Abstract
Trypanosomosis is a common disease in camels and other ruminants, horses, dogs, and of the most important diseases in camels causing huge losses in different countries. Two camel herds in mid-summer of 2014 were involved by wasting infectious disease with cachexia and progressive weakness in southwestern Iran. Upon clinical examination, tachycardia, fever, edema and facial swelling, keratitis, hypopyon, pale mucous membranes, alopecia, dehydration, enlarged lymphatic nodes, and reluctance to move were submitted in a large number of camels. Finally, according to the clinical signs, hematological and biochemical changes, and also observing trypanosomes in peripheral blood smears of camels, trypanosomosis was confirmed. For detection of trypanosome and assuring the virulence of current strain, laboratory-bred mice were inoculated intraperitoneally and subcutaneously with 0.5 ml of buffy coat or whole blood of each smear-positive camel. Parasitemia with anemia was observed in experimentally inoculated mice. Furthermore, the DNA was extracted from the blood and tissue samples of the camels and aborted fetus respectively, and analyzed by nested polymerase chain reaction (nPCR) using specific primers. Disease confirmed and the camels in both herds with the same clinical signs were treated by trypanocide drugs. The signs of trypanosomosis were obviated in all involved camels. It was concluded that trypanosomosis was the cause of abortion and the other observed signs in the camels of Khuzestan province, Iran.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trypanosoma evansi is the most widely distributed of the pathogenic African trypanosomes of animals, affecting domestic livestock and wildlife in Asia, Africa, and Latin America (Luckins and Dwinger 2004). This parasite is widespread throughout tropical and subtropical areas. However, in Africa, where camels may contact tsetse-transmitted trypanosomes, infections may also occur with T rypanosoma brucei, Trypanosoma congolense, Trypanosoma vivax, and Trypansoma simiae (Wernery and Kaaden 2002). T. evansi is transmitted by a number of species of hematophagous biting flies including Tabanus, Stomoxys, Lyperosia, and Haematobia (Rutter 1967). In addition to the name surra, other names such as “murrina,” “mal de caderas,” or “derrengadera” are used to describe similar diseases caused by trypanosomes indistinguishable from T. evansi in the other countries (Losos 1980; Luckins 1988). The degree of the disease may be acute, subacute, chronic, or inapparent, but generally, chronic form is the most common (Ngaira et al. 2003). In the acute form, trypanosomes are present in the blood and the disease is almost always fatal (Rutter 1967). In chronic forms, a huge production loss occurs due to lower milk and meat yields in adults. Abortions, premature births, and an inability to feed the young all greatly reduce the reproductive potential in affected herds (Yagil 1982). Natural and experimental camel trypanosomosis have been described in different parts of the world (Dia 1997; Al-Rawashdeh et al. 2000a, b; Pacholek et al. 2001; Njiru et al. 2002).
Severe outbreaks occurred in different parts of the world where several thousand animals affected in the 1970s and, of late, in 1994, 1995, 1998, 1999, 2000, 2004, and 2008. For instance, those in Pantanal, Brazil, UAE, Jordan, Canary Island, Chad, and France have also been well documented (Luckins 1988; Chaudhary and Iqbal 2000; Abo-Shehada et al. 1999; Gutierrez et al. 2000; Delafosse and Adoum Doutoum 2004; Desquesnes et al. 2008). Trypanosomosis was reported for the first time in Iran by Rafiee (1979). Derakhshanfar et al. (2010) in southern, Khosravi et al. (2013) in southeastern, Ahmadi-Hamedani et al. (2014), and Sazmand et al. (2011) in central regions of Iran reported outbreaks and prevalence of the disease, but based on our knowledge, this is the first documented outbreak in Khuzestan province, southwestern Iran. In this communication, we intend to determine the probability of the alteration in clinical, hematological, and some biochemical parameters of naturally infected camels (Camelus dromedarius) with T. evansi in the tropical regions of Iran and confirmed virulence of the present strain in Khuzestan province by inoculation test in the laboratory-bred mice.
