Abstract
Several studies have demonstrated asymbiotic growth and development of arbuscular mycorrhizal (AM) fungi, although AM fungi are regarded as obligately symbiotic root-inhabiting fungi. Phytohormones, root exudates, and volatiles are important factors regulating the host-AM fungi interaction. However, the effects of phytohormones, root exudates, and volatiles on asymbiotic (without roots present) or pre-symbiotic (with roots present but no colonization) sporulation of AM fungi are unexplored. In this study, we tested the asymbiotic sporulation of Rhizophagus irregularis DAOM 197198 and further investigated the influences of abscisic acid (ABA), the exudates, and volatiles of tomato hairy roots on asymbiotic or pre-symbiotic sporulation in vitro. Results indicated that mother spores asymbiotically and pre-symbiotically produced daughter spores singly or in pairs. Compared with symbiotically produced spores, pre-symbiotically produced spores were significantly smaller (43.1 μm vs. 89.2 μm in diameter). Exogenous ABA applied to mother spores significantly increased the number of daughter spores, and root volatiles also significantly promoted pre-symbiotic sporulation. Our results provide the first evidence that exogenous ABA can promote AM fungal asymbiotic and pre-symbiotic sporulation, which highlights the potential role of phytohormones in AM fungal propagation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytohormones are essential internal factors regulating plant growth and development. Intriguingly, phytohormones are also involved in the regulation of plant-microbe interactions, such as pathogenesis and symbiosis (Cao et al. 2018; Fousia et al. 2018; Zhang et al. 2018). Arbuscular mycorrhizal (AM) fungi are ubiquitous symbiotic fungi, which belong to the subphylum Glomeromycotina (Spatafora et al., 2016), and form mycorrhizas in association with most terrestrial plants (Powell and Rillig 2018). The regulation of the establishment of this symbiosis by phytohormones has been intensively studied and recognized for decades (Liao et al. 2018). For example, application of synthetic auxin analog (2,4-D or NAA) at 10−10 M to roots significantly increased root length colonization and arbuscule abundance in tomato, alfalfa, and rice plants (Etemadi et al. 2014). In contrast, application of exogenous gibberellin (GA3) at 10−6 M to roots significantly suppressed root length colonization and arbuscule abundance in Lotus japonicus (Takeda et al. 2015). Similar to auxin, abscisic acid (ABA) exhibited a positive effect on root length colonization and arbuscule abundance in tomato plants, which was revealed by using the ABA-deficient sitiens mutant (Herrera-Medina et al. 2007), probably with a different action mode from that of auxin (Charpentier et al. 2014). Although AM fungi can regulate ABA biosynthesis in roots (Martín-Rodríguez et al. 2011), no direct evidence indicates the biosynthesis of ABA in AM fungi thus far. Therefore, the effect of ABA on AM fungal sporulation is far from being well understood. Strigolactones, firstly isolated and identified from root exudates of Lotus japonicus and recently identified as novel phytohormones (Al-Babili and Bouwmeester, 2015), showed a strong stimulatory effect on branching of AM fungal germ tubes (Akiyama et al. 2005).
Root exudates (excluding root volatiles) contain a variety of compounds regulating the establishment of AM symbiosis. Although root exudates also contain phytohormones, including ABA (Vives-Peris et al., 2017), other compounds in root exudates can affect the formation of arbuscular mycorrhizas. In order to distinguish the effects of phytohormones from the effects of other compounds, we refer to those compounds other than phytohormones as “root exudates” hereafter. So far, a series of compounds in root exudates have been demonstrated to affect spore germination, germ tube growth, and host penetration by AM fungi (Graham 1982; Nair et al. 1991; Scervino et al. 2005, 2006). Flavonoid apigenin, commonly present in root exudates of most plants, significantly increased spore germination of Gigaspora rosea (Scervino et al. 2006).
In general, studies indicate that root exudates including phytohormones are active in regulating AM symbiosis and that root-associated microbes as well can produce diverse phytohormones (Egamberdieva et al. 2017). However, most such experiments focused on pre-symbiotic (with roots present but no colonization) or symbiotic (with root colonization) status, and their effects in asymbiotic (without roots present) status are largely unknown. Pre-symbiotic status can be much different from asymbiotic status in terms of spore germination and subsequent germ tube growth of AM fungi because the signaling molecules from roots are absent in asymbiotic status. It is well known that AM fungi are obligately symbiotic fungi, exclusively depending on their hosts for carbon resources. Intriguingly, however, AM fungal sporulation in asymbiotic status has been reported for two fungal species, e.g., Glomus intraradices and Rhizoglomus irregulare (also known as Rhizophagus irregularis) (Hildebrandt et al. 2002, 2005; Kokkoris et al. 2019). These studies encouraged us to test whether ABA and root exudates promote AM fungal sporulation in asymbiotic or pre-symbiotic status. Given that AM fungi and hosts have established their intimate relationship during 450 million years of evolution (Redecker et al. 2000), we hypothesized that phytohormones might exert a direct influence on AM fungi besides the indirect one via host plants. Particularly, we hypothesized that ABA and root exudates could directly regulate AM fungal sporulation in asymbiotic and pre-symbiotic status. This study aimed to investigate the influence of exogenously applied ABA on AM fungal sporulation without the establishment of symbiosis. Moreover, the effects of root exudates and volatiles also were evaluated by comparing effects of the presence and absence of host roots.
Materials and methods
Biological materials
We established AM symbiosis with tomato (Solanum lycopersicum Xinjinfeng No. 1) and Rhizophagus irregularis DAOM 197198. R. irregularis DAOM 197198 was commercially obtained from Premier Tech Co., Québec, Canada, and propagated in vitro in symbiosis with tomato hairy roots. Mature spores were harvested from this system and used in this study. Hairy roots were transformed with Agrobacterium rhizogenes ACCC 10060 according to the method by Bécard and Piché (1992). MSR medium was used as substrate for the growth of hairy roots and spores (Declerck et al. 1998).
Experimental setup
Experiment 1: Observation of asymbiