Abstract
Pistacia atlantica hulls and Quercus infectoria galls have been used in traditional medicine for treatment of many disorders. In the current study, excision wound model was used for the assessment of wound-healing activity of topical co-administration of hydroethanolic extracts of Pistacia atlantica hulls (P. atlantica) and Quercus infectoria galls (Q. infectoria) in streptozotocin-induced diabetic mice. Four groups of diabetic mouse model were used; control group (received soft yellow paraffin), treated groups P. atlantica 5%; Q. infectoria 5% and Q. infectoria 5% + P. atlantica 5% mixed soft yellow paraffin. Two circular, full-thickness skin wounds with 5 mm diameter were created on the back of each of the mice. During the healing time, wound rate was measured and wound sample was obtained at the end of days 3, 7, and 14 for histopathological evaluation. Moreover, immunohistochemistry staining for GLUT-1 and GPC3 was done. According to the results, topical application of each hydroethanolic extract of pistachio and quercus extract alone and co-administrated together cause improved wound-healing activity in diabetic mice via decrease in inflammation phases with decrease of edema and immune cell migration scores, and proliferation stage with increase in new vessels formation, fibroblast infiltration, collagen synthesis scores, and GLUT-1- and GPC3-positive cells in diabetic mice. These results suggest that the topical application of P. atlantica hulls and Q. infectoria galls hydroethanolic extracts has beneficial effect on full-thickness wound-healing activity in diabetic mice and it might be useful for treating various types of chronic wounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a major health problem with high morbidity that has several social and economic consequences (Silva et al. 2007). This disease is the third largest killer of humans after cancer and cardiovascular disease (Li et al. 2004). Diabetes mellitus causes delay in the procedure of wound healing as a result of hyperglycemia, oxidative stress vascular insufficiency, and microbial infections (Abu-Al-Basalc 2009). Other complications of diabetes mellitus such as atherosclerosis, nephropathy, neuropathy, and foot deformities contribute to make the wounds chronic resulting in ulceration, necrosis, and amputation (Hirsch et al. 2008).
Glypicans as a member of proteoglycans family are enclosed to the cell surface by a glycosyl-phosphatidylinositol anchor. Considering the glypicans role in promoting several growth factors, estimating the expression of Glypican-3 (GPC3) in experimentally induced wounds seems to be important. Indeed, the glypicans are involved in physiologic interactions of several growth factors, including Hedgehogs (Song et al. 2005; Lum et al. 2003), bone morphogenetic proteins, and fibroblast growth factors (FGFs) (Capurro et al. 2008; Akiyama et al. 2008). In line with this issue, we here in present study tried to uncover the expression of GPC3, as a member of glypicans family, in experimentally induced diabetic wound model.
On the other hand, the intact/physiologic glucose up-take energizes the keratinocytes to be more mobile at the final stages of the healing that in turn facilitate the healing process (Takahashi et al. 2000; Shehu et al. 2013). Thus, it is hypothesized that migrating epithelium would require high levels of glucose to provide a substrate for glycolysis. It is well established that glucose is transported into all mammalian cells via the facilitated glucose transport proteins nominated as glucose transporters (GLUTs) (Takahashi et al. 2000). These proteins are involved in transporting the glucose into various cell types in different tissues. Seven isoforms have been identified and named based on the order of cloning (GLUT1–7). The expression of these isoforms is mainly inducible by insulin (Takahashi et al. 2000). Among these isoforms, GLUT-1 (50–55 kDa) is the most abundant and is expressed at high levels (Burant et al. 1993). GLUT-1 plays a critical role in cell survival, and its expression is enhanced when cells are starved for it (Haspel et al. 1986).
The Pistacia atlantica belongs to Anacardiaceae family, which grows annually in the Mediterranean basin to central Asia, especially in Iran, Turkey, Iraq, and Saudi Arabia. Many studies show that this herb has some properties such as sedative, anti-inflammatory, antimicrobial, antifungal, and anti-hyperlipidemia features (Farahpour et al. 2015; Farahpour and Fathollahpour 2015); on the other hand, it has been reported that it has useful effects on full-thickness wound healing and burn wound treatment (Farahpour et al. 2015; Farahpour and Fathollahpour 2015; Haghdoost et al. 2013).