Material and methods
Clinical examination
In mid-summer of 2014, two separate herds of 300 camels in rural areas of Ahvaz city with a history of death, abortion, stillbirth, cachexia, weakness, anorexia, depression, lack of production, diarrhea, and in some cases constipation had begun a month ago referred to Veterinary Teaching Hospital (VTH) of Shahid Chamran University of Ahvaz, Iran. Ahvaz is located in the southwest of Iran between 31° 19′ 21 N latitude and 48° 40′ 15 E longitude, and it is situated at elevation 23 m above sea level. It has a desert climate with long, very hot summers and mild, short winters. This city is consistently one of the hottest cities on the planet during the summer, with summer temperatures regularly at least 45 °C, sometimes exceeding 50 °C with many sandstorms and dust storms common during the summer period.
After clinical examination, the same signs included tachycardia, fever, edema and facial swelling, keratitis, hypopyon, pale mucous membranes, alopecia, dehydration, enlarged lymphatic nodes, sternal recumbency, and reluctance to move were submitted in the most of animals. The camels were treated with broad-spectrum antibiotic (Oxytetracycline) and nonsteroidal anti-inflammatory drug (Flunixin meglumine) for 4 days but no signs of recovery were noticed. Our veterinary team visited the affected herds and examined sick camels in order to obtain an accurate and complete assessment.
Sample collection
Camels with clinical signs and history of abortion, stillbirth, weight loss, lack of production, anorexia, and previous antibiotic treatment were considered for blood sampling and marked using the color spray, and information on age, sex, and parity number were recorded. For more evaluation, two separate blood samples of jugular vein were collected from 68 infected camels with clinical signs and 20 apparently healthy camels with approximately 4 years old, though most camels were females (78.1 % of the affected camels and 80 % of apparently healthy camels). One sample was collected into a tube containing EDTA for hematological parameters, parasitological survey, and total nucleic acid extraction for nested PCR (nPCR) amplification, another into a clot activator tube for biochemical parameters. The serum samples were collected and preserved at −20 °C until its processing. Also, a fresh tissue sample of some aborted fetus was randomly collected for DNA extraction and nPCR amplification.
Serological evaluation
To disprove the presence of other important abortive microorganisms in these camel herds, serum samples were analyzed for the presence of Brucella abortus to find Brucella-free camels (an endemic disease in Iran). Antigen for Rose Bengal plate test (RBPT) and serum agglutination test (SAT) was prepared and performed according to the method of Alton et al. (1975). B. abortus strain 99 was obtained from Razi Institute, Karaj. In RBPT, the results were taken as 1+ if the agglutination was visible after 4 min of mixing of serum and antigen, 2+ if the agglutination took place immediately, and negative if there was no agglutination. In SAT, the titers of 1/80 or above were considered positive.
Parasitological examination
Blood smears prepared using blood were taken aseptically from the ear vein of 68 affected camels and 20 apparently healthy camels (negative control group) on site, air dried, fixed with methanol, and in the parasitology laboratory stained with the conventional Giemsa stain (Baharafshan Co., Iran) and observed microscopically to investigate the presence of T. evansi.
To detect trypanosome and also to confirm the virulence of circulating pathogen in the camel herds, 20 laboratory-bred mice (about 5–7 weeks old, purchased from the Pasteur Institute, Iran) per any were selected for inoculation test by infected blood sample with T. evansi that confirmed microscopically. At the first, 20 mice randomly were divided into two groups with a normal diet, ad libitum water access, and similar condition, and five healthy mice were allocated as the control group. One group consisting of ten mice were inoculated intraperitoneally (IP) with 0.5 ml of buffy coat after centrifugation of infected blood and another group with ten mice inoculated subcutaneously (SC) with 0.5 ml of infected whole blood. After inoculation, mice were checked visually daily and by thin and thick wet smear examination of the tail-tip blood every 2 days in the presence of T. evansi.
