report were having normal weight and less colloidal content. However, this is in agreement with the finding of Doige and Mclaughlin (1981), who reported a non-enlarged thyroid gland in a new born foal with hyperplastic goiter.
Hurthle cells, were frequently seen either lining the follicles or dispersing in sheets or cords in the parenchyma along with other follicular or parenchymatous cells. The presence of these large cells with their abundant mitochondria may indicate cellular stress (Wikipedia 2016), reflecting the harsh environment where these animals live. Previous reports indicated the common occurrence of Hurthle cells in nodular goiter, toxic goiter, Hashimoto, Graves’ and neoplastic diseases of human thyroids (Kathleen and Zubair 2008; Wikipedia 2016), and animal thyroid adenoma (Capen 1978; Jubb et al. 1985).
The majority of the thyroid lesions were papillary hyperplastic nodules mostly non capsulated or encaged within a thin fibrous capsule and with scanty or no colloid. The cells in these lesions were cuboidal with normal nuclei. It is remarkable that all psammoma-like bodies were without lamellation and either in the papillary hyperplastic or nodular colloid goiter and associated with intracytoplasmic inclusion bodies. The psammoma-like bodies normally formed by focal areas of infarction at the tips of papillae, with calcium deposition on the dying cells with subsequent lamellation with repeated cycles (Deepa and Krishnaraj 2014). Despite the fact that the presence of psammoma-like bodies in the thyroid interstitial tissues alerts to the high probability of papillary carcinoma elsewhere in the gland (Kathleen and Zubair 2008), we could not report such tumor in any of the cases. The adenomatoid hyperplastic nodules differ from the papillary type where in this category, the follicles attained adenoma-like shapes with crowded polymorphic nuclei and entrapped within a thick capsule. The presence of the degenerative changes within the adenomatoid nodules was most likely an indication of hyperplasia rather than true adenoma (Suster 2006), and the optically clear nuclei might have been an expression of reactive change in the follicular cells that had been entrapped by fibrous tissue (Rosai et al. 2006).
In humans, the risk of nodules harboring papillary carcinoma is proportional to the solid tissue component inside the cyst, and generally the incidences vary between 4 and 5% in large nodule with small solid components (Lee et al. 2009). In this report, the pathology and immunohistochemistry profile do not fulfill the criteria for malignancy. Thyroid immunohistochemistry tumor markers such as CK 19, GAL 3, Calcitonin, CD56, HBME1, thyroglobulin, thyroid transcription factor-1 (TTF) were negative for thyroid malignancies in all suspected cases. This is despite the limited vascular and capsular invasion seen in one case and the optically clear nucleus which has been claimed as an important diagnostic feature of papillary carcinoma (Zubair and Virginia 2002). Generally, the thyroid tumors are infrequent in domestic animals other than dogs, cats, and horses (Capen 1978), i.e., only ten cases of thyroid tumors among 370 tumors were reported in cattle within 10 years (Vitovec 1976), and only one case of follicular and papillary adenomas was reported in a slaughtered camel (Tageldin et al. 2016).
The cause of goiter in the region is not well understood; however, it could be multifactorial including low iodine content combined with low selenium level, goitrogenes, and the highly alkaline calcareous soil. The magnitude and duration of these different causative agents along with individual variation may have been the causes of the variety of lesions seen in the thyroids of these camels. It is believed that iodine is borderline deficient in the area and that the nodules eventually form as a sequence of repetitive cycles of iodine depletion and repletion or decreased requirement of thyroid hormones as an adaptational mechanism (Jubb et al. 1985; Madeiros-Neto 2013). The total T3 and T4 values in the Dhofari camels were lower than levels reported by Nazifi et al. (2009a, b), Saeb et al. (2010), and Tajik et al. (2013). Again the fT3 and fT4 levels in serum of the camels slaughtered in Dhofar were significantly lower than the basal data collected from UAE, and fT3 and fT4 values reported for normal camels (Nazifi et al. 2009a, b; Saeb et al. 2010; Rejeb et al. 2012). This is not surprising as the samples were collected from an area where IDD prevalence is 52.7% among school children aged 9–12 years with an overall country average of 49.8% school children with urine samples low (< 100 μg/l) in iodine (WHO 2006).