Quercus infectoria is a species of oak, bearing galls that have been traditionally used for centuries in Asia medicinally. Many studies report that the galls of Quercus infectoria due to tannin, gallic acid, and ellagic acid have also pharmacological properties to possess astringent, antidiabetic (Hwang et al. 2000), anti-inflammatory (Kaur et al. 2004), and wound-healing activities (Jalalpure et al. 2002; Umachigi et al. 2008).
Despite the therapeutic effects of Pistacia atlantica hulls and Quercus infectoria galls co-administration, its role in experimental cutaneous wound healing in diabetic experimental animal is not studied. Therefore, the present study was designed to investigate effect of hydroethanolic extracts of Pistacia atlantica hulls and Quercus infectoria galls co-administration, as the new topical herbal formulation, on wound-healing process in diabetic mice. For this purpose, the possible alterations in expression of GLUT-1 and GPC3 and tissue histomorphometric parameters were evaluated.
Methods and materials
Plant material and extract preparation
Pistacia atlantica hulls were collected and picked by hand from the outskirts of Urmia, West Azerbaijan Province, Iran, in July 2015, latitude of 37 34′, longitude of 44 58′. Moreover, Quercus infectoria gall were collected and picked by hand from the outskirts of the Sardasht, Kordestan Province, Iran, in July, 2015, latitude of 367 9′, longitude of 45 29′. The plant was authenticated by the Department of Botany Sciences, Agriculture and Natural Resources Research Center, Urmia, Iran. Around 600 g of fresh Pistachio hulls and Quercus gall individually were dried naturally in shade on laboratory benches at room temperature (23–24 °C) for 6 days until crisp and powdered in an electric blender. Then, 150 g of the plant powder was suspended in 600 ml of hydroethanolic solution for 96 h at room temperature. The mixture was filtered using a fine muslin cloth followed by filter paper (Whatman No. 1). The filtrate was placed in an oven to dry at 40 °C. The obtained clear residue was used for the study. The extracts were kept at −20 °C until they were used in the experiment (Farahpour et al. 2015; Farahpour and Fathollahpour 2015).
Animals and induction of diabetes
Fifty-four mice aged 8 to 12 weeks were used in order to induce diabetes. The appropriate volume of Streptozotocin (Sigma-Aldrich Chemie Gmbh Munich, Germany) was dissolved in sterile citrate buffer (0.05 mol/L sodium citrate, pH 4.5, 55 mg/kg) and then was injected intraperitoneally for four consecutive days. Serum level of Glucose was analyzed 5 days after last administration of streptozotocin and then monitored closely for 2 weeks by using AlphaTrak glucose meter and strips. Diabetic mice (with fasting blood glucose levels >400 mg/dl) were used for experiment (Moura et al. 2014).
Wound induction, treatment, and measurement
All mice were anesthetized by intraperitoneal administration of ketamine 5%, 70 mg/kg (Ketaset 5%; Alfasan, Woerden, The Netherlands), and xylazine hydrochloride 2%, 10 mg/kg (Rompun 2%, Bayer, Leverkusen, Germany). The dorsal area was prepared aseptically, and the predetermined area was marked on the back of animals. Following surgical preparation, two 5 mm diameter circular full thickness wounds were created with a biopsy punch (Moura et al. 2014). Following wound induction, all mice were labeled by non-toxic color and divided into three groups including control and treatment (No = 12 for each group). The soft yellow paraffin as base formulation of ointment was administrated in control group. The animals in each test group were tropically treated with ointment containing Pistachio hulls hydroethanolic extract 5% (P. atlantica 5%), Quercus gall hydroethanolic extract 5% (Q. infectoria 5%), and combination of Pistachio hulls + Quercus gall hydroethanolic extract 5% (Q. infectoria 5% + P. atlantica 5%). The ointments were applied once a day for 14 successive days (Farahpour et al. 2015).