Hemato-biochemical investigation
Red blood cell (RBC) count, hematocrit (HCT), hemoglobin (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), and white blood cell (WBC) count of sampled camels were analyzed using a BC-2800 Vet hematology analyzer (Mindray Co., China). For differential white blood cell counting, a blood smear was prepared and the number of neutrophils, lymphocytes, monocytes, and eosinophils were microscopically enumerated. In addition, above-mentioned hematological parameters in allocated mice were analyzed to survey the pathogenicity of the present strain of T. evansi.
Serum concentrations of fibrinogen, urea, creatinine, glucose, calcium, phosphorous, albumin, alkaline phosphatase (ALP), total protein, total bilirubin, direct bilirubin, and iron were measured using an automated clinical chemistry analyzer (Ebra Mannheim, XL 200, Germany) with commercially available kits (Pars Azmoon Co., Iran). Globulin concentration was calculated by subtracting the total protein value from albumin.
Extraction of DNA from blood and tissue samples
Total genomic DNA was extracted from the blood by the QIAGEN kit (QIAGEN Inc., Germany) according to the manufacturer’s instructions. Briefly, 200 μl of whole blood, 50 μl of proteinase K, and 200 μl of lysis buffer were added and incubated at 37 °C for 30 min, then at 70 °C for 10 min. Two hundred microliter of absolute ethanol was added to each sample and mixed by vortexing and spinning, then transferred to the column which was placed in a 2 ml collection tube and centrifuged at 8000g for 1 min. The QIAspin column was washed twice using 550 μl of washing buffers. The QIAamp spin column was then placed in a clean 1.5 ml tube and the DNA was eluted with 100 μl elution buffer preheated at 70 °C. Maximum DNA yield was obtained by spinning at 12,000g for 1 min. The DNA concentration was determined by spectrophotometry at 260 nm wavelength. Five microliter of the extracted DNA was used for PCR amplification.
Moreover, 1 gr of each tissue sample was collected from the liver and spleen of aborted fetus eluted in 5 ml distilled water and transported to the laboratory immediately. Tissue samples were mechanically broken down by homogenizer, then treated by freezing and thawing and finally centrifuge at 3000 RPM for 10 min. Two hundred microliter of the supernatant was used for DNA extraction according to the procedure which described before.
Primers selection
For detection, we used nPCR and the primer sets are as follows. Highly conserved regions of the published sequence of the nuclear repetitive gene of T. evansi were used for primer selection as F1 (5)-AGG ACG CAG AAA TAG CAG TA-(3) and R1 (5)-ATT TAA TTG AGT GGC GTG AG-(3) (Artama et al. 1992) with PCR product size 810 bp as external primers and for the internal sets of the primers F2 5-CTT TTA TAC GAG GAG AGG GA-3 and R2: 5-TAT GGG CGT GCA GAT TTC AC-3 that results in 270-bp fragment.
External and internal PCR
Two hundred fifty microliter of 10× PCR buffer plus 100 μl of magnesium chloride and 12.5 μl of dNTPs were mixed tougher thoroughly. The primers were used at 20 pg/μl concentration, and double distilled water was added to bring the volume of the stock buffer solution to 1.5 ml. Two microliter of primers, 5.0 μl of the target DNA, and 42 μl of the stock solution were added into 0.5 ml PCR tubes and mixed by shaking gently. One microliter of Taq DNA polymerase (CinnaGen Co.) was added at a concentration of 5.0 U/μl in a final volume of 50 μl. The thermal cycling program was 2 min initial incubation at 95 °C, followed by 40 cycles of 95 °C for 1 min, 55 °C for 30 s, and 72 °C for 45 s, and a final incubation at 72° for 10 min on a board thermocycler. Fifteen microliter of each PCR products was loaded onto 1.0 % agarose gels stained with safe stain (CinnaGen Co.) and electrophoresed and visualized by Gel doc under UV. Also for internal nested PCR, 2.0 μl of the primary PCR product resulted from F1R1, transferred to 0.5-ml PCR tube containing 2 μl of internal primers, 42 μl of the stock PCR buffer, and 5.0 U/μl of Taq DNA polymerase in a final volume of 50 μl. The PCR program was as follows: 2 min incubation at 95 °C, followed by 30 cycles of 94 °C for 1 min, 55 °C for 30 s, and 72 °C for 45 s, and a final incubation at 72 °C for 10 min. Fifteen microliter of each PCR product loaded onto 1.5 % agarose gel and electrophoresed. The gel was stained with safe stain and the nested PCR products were easily identified following observation under UV light (Fig. 1).