Low selenium levels can play an important role in determining the severity of hypothyroidism associated with low I (Beckett et al. 1987, 1993). Se, as selenocysteine is a prosthetic group of the iodothyronine deiodinases iso-enzymes (1, 11, 111) which convert T4, into T3 or rT3 (reverse triiodothyronine) in target cells (Beckett et al. 1987; Drutel et al. 2013). The Dhofar area with its sandy/rocky nature of soil is deficient in most micronutrients including Se (ANON 1992). Indeed, the serum Se value of camels in Dhofar was comparatively low compared to blood Se value (100 ng/ml) considered proper for metabolic functions in camels (Hamliri et al. 1990; Barri and Al-Sultan 2007), i.e., 25% of cases were having serum Se below 64 ng/ml. The low Se in the serum along with low iodine may be implicated in the formation of the thyroid nodules in the affected camels. In humans, multinodular goiter has been associated with low iodine along with low plasma Se concentration (Samir and El-Awady 1998; Rasmussen et al. 2011; Drutel et al. 2013).
Although the Dhofar area is quite near to the sea coast and expected to be adequate in iodine, subclinical goiter in camels was very high. It is possible that the condition is accentuated by the calcareous soil of the region, which is very alkaline and with high levels of CaCO3, beside the low iodine and Se. In summer, the camels depend on the water from deep wells and graze on sparse bushes and shrubs growing in these soils beside the supplemented fodders. It is possible that the water, bushes, and soils consumed may have added to the existing situation. It worth mentioning that endemic goiter has been reported in Derbyshire, UK, a carboniferous lime stone region residing few kilometers from the sea and with adequate iodine in soil, water, and plants (Saikat et al. 2004).
Dietary goitrogenic substances interfere with iodine accumulation in the thyroid and hormonal synthesis (Doige and Mclaughlin 1981; Ong et al. 2014). The role of these substances in this condition could not be ruled out, as in the dry season, the camels are offered different sorghum fodders and vegetable residues of Brassica species. These contain cyanogenetic glycosides and different types of glycosylflavones which may be implicated in the condition.
Goiter in the camels has been associated with elevated levels of CK and AST activities in serum (Table 5). Previous reports in humans had shown that more than 90% of hypothyroid patients have evidence of elevated CK activity due to myopathy (McKeran et al. 1975). Myopathy was also reported in dogs with primary hypothyroidism (Braund et al. 1981). The effect is mainly due to severe lesions in type 11 muscle fibers leading to either their complete loss or pronounced reduction in their diameter. This effect may have a serious implication in camel racing which is very popular in the Arabian Peninsula.
We concluded that subclinical goiter is prevalent in Dhafar region and the cause is speculated to be multifactorial. Further studies are deemed essential to elucidate the causes and both iodine and Se may need to be supplemented. The camel is very sensitive to iodine deficiency and has much similarity to human goiter pathology and thus may serve as a model for iodine studies.
Change history
15 December 2018
The original published version of this article contained a mistake in the name of Remya R. Nair. It was incorrectly presented as Remya R. Nir.
15 December 2018
The original published version of this article contained a mistake in the name of Remya R. Nair. It was incorrectly presented as Remya R. Nir.