The wound area was measured by immediately placing a transparent paper over the wound and tracing it out (on days 3, 6, 9, and 12); the area of this impression was calculated using the graph sheet (Farahpour and Fathollahpour 2015).
Histopathological analyses
Following 3, 7, and 14 days after wound creation, the animals were euthanized and wound area was dissected out along with 1 to 2 mm from surrounding normal skin and a depth of approximately 3 mm. Specimens were then fixed in neutral-buffered formalin 10%. Tissue samples were routinely processed, paraffin wax embedded, sectioned at 5 μm, and stained with Masson’s trichrome and examined under light microscopy (Olympus CX31RBSF attached cameraman) to assess the predominant stage of wound healing. Three sections were prepared from each specimen and stained by Masson’s trichrome technique. Edema, cellular infiltration (immune cells, fibroblasts), and collagen deposition were analyzed. The results for collagen intensity and edema scores were presented in semi-quantitative format including negative (−), mild (+), mild to moderate (++), moderate (+++), and intensive (++++). All parameters were analyzed under ×400 magnification (Farahpour et al. 2015; Farahpour and Fathollahpour 2015).
Immunohistochimie staining technique for GLUT-1 and GPC3
Tissue section slides were heated at 60 °C for 25 min in a hot air oven (Venticell, MMM, Einrichtungen, Germany). The tissue sections were de-paraffinized in xylene and rehydrated using alcohol gradient. The antigen retrieval process was performed in 10 mM sodium citrate buffer. Immunohistochemical staining was conducted according to the manufacturer’s protocol (Biocare, USA). Briefly, endogenous peroxidase was blocked in a peroxidase blocking solution (0.03% hydrogen peroxide containing sodium azide) for 5 min. Tissue sections were washed gently with washing buffer and subsequently incubated with GLUT-1 and GPC3 (Biocare, USA) biotinylated primary antibodies (Rabbit/anti-mouse, 1:500) for 15 min. The sections were rinsed gently with washing buffer and placed in a buffer bath. Slides were placed in a humidified chamber with streptavidin–HRP (Streptavidin conjugated to horseradish peroxidase in phosphate-buffered saline containing an anti-microbial agent). Slides were incubated for 15 min. Subsequently, tissue sections were rinsed using washing buffer and placed in a buffer bath. A DAB chromogen was added to the tissue sections and incubated for 5 min, followed by washing and counterstaining with hematoxylin for 5 s. The sections were dipped in weak ammonia (0.037 M/L) 10 times, rinsed with distilled water, and cover slipped. Positive immunohistochemical staining was observed as brown stains under a light microscope.
Statistical analyses
Results were reported as mean ± SD. All analyses were performed using PASW version 18.0. PModel assumptions were evaluated by examining the residual plot. One-way ANOVA was used to analyze the results. Dunnett’s test for pair-wise comparisons was used to assess the effect of time and treatments. Differences were considered significant if < 0.05.
Result
Wound-healing rate
According to the results in Table 1, a significant (P < 0.05) increase was observed on wound contraction rate in all treated groups compared with control group at all days. Interestingly, there was no significant (P > 0.05) effect between 5% P. atlantica extract-treated group and 5% Q. infectoria extract-treated group, while co-administration of P. atlantica + Q. infectoria had better effect on reducing wound area compared to lone extract-treated groups on days 8, 12, and 14 post-wound creation (P < 0.05).
Histopathological findings
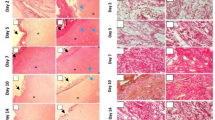
Observations demonstrated that animals treated with Q. infectoria 5%, P. atlantica 5% extracts, especially in co-administrated Q. infectoria 5% + P. atlantica 5% group, in the third and seventh days after the wound creation, showed lower edema score and immune cells infiltration compared to control animals (Table 2). Moreover, at the third and seventh day after the wound creation, the number of new vessels, fibroblast, and fibrocyte increased in all treated animals, especially in animals treated with combination of Q. infectoria 5% + P. atlantica 5%, compared to control animals (Table 2). Comparing the results at day 7 and 14 between groups showed that collagen biosynthesis score and Epithelial thickness increased in all treated animals, especially animals treated with combination of Q. infectoria 5% + P. atlantica 5%, compared to control animals (Table 2, Fig. 1).