Results
Out of 88 tested blood samples, four cases (4.54 %) were serologically positive for brucellosis that were crossing checked by SAT. All remaining Brucella-free samples were selected for further studies and positive samples eliminated.
The definitive and relative frequencies of parasitological examination, epidemiological parameters, and history of two affected camel herds are shown in Table 1 in details. The parasitological results showed 75 % of tested camels had T. evansi smear-positive. In this study, rate of morbidity, mortality, and stillbirth were 20.3, 3.2, and 2.33 %, respectively. Also, 16 (5.3 %) and 20 heads (6.66 %) had abortion and retained placenta history in recent parturition, respectively (Table 1).
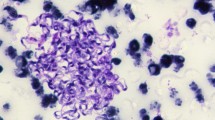
Clinical signs and biochemical parameters of the affected camels which were recorded and statistically compared based on the three groups of camels (negative control, smear-positive, and nPCR-positive camels) are shown in more detail in Table 3. The results of Table 3 illustrated that clinical signs of smear-positive camels were much more intense than nested PCR-positive camels and, in the majority of biochemical parameters, there was statistical difference between infected and control groups. In the present study, significant changes were found in terms of serum concentrations of urea (P < 0.01), fibrinogen (P < 0.001), glucose (P < 0.01), ALP activities (P < 0.05), total bilirubin (P < 0.05), direct bilirubin (P < 0.05), and iron (P < 0.001) in the group of smear-positive camels, while mean values of serum calcium, phosphorus, and creatinine had not changed significantly between groups. Besides, biochemical parameters in smear and nPCR-positive camels did not show a remarkable difference (Table 3). Also, due to observing trypanosome in peripheral blood samples of tested camels, parasitemia was confirmed (Fig. 2).
Fifty-seven (89.1 %) out of 64 tested blood samples investigated by nPCR were positive. External primer sets amplify our target sequence and internal primers specially amplify our target, and because internal primers have extra an annealing site out of target, we used nPCR to simplify the desired sequence exactly. The amplification of positive nPCR in four camels was shown in Fig. 1.
In the hematological survey of smear-positive camels, severe leukocytosis, neutrophilia, and decrement of HGB (P < 0.01), HCT (P < 0.01), and RBC concentrations (P < 0.05) were seen that refer to anemia as shown in Table 2.
Furthermore, in the laboratory bred-mice under study, on the fifth day in IP inoculated mice group with buffy coat and on the seventh day in SC inoculated mice group with the whole blood, for the first time T.evansi in the smears of the tail-tip blood detected and parasitic infection in both groups was confirmed. The mouse inoculation test (MIT) results were showed; IP inoculated mice with buffy coat had low mean values of RBC (P < 0.05), HCT (P < 0.05), and neutrophils (P < 0.05) and high mean values of MCHC (P < 0.05), WBC (P < 0.01), lymphocytes (P < 0.05), monocytes (P < 0.05), eosinophils (P < 0.01), and basophils (P < 0.01) compared to SC inoculated mice with whole blood and negative control mice (Table 2). Out of ten IP inoculated mice with buffy coat 100 % (10/10) and out of ten SC inoculated mice with whole blood 70 % (7/10) became infected, and trypanosomosis was observed in the tail-tip blood microscopically (Figs. 3 and 4).