References
Abdel Gadir WS, Adam SEI (1999) Development of goiter and enterohepatonephropathy in Nubian goats fed with pearl millet (Pennisetum typhoides). Vet J 157:178–185
Abu Damir A, Barri MES, Tageldin MH, Idris OF (1990) Clinical and subclinical colloid goiter in adult camels (Camelus dromedarius) at Kordofan Region of the Sudan. Br Vet J 146:219–227
Al-Mishakhi MSA, Koll EHBA (2007) Country pasture/forage resource profile. Oman, FAO Technical Report, 23 p
ANON (1992) Soil survey and land classification project, OMA/87//011.Salalah integrated study: farming survey report. Ministry of Agriculture and Fisheries, SOO, and FAO, Muscat, pp 82
Barri MES, Al-Sultan SI (2007) Studies on Selenium and Vitamin E status of young Megheem dromedary camels at Al-Hasa province. J Camel Pract Res 14:51–53
Beckett GJ, Beddows SE, Morrice PC, Nicol F, Arther JA (1987) Inhibition of hepatic type-1 iodothyronine deiodination of thyroxin caused by selenium deficiency in rats. Biochem J 248:443–447
Beckett GJ, Nicol F, Rae PW, Beech S, Guo Y, Arther JA (1993) Effects of combined iodine and selenium deficiency on thyroid hormone metabolism in rats. Am J Clin Nutr 57(Supplement):240S–243S
Bogin E (2000) Clinical pathology of camelides: present and future. Revue Med Vet, 151:563–568
Bourdoux P, Delange F, Gerald M (1978) Evidence that cassava ingestion increase thiocyanate formation: A possible etiologic factor in endemic goiter. J Clin Endocrinol Metab 4:613–621
Braund KG, Dillon AR, August JR, Ganjam VK (1981) Hypothyroid myopathy in two dogs. Vet Pathol 18:589–598
Capen CC (1978) Tumors of the endocrine glands. In: Moulton JE (ed) Tumors in domestic animals, 2nd edn. University of California Press, Berkeley, Los Angeles, London, pp 372–429
Decker RH, Hruska JC, McDermid AM (1979) Colloid goiter in a newborn camel and an aborted foetus. J Am Vet Med Assoc 175:968–969
Deepa TK, Krishnaraj U (2014) Histopathological features of papillary thyroid carcinoma with special emphasis on the significance of nuclear features in their diagnosis. Arch Med Health Sci 2:16–22
Doige CE, Mclaughlin BG (1981) Hyperplastic goiter in new born foals in Western Canada. Can Vet J 22:42–45
Drutel A, Archambeaud F, Caron P (2013) Selenium and thyroid gland. Clin Endocrinol 78:155–164
El-Sheikh MA (2013) Weed vegetation ecology of arable land in Salalah, Southern Oman. Saudi J Biol Sci 20:291–304
Faye B, Seboussi R (2009) Selenium in camel - A review. Nutrients, 1:30–49
Hamliri A, Khallaayoune K, Johnson DW, Kessabi M (1990) The relationship between concentration of the selenium in the blood and the activity of glutathione peroxidase in the erythrothytes of the dromedary camels (camelus dromedaries). Vet Res Commun 14:27–30
Harijoko A, Warmada IW, Sudargo T, Widagdo D, Watanabe K (2002) Factor controlling iodine deficiency disorders (IDD) incident in communities living within volcanic landscape www.cprm.gov.br/331GC/12872.htm
Jubb KVF, Kennedy PC, Palmer N (1985) Pathology of domestic animals, vol 3, 3rd edn. Academic Press, New York
Karmarkar MG, Deo MG, Kochupillai N, Ramalingaswami V (1974) Pathophysiology of Himalayan endemic goiter. Am J Clin Nutr 27:96–103
Kathleen TM, Zubair WB (2008) The thyroid Hurthle (Oncocytic) cell and its associated pathologic conditions. Arch Pathol Lab Med 132:1241–1250
Lee MJ, Kim EK, Kwak JY, Kim MJ (2009) Partially cystic thyroid nodules on ultrasound. Probability of malignancy and sonographic differentiation. Thyroid 19:341–346
Madeiros-Neto G (2013) Multinodular goiter www.thyroidmanager.org, chapter 17
McKeran RO, Lavin GS, Andrews MT, Ward P, Mair WGP (1975) Muscle fibre type changes in thyroid myopathy. J Clin Path 28:659–663
Ministry of Agriculture, Oman (MOA) (2008) The state of plant genetic resource for food andagriculture in Oman. Report 1–34. http://www.fao.org/docrep/013/i1500e/Oman.pdf
Nazifi S, Mansourian M, Nikahval B, Razavi SM (2009a) The relationship between serum level and thyroid hormones, trace elements and antioxidant enzymes in dromedary camels (Camelus dromedarius). Trop Anim Health Prod 41:129–134