Cross section from skin. a Control. b Q. infectoria 5%. c P. atlantica 5%. d Q. infectoria 5% + P. atlantica 5%. In first row, see well-formed dermis in Q. infectoria 5% and P. atlantica 5%-treated cross sections on day 14 after wound induction. Well-formed re-epithelialization is exhibited in Q. infectoria 5% + P. atlantica 5%-treated group. In second row, the dermal tissue of all groups is presented. The cross section from control group presents low collagen bundle generation. Meanwhile, the cross sections from Q. infectoria 5% and P. atlantica 5%-treated groups both alone and in simultaneous forms exhibited well-organized collagen biosynthesis as well as bundles. Masson-trichrome staining, ×100 and ×400 magnification
Observations demonstrated that Q. infectoria 5%, P. atlantica 5% extracts, and Q. infectoria 5% + P. atlantica 5% co-administration significantly (P < 0.05) enhanced the GPC3 and GLUT-1 expression versus non-treated animals, at days 7 and 14 after wound-induction (Figs. 2, 3, and 4). Comparing the alone forms of administration (5% P. atlantica and 5% Q. infectoria) with each other and with co-administrated groups showed no remarkable statistical differences between alone forms, while analyses exhibited a remarkable difference between alone forms and co-administrated ones.
Cross section from skin. a Control. b Q. infectoria 5%. c P. atlantica 5%. d Q. infectoria 5% + P. atlantica 5%. See increased biosynthesis of GPC3 in P. atlantica 5% and Q. infectoria 5% + P. atlantica 5%-treated groups (brown stained cells; first row). Immunohistochemical staining for GPC3, ×400 magnification. Note increased biosynthesis of GLUT-1 in keratinocytes and connective tissue cells in both Q. infectoria 5% and P. atlantica 5%-treated groups (arrows, second row). Cross section from Q. infectoria 5%-treated group presented intensive GLUT-1 expression in connective tissue cells compared with P. atlantica 5%-treated cross section. Immunohistochemical staining for GLUT-1, ×100 magnification
Discussion
Diabetes is a chronic disease in which the cells are not able to physiologically consume the serum glucose the same as intact ones do. Consistent with severe problems in up-taking the glucose, the diabetes adversely affects the wound-healing process by promoting infection. Indeed, the diabetes results in considerable impairments including nerve damage (neuropathy), weakened immune system, and vasoconstriction.
The natural wound-healing process is delayed when a wound is exposed to external environment since it will be more prone to being attacked by microbes through the skin. In fact, some therapeutic approaches as appropriate washing of skin wounds, administrating antibiotics and in some cases debridement of ulcers from wound area, are considered for diabetic conditions. Although mentioned methods are widely used for diabetic wound, the diabetes-induced infection, complexity of the wound-healing process in diabetic patients, and considerable immune system suppression results in delayed treatment. Thus, various trials have been performed in order to find out appropriate therapeutic intervals for accelerating healing process in diabetic patients.
Generally, wound healing is a dynamic process and consists of three phases including inflammatory, proliferative, and maturation that are largely impressible to the type and extent of damage, the ability of the tissue to repair, and different inflammatory factors (Farahpour et al. 2015; Farahpour and Fathollahpour 2015). Moreover, various chemotactic factors drive polymorphonuclear and mononuclear immune cells into the injured tissue where they can during healing inflammation phase.
Reactive oxygen species (ROSs), simply known as “oxidants,” consist of radical and non-radical oxidants which can provide a vital part of healing by serving as cellular messengers that induce numerous aspects of molecular and cell biology. Various beneficial pathways of wound healing can be triggered by ROS (Dunnill et al. 2015). On the other hand, microorganisms are located, identified, phagocytized, killed, and digested by these polymorphonuclear and mononuclear immune cells and wound debris is eliminated through phagocytosis and their characteristic activity of “respiratory burst” (Clark and Moon 1999). Thus, the wound site will be rich in oxidants together with their derivatives including chloramines (Sen et al. 2002). As a result, diabetic patients exhibit delayed healing process due to reduced transportation of glucose into the cells, which enhances the infection risk in these patients. Hence, wound-healing process can be accelerated by appropriate therapeutic agents incorporating a compound with antioxidant potential and antimicrobial activity.