The infected camels and camels with the same clinical signs were treated with diminazene aceturate 7 % (Rooyan Darou Pharmaceutical Co., Iran), 5 mg/kg BW, IM, q48 hours, flunixin meglumine (Flumax M®, Rooyan Darou Pharmaceutical Co., Iran), 2.2 mg/kg BW, IM, q12 hours, and vitamin B complex (B Coject®, Rooyan Darou Pharmaceutical Co., Iran), 0.1 ml/kg BW, IM, q24h. After 1-week, the affected camels re-examined and blood samples were taken to investigate blood parasite and hematological parameters, but signs of parasitemia and anemia had been disappeared. After 1 month gradually, abortion, stillbirth and mentioned signs were eliminated in the infected herds and production of camels return to its initial state.
Discussion
Camels are currently breeding in several provinces of Iran and represent populations are 156,000 heads. In 2009, there were 7832 heads of camel in Khuzestan Province which approximately included 8.7 % of the livestock population (FAO, 2009).
The significance and pathogenesis of trypanosome are mostly related to productivity decrement and progressive cachexia in the camels. It also can cause hump loss and muscle atrophy. Tracheal edema was noticed with a few purulent edemas. In the acute stage of the disease, diarrhea, abortion, and mortality are probably noticing in infected herds (Al-Rawashdeh et al. 2000a, 2000b). The general clinical signs of T. evansi infection are not sufficiently pathognomonic for diagnosis; therefore, laboratory tests are required for detecting the parasite. In early stages or acute cases, when parasite counts are high, examination of wet blood films, stained blood smears, or lymph node materials might reveal the trypanosomes. In more chronic cases or more generally when the parasitemia is low, the examination of thick blood smears, as well as methods for measuring parasite concentration along with the inoculation of laboratory rodents, is indispensable. In apparently healthy carriers, parasites are rarely observed and the MIT gives the best results (OIE Terrestrial Manual 2012).
Inoculation of the causative agent of disease in laboratory rodents is a highly sensitive method for the diagnosis of T. evansi infection (El-Baky and Salem 2011). Evaluation of hematological changes in experimentally infected mice that as evidence of the disease in camels also indicates anemia and leukocytosis in them. The results of the current study in Khuzestan province were in agreement with the results of Omer et al. (2007) and Raoofi et al. (2009), which both of them inoculated the blood of infected camels in laboratory mice and examined hematological alterations. Zia-ur-Rehman (1992) demonstrated neutropenia, lymphocytosis, and eosinophilia in one-humped camels infected with trypanosome, but his results are in contrast to the results of the present study. Perhaps, this difference is related to the stage of the disease. In the acute form of trypanosomosis or if the disease was diagnosed quickly, it will not become chronic; as a result, the signs of immune suppression and neutropenia will not be seen.
Sensitivity of different parasitological detection methods in the diagnosis of T. evansi in camels has been investigated by other authors; the sensitivity of blood examination was 45.0 % (Godfrey and Killick-Kendrick 1962), 26.0 % (Pegram and Scott 1976), and 50.0 % (Nantulya and Lindqvist 1989), respectively, but the sensitivity of MIT in all studies was 100 %. The ability of the MIT to detect T. evansi infection declined with decreasing parasite level in the blood, i.e., 96, 100, 95, and 80 % at 1000, 125, 25, and 12.5 T. evansi per milliliter, respectively (Reid et al. 2001). Substituting buffy coat for blood in the MIT increased its sensitivity for detecting T. evansi at least ten-fold. However, more mice should be tested with buffy coat to obtain an accurate estimate on the number of mice that would be required to ensure a 95 % probability of detecting infection with fewer than one T. evansi per milliliter of blood (Reid et al. 2001). In the present study, sensitivity of blood examination test, IP and SC inoculated mice were 100, 100 and 70.0 %, respectively (Table 1).
To assure the correct diagnosis, we sequenced two random PCR products and the blast result confirmed our detection (Fig. 1). The nPCR assay was a simple and rapid procedure which efficiently detected all T. evansi strains under the stringency conditions. The laboratory detection indicated that the nPCR protocol was capable of detecting the amount of more than 10 fg of total T. evansi genomic DNA. This could be very detrimental in the diagnosis of cryptic and latent carriers where parasite concentration is very much lower to be amplified by a simple PCR technique or by conventional serological and microscopic techniques, hence nPCR method is very accurate and sensitive for detection of acute and intense T. evansi infection.
In this study, by investigating hematological parameters, we found a significant reduction in the amount of erythrocytes, hematocrit, and hemoglobin concentration occurred in the infected camels with T. evansi compared to apparently healthy camels of the control group. Due to the remarkable increasing amount of MCV in the infected camels (P < 0.05), in term of cytological and etiological aspect, observed anemia was macrocytic and hemolytic, respectively. The results of present research in Khuzestan province were in agreement with the results of other authors (Chaudhary and Iqbal 2000; Saleh et al. 2009; El-Baky and Salem 2011). Hemolytic anemia that happens in T. evansi infection usually occurs after hemolysis due to a reduction in RBC life span and increase in erythro-phagocytosis. In addition, other factors such as immunological mechanism, coagulopathies, suppression of hematopoiesis, and release of sialidase enzyme from trypanosomes lead to hydrolysis of sialic acid (derived from N-acetyl neuraminic acid) in the membrane of RBCs and, as a consequence, lead to the destruction of red blood cells and anemia, which was mentioned clearly in the study of Ahmadi-hamedani et al. (2014).
With counting circulating leukocytes, there was a significant increase in the WBC level with leukocytosis in the infected camels (P < 0.01) than control group. Leukocytosis may be seen following neutrophilia in trypanosomosis as a result of increased activity of the phagocytic system. In the early stages of acute trypanosomosis, a significant increase in the number of neutrophils and lymphocytes occurs, because these cells are the first line of defense against T. evansi infection. The results of this study (Table 2) are in agreement with the findings of Chaudhary and Iqbal (2000) in the respect of differential WBC counting.
The results of biochemical parameters in the present study represent hypoalbuminemia with hyperglobulinemia in the infected camels (Table 3). In this disease, the presence of trypanosome in blood circulation stimulates the immune system, leading to more immunoglobulin secretion which results is hyperglobulinemia. In addition to the above-mentioned reason, hypoalbuminemia may be induced due to severe degenerative changes in the liver that is associated with the incidence of hepatic necrosis. Also, following hyperglobulinemia in the infected camels with T. evansi, hypoalbuminemia may be a compensatory mechanism to maintain the blood osmolarity. It should be noted that alteration of albumin/globulin ratio in the current study occurred due to the presence of hyperglobulinemia and hypoalbuminemia, so as a result, no changes was seen in serum concentration of total protein which is consistent with the results of Chaudhary and Iqbal (2000) and Ahmadi-hamedani et al. (2014). Hyperfibrinogenemia which occurred in our study refers to the presence of acute inflammation and active response of the immune system to T. evansi infection. Measurement of biochemical parameters in this study showed hyperfibrinogenemia, hyperbilirubinemia, uremia, hyperglycemia, and increasing ALP activity of serum in the smear-positive camels. Increased concentration of serum total bilirubin will occur after severe hemolytic anemia since the intensity of hemolysis is more than the conjugation capacity of the liver which leads to hyperbilirubinemia. Reduced serum ALP activity during parasitemia was reported by Boid et al. (1980) that came back to the normal range after treatment. The remarkable reduction in iron concentration was found in infected camels of the present study that can be regarded as a defense mechanism in the body which prohibits the parasite using from iron by sending it into the cells. Also, this decline may occur due to a decrement in the concentration of iron transportation protein (i.e., ferritin). This results about a significant reduction of iron concentration were in consistent with Chaudhary and Iqbal (2000).