Nazifi S, Nikahval B, Mansourian M, Razavi SM, Farshneshani F et al (2009b) Relationships between thyroid hormones, serum lipid profile and erythrocyte antioxidant enzymes in clinically healthy camels (Camelus dromedarius). Revue Med Vet 160:3–9
Ong CB, Thomas HH, Scott DF (2014) Hyperplastic goiter in two adult dairy cows. J Vet Diagn Investig 26:810–814
Rasmussen LB, Schomburg L, Kohrle J, Pedersen IB, Hollenbach B, Hog A, Oversen L, Perrild H, Laurberg P (2011) Selenium status. Thyroid volume and multiple nodule formation in an area with mild iodine deficiency. Eur J Endocrinol 164:585–590
Rejeb A, Amara A, Rezeigui H, Crespeau F, De Lverdier M (2012) Pathological and hormonal study of the goiter in the dromedary (Camelus dromedarius) in South of Tunisia. Revue Med Vet 163:242–249
Rosai J, Kuhn E, Carcangiu ML (2006) Pitfalls in thyroid tumor pathology. Histopathology 49:107–120
Saeed A, Khan IA, Hussein MM (2009) Changes in biochemical profile of pregnant camels (Camelus dromedarius) in term. Comparative Clinical Pathology, 18:139–143
Saeb M, Baghshani H, Nazifi S, Saeb S (2010) Physiological response of dromedary camels to road transportation in relation to circulating levels of cortisol, thyroid hormones and some serum biochemical parameters. Trop Anim Health Prod 42:55–63
Saikat SQ, Carter JE, Mehra A, Smith B, Stewart A (2004) Goiter and environmental iodine deficiency in the UK-Derbyshire. A review. Environ Geochem Health 26:395–401
Samir M, El-Awady MY (1998) Serum Selenium levels in multinodular goiter. Clin Otolaryngol Allied Sci 23:512–514
Suster S (2006) Thyroid Tumors with a follicular growth pattern: problems in differential diagnosis. Arch Pathol Lab Med 130:984–988
Tageldin MH, ElSawi ASA, Ibrahim SG (1985) Observation on colloid goiter of dromedary camels in the Sudan. Rev Elev Med Vet Pays Trop 38:394–397
Tageldin MH, Abu Damir H, Omer EA, Ali MA, Adam AM (2016) Follicular adenoma associated with spindle cell proliferation, papillary adenoma and colloid goitre in a dromedary camel. Comp Clin Pathol 25:241–245
Tajik J, Sazmand A, Moghaddam SHH, Rassoli A (2013) Serum concentrations of thyroid hormones, cholesterol and triglyceride, and their correlations together in clinically healthy camels (Camelus dromedarius): effects of season, sex and age. Vet Res Forum 4:239–243
Vargas-Uricoechea H, Bonelo-Perdomo A, Sierra-Tares CH (2016) Iodine and the thyroid. In: Imam SK, Ahmad SI (eds) Thyroid Disorders. Basic Science and Clinical Practice. Springer, Netherland, pp 27–48
Vitovec J (1976) Statistical data on 370 cattle tumors collected over the years 1964-1973 in South Bohemia. Zentralbl Veterinarmed A 23:445–453
WHO (2006) Vitamin and Mineral Nutrition Information System (VMNIS) World global data base on iodine deficiency World Health Organization, Geneva, Switzerland, www.who.int/vmnis/database/iodine/countries/en
Wikipedia (2016) Hurthle cell, http://en.wikipedia .org
Zubair WB, Virginia AL (2002) Etiology and significance of the “optically clear nucleus”. Endocr Pathol 13:289–299
Acknowledgements
We would like to express our appreciation and gratitude for professor A.M. El Hassan, Faculty of Medicine, University of Khartoum for the second opinion; Abu Dhabi Food Control Authority, for the approval to use Al Qattara Veterinary Lab facilities; the staff in Salalah Veterinary Hospital for the help during specimen collection; the staff in the Pathology Department College of Medicine and Health Sciences, Sultan Qaboos University, Sultanate of Oman for the help in the preparation and staining of the slides; the Department of Defense Armed Forces Institute of Pathology, Washington DC, for the second opinion in some tumor markers; and the UAE University lab staff for some specimens analysis.
Funding
This study was funded by College of Agricultural and Marine Sciences, Sultan Gaboos University, Sultanate of Oman (grant number IG/AGR/ANVS/10/01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Tageldin, M.H., Abu Damir, H., Hussein, M.F. et al. Subclinical nodular goiter associated with Hurthle cell, papillary, and adenomatoid hyperplasic nodules in the dromedary camel in the Sultanate of Oman. Comp Clin Pathol 27, 135–145 (2018). https://doi.org/10.1007/s00580-017-2565-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2565-5