Therefore, it can be assumed that infiltration of immune cells into wound area results in controlling the infection. In accordance with this issue, we analyzed immune cell infiltration in the wound area at different days after wound creation. Observations revealed infiltration of treated animal’s intensive immune cells, which was significantly decreased on all day’s post-wound creation. Considering rapid reduction of immune cells infiltration at days 3 and 7 indicates that Pistachio hulls extract only and combination with Quercus gall’s extract resulted in rapid and shortened inflammatory stage during wound healing.
Pistachio hulls extract has high levels of antibacterial and antioxidant properties due to high flavonoid and phenolic compound content (Farahpour et al. 2015; Farahpour and Fathollahpour 2015). Moreover, Quercus gall’s extract due to its high levels of tannins and phenolic compounds to a lesser extent has antioxidant, antibacterial, and free radicals-reducing properties (Jalalpure et al. 2002; Umachigi et al. 2008). To a cross link between the accelerative effect of slight oxidative stress in wound healing and promoting effect of administrated antioxidants, one should note that diabetic wounds exhibit massive ROS generation versus common wound models. Therefore, accelerated wound healing in extracts-treated groups may be partially attributed to their antioxidant properties.
In this respect, the variations at the third day can be attributed to the high anti-inflammatory properties in treatment groups, especially the groups that were treated with Pistachio hull extract. Interestingly, combined pistachio and Quercus gall’s hydroethanolic extracts had a synergistic effect so that the tannins in Quercus gall’s hydroethanolic extract along with antioxidant properties of pistachio hull extract accelerated the wound healing in diabetic mice compared with the other groups. This effect may be due to prevention of oxidative stress and the increase of free radicals scavenging in the granulation and healing tissues in topical application. Moreover, shortened inflammatory stage in treated groups may be attributed to pistachio and Quercus gall’s hydroethanolic extract-induced antibacterial properties. Accordingly, diminished bacterial contamination (following administration of extracts) could fairly shorten the inflammatory reactions, leading to shortened inflammatory stage.
At the next phase of the wound-healing process, healing is characterized by an increased cellularity (number of fibroblasts and fibrocyte). In order to understand how diabetes negatively affect the healing process, one should note that there is a positive cross link between insulin, GLUT-1, GPC3, and cellularity. Indeed, the GPC3 has also been reported to bind Insulin-like growth factor-II, which is responsible for participating in cell proliferation (Pilia et al. 1996), G1 cell cycle progression (Pietrzkowski et al. 1993), and prevention of apoptosis (Resnicoff et al. 1995). Considering the role of insulin-like growth factor in diabetes, we can hypothesize that Q. infectoria and P. atlantica extracts alone and in co-administration together by enhancing the GPC3 expression significantly provoked the interaction of insulin-like growth factor, which in turn improved cellularity and inhibited apoptosis. Increased cellularity (fibroblast, fibrocytes) in Q. infectoria 5%, and P. atlantica 5%-treated animals versus control animals confirms this hypothesis. Observations demonstrated that Q. infectoria and P. atlantica extracts significantly upregulated the GPC3 expression versus control animals. Thus, it can be inferred that Q. infectoria and P. atlantica extracts by enhancing the expression of GPC3 improved the interaction of different growth factors impact with their receptors (Lum et al. 2003; Akiyama et al. 2008), which in turn accelerated proliferation phase of wound-healing process.
At this stage, fibroblasts are the main cell source of connective tissue that plays the main role in the synthesis and secretion of collagen (Farahpour et al. 2015; Farahpour and Fathollahpour 2015). Examinations in the seventh day showed a significant increase in the fibroblasts infiltration to the wound site in the treatment groups compared with the control group. Concurrent with increase in the collagen synthesis and decrease in the wound size, epithelial cells are synthesized and move from the wound edges towards the center of the wound (Farahpour et al. 2015).