In conclusion, the virulence of circulating strain of T. evansi in the present outbreak of Khuzestan province was confirmed and the alterations pattern of hematological parameters in experimentally infected mice and hemato-biochemical parameters with clinical signs in naturally infected camels (Camelus dromedarius) showed.
References
Abo-Shehada MN, Anshassi H, Mustafa G, Amr Z (1999) Prevalence of Surra among camels and horses in Jordan. Prev Vet Med 38:289–293
Ahmadi-hamedani M, Ghazvinian K, Mehdi Darvishi M (2014) Hematological and serum biochemical aspects associated with a camel (Camelus dromedarius) naturally infected by Trypanosoma evansi with severe parasitemia in Semnan, Iran. Asian Pacific J Trop Biomed 4(9):743–745
Al-Rawashdeh OF, Sharif LA, AL-Qudah KM, AL-Ani FK (2000a) Trypanosoma evansi infection in camels in Jordan. Revue Elev Med Vet Pays Trop 20:233–237
Al-Rawashdeh OF, Al-Ani FK, Sharrif LA, Al-Qudah KM, Al-Hami Y, Frank N (2000b) A survey of camel (Camelus dromedarius) diseases in Jordan. J Zoo Wildl Med 1:335–338
Alton GG, Jones LM and Pietz, DE (1975) Laboratory techniques in brucellosis. In: WHO monograph series No. 55, 2nd edn. WHO, Geneva
Artama WT, Agey MW, Danelson JE (1992) DNA comparison of Trypanosoma evansi (Indonesia) and Trypanosoma brucei spp. Parasitol 104:67–74
Boid R, Mahmoud M, Gray A (1980) Changes in the levels of some serum enzymes in dromedary camels infected with Trypanosoma evansi. Res Vet Sci 28(3):336–340
Chaudhary Z, Iqbal J (2000) Incidence, biochemical and hematological alterations induced by natural trypanosomosis in racing dromedary camels. Acta Trop 77:209–213
Delafosse A, Adoum Doutoum A (2004) Prevalence of Trypanosoma evansi infection and associated risk factors in camels in eastern Chad. Vet Parasitol 119:155–164
Derakhshanfar A, Mozaffari AA, Mohaghegh Zadeh A (2010) An outbreak of trypanosomiasis (Surra) in camels in the southern Fars province: clinical, hematological, and pathological findings. Res J parasitol 5(1):23–26
Desquesnes M, Bossard G, Patrel D, Herder S, Patout O, Lepetitcolin E, Thevenon S et al (2008) The first outbreak of Trypanosoma evansi in camels in metropolitan France. Vet Rec 162:750–752
Dia ML (1997) Epidémiologie de la trypanosomose cameline à T. evansi en Mauritanie. Thèse Doct. Sci. University of Montpellier I, France
El-Baky AAA, Salem SI (2011) Clinicopathological and cytological studies on naturally infected camels and experimentally infected rats with Trypanosoma evansi. World App Sci J 14(1):42–50
Godfrey DG, Killick-Kendrick R (1962) Trypanosoma evansi of camels in Nigeria: a high incidence demonstrated by the inoculation of blood into rats. Ann Trop Med Parasite 56:14–19
Gutierrez C, Juste MC, Corbera JA, Magnus E, Verloo D, Montoya JA (2000) Camel trypanosomosis in the Canary Islands: assessment of seroprevalence and infection rates using the card agglutination test (CATT/T. evansi) and parasite detection tests. Journal of Vet Parasitol 90:155–159
Khosravi A, Hakimi Parizi M, Bamorovat M, Borhani Zarandi M, Mohammadi MA (2013) Prevalence of Trypanosoma evansi in camels using molecular and parasitological methods in the southeast of Iran. J Parasitic Dis 39:422–425
Losos GJ (1980) Diseases caused by Trypanosoma evansi: a review. Vet Res Comm 4:165–181
Luckins AG (1988) Trypanosoma evansi in Asia. Parasitol Today 4:137–142
Luckins AG, Dwinger RH (2004) Non-tsetse-transmitted animal trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA (eds) The trypanosomiases. CABI Publishing, pp 269–282
Nantulya VM, Lindqvist KJ (1989) Antigen detection enzyme immunoassays for the diagnosis of Trypanosoma vivax, T. congolense and T. brucei infections in cattle. Trop Med Parasitol 40:267–272
Ngaira JM, Bett B, Karanja SM, Njagi ENM (2003) Evaluation of antigen and antibody rapid detection tests for Trypanosoma evansi in camels in Kenya. Vet Parasitol 114:131–141
Njiru ZK, Bett B, Mapeny-Ole IM, Githiori JB, Ndung’u JM (2002) Trypanosomosis and helminthosis in camels: comparison of the ranch and traditional camel management systems in Kenya. Camel Pract Res 9:67–71
OIE Terrestrial Manual (2012) Trypanosoma evansi infection (surra). Chapter 2.1.17
Omer OH, Mousa HM, Al-Wabel N (2007) Study on the antioxidant status of rats experimentally infected with Trypanosoma evansi. Vet Parasitol 145:142–145
Pacholek X, Gamatic D, Franek SG, Tibayrene R (2001) Prevalence of Trypanosoma evansi trypanosomosis in young camels in west Niger. Revue Elev Med Vet Pays Trop 44:177–182
Pegram RG, Scott JM (1976) The prevalence and diagnosis of Trypanosoma evansi infection in camels in southern Ethiopia. Trop Anim Hlth Prod 8:20–27
Rafiee A (1979) Comparative veterinary protozology, 1st edn. Research council press of ministry of sciences and technology, Iran
Raoofi A, Kazempoor R, Akbarinejad V, Shojaei M, Tabatabaei SS (2009) Natural trypanosomosis in a Bactrian camel (Camelus bactrianus) in Iran. J Camel Prac and Res 16(2):237–239
Reid SA, Husein A, Copeman DB (2001) Evaluation and improvement of parasitological tests for Trypanosoma evansi infection. Vet Parasitol 102:291–297
Rutter TEG (1967) Trypanosomiasis in camels. Vet Bull 37:611
Saleh MA, Al-Salah MB, Sanousi SA (2009) Oxidative stress in blood of camels (Camelus dromedaries) naturally infected with Trypanosoma evansi. Vet Parasitol 162:192–199
Sazmand A, Rasooli A, Nouri M, Hamidinejat H, Hekmatimoghaddam S (2011) Serobiochemical alterations in subclinically affected dromedary camels with Trypanosoma evansi in Iran. Pak Vet J 31:223–226
Wernery U, Kaaden OR (2002) Infectious diseases in camelids, 2nd edn. Blackwell Wissenschaftsverlag, Berlin
Yagil R (1982) FAO animal production and health paper no. 26. Camels and camel milk. Food and Agriculture Organization of the United Nations, Rome, p. 41
Zia-ur-Rehman (1992) Serum biochemical, enzymes and hematological changes in one-humped camels infected with Surra. Proceedings of the First International Camel Conference, Dubai , p. 405February 2-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed were in accordance with the ethical standards of the ethical research committee of Shahid Chamran University of Ahvaz and were in full compliance of the principles of animal welfare.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zakian, A., Nouri, M., Safaei, P. et al. An acute outbreak of natural Trypanosoma evansi infection in camel (Camelus dromedarius) herds in the southwestern Iran. Comp Clin Pathol 26, 51–59 (2017). https://doi.org/10.1007/s00580-016-2345-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-016-2345-7