The glucose transportation across the cell plasma membrane facilities by accelerated diffusion is controlled by a family of transmembrane glycoproteins named as GLUTs (Olson and Pessin 1996; Cushman et al. 1998). Stimulation uptake of glucose, especially by insulin, is controlled via translocation of the GLUTs from the cytoplasm to the plasma membrane (Cushman et al. 1998). Studies on human keratinocytes have shown that these cells seem to express only GLUT 1 (Shen et al. 2000). Considering the important role of GLUT-1 in enhancing the cellular proliferation, we here in the present study tried to show the effect of OB- and PS-treated animals on GLUT-1 expression in connective cell lines. Immunohistochemical staining results at 7 and 14 days showed that Q. infectoria and P. atlantica-treated animals, alone and co-administrated together, significantly increased the GLUT-1 expression. Therefore, we can conclude that the Q. infectoria and P. atlantica-treated animals by up-regulating the GLUT-1 expression facilitated the glucose transport via cell membrane.
This reduces the wound surface that occurs with increased epithelial cell migration and collagen biosynthesis. Examinations on days 7 and 14 showed a significant increase in the collagen biosynthesis and epithelial cell migration and thickness score in the regenerative tissue samples in the treatment groups, especially in group treated with the combined ointment, compared with the control group.
In conclusion, the present study showed that Pistachio hulls and Quercus gall’s hydroethanolic extracts alone and in combination together accelerate the healing process in diabetic animal model through reducing the inflammatory phase, increasing the proliferation of connective tissue cells and collagen synthesis that result in shortening of the wound-healing time.
References
Abu-Al-Basalc MA (2009) In vitro and in vivo anti-microbial effects of Nigella sativa Linn. Seed extracts against clinical isolates from skin wound infections. Am J App Sci 6(8):1440–1445
Akiyama T, Kamimura Firkus C, Takeo S, Shimmi O, Nakato H (2008) Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol 313:408–419
Burant CF, Takeda J, Gould GW, Pendino KJ, Gardner CR, Laskin JD, Laskin DL, Miyata KS, Zhang B, Marcus SL (1993) Structure and function of mammalian facilitative sugar transporters. J Biol Chem 268:19161–19164
Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J (2008) Glypican-3 inhibits hedgehog signaling during development by competing with patched for hedgehog binding. Dev Cell 14:700–711
Clark LA, Moon RE (1999) Hyperbaric oxygen in the treatment of life-threatening soft-tissue infections. Respir Care Clin N Am 5(2):203–219
Cushman SW, Goodyear LJ, Pilch PF, Ralston E, Galbo H, Ploug T, Kristiansen S, Klip A (1998) Molecular mechanisms involved in GLUT4 translocation in muscle during insulin and contraction stimulation. Adv Exp Med Biol 441:63–71
Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos NT (2015) Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Inter Wound J. doi:10.1111/iwj.12557
Farahpour MR, Fathollahpour S (2015) Topical co-administration of flaxseed and pistachio ointment promoted wound healing; evidence for histopathological features. Comp Clin Pathol 24(6):1455–1461
Farahpour MR, Mirzakhani N, Doostmohammadi J, Ebrahimzadeh M (2015) Hydroethanolic Pistacia atlantica hulls extract improved wound healing process; evidence for mast cells infiltration, angiogenesis and RNA stability. Inter J Surg 31(17):88–98
Haghdoost F, Baradaran Mahdavi MM, Zandifar A, Sanei MH, Zolfaghari B, Javanmard SH (2013) Pistacia atlantica resin has a dose-dependent effect on angiogenesis and skin burn wound healing in rat. Evid Based Complement Alternat Med 27–33
Haspel HC, Wilk EW, Birnbaum MJ, Cushman SW, Rosen OM (1986) Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem 261:6778–6789
Hirsch T, Spielmann M, Zuhaili B, Koehler T, Fossum M, Steinau HU, Yao F, Steinstraesser L, Onderdonk AB, Eriksson E (2008) Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg 8(1):5–9
Hwang JK, Kong TW, Baek NI, Pyun YR (2000) Alpha-glycosidase inhibitory activity of hexagalloylglucose from the galls of Quercus infectoria. Planta Med 66(3):273–274
Jalalpure SS, Patil MB, Alagawadi KR (2002) Wound healing activity of the galls of Quercus infectoria Olivier. J Nat Remed 2(1):54–58
Kaur G, Hamid H, Ali A, Alam MS, Athar M (2004) Anti-inflammatory evaluation of alcoholic extract of galls of Quercus infectoria. J Ethnopharmacol 90(2):285–292
Li WL, Zheng HC, Bukuru J, De Kimpe N (2004) Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92(1):1–21
Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, Beachy PA (2003) Identification of hedgehog pathway components by RNAi in drosophila cultured cells. Science 299:2039–2045
Moura LI, Dias AM, Suesca E, Casadiegos S, Leal EC, Fontanilla MR, Carvalho L, de Sousa HC, Carvalho E (2014) Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. BBA-Mol Basis Dis 1842(1):32–43
Olson AL, Pessin JE (1996) Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr 16:235–256
Pietrzkowski Z, Mulholland G, Gomella L, Jameson BA, Wernicke D, Baserga R (1993) Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res 53(5):1102–1106
Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D (1996) Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nature Genet 12(3):241–247
Resnicoff M, Abraham D, Yutanawiboonchai W, Rotman HL, Kajstura J, Rubin R, Zoltick P, Baserga R (1995) The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res 55(11):2463–2469
Sen CK, Khanna S, Gordillo G, Bagchi D, Bagchi M, Roy S (2002) Oxygen, oxidants, and antioxidants in wound healing. Ann N Y Acad Sci 957(1):239–249
Shehu A, Lu J, Wilson H, Bach DQ, Shipp D, Randeria P, Mirkin C, Paller AS (2013) Cytoplasmic sequestration of keratinocyte GLUT1 by ganglioside GM3 mediates impaired diabetic wound healing. J Invest Dermatol 133:S247–S259
Shen S, Wertheimer E, Sampson SR, Tennenbaum T (2000) Characterization of glucose transport system in keratinocytes: insulin and IGF-1 differentially affect specific transporters. J Invest Dermatol 115:949–954
Silva SY, Rueda LC, Márquez GA, López M, Smith DJ, Calderón CA, Castillo JC, Matute J, Rueda-Clausen CF, Orduz A, Silva FA (2007) Double blind, randomized, placebo controlled clinical trial for the treatment of diabetic foot ulcers, using a nitric oxide releasing patch. PATHON 8(1):26–30
Song HH, Shi W, Xiang Y, Filmus J (2005) The loss of Glypican-3 induces alterations in Wnt signaling. J Biol Chem 280:2116–2125
Takahashi H, Ohara K, Ohmura T, Takahashi R, Zieske JD (2000) Glucose transporter 1 expression in corneal wound repair under high serum glucose level. Jpn J Ophthalmol 44:470–474
Umachigi SP, Jayaveera KN, Kumar CA, Kumar GS, Kumar DK (2008) Studies on wound healing properties of Quercus infectoria. Trop J Pharm Res 7(1):913–919
Acknowledgements
The authors are grateful to Ayande lab for histopathology study and Mohammad Aghaei (Dr) for the preparation of extracts and formulations.
Author information
Authors and Affiliations
Ethics declarations
Ethical approval
All animal experiments strictly complied with the guidelines of the Institutional Animal Ethical Committee no 2015/iau/111.
Funding
This study was the result of a thesis research project and was supported by Author’s own work (grant number 870,064,555).
Conflict of interest
Author A declares that he/she has no conflict of interest. Author B declares that he/she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Bonab, F.S., Farahpour, M.R. Topical co-administration of Pistacia atlantica hull and Quercus infectoria gall hydroethanolic extract improves wound-healing process. Comp Clin Pathol 26, 885–892 (2017). https://doi.org/10.1007/s00580-017-2473-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2473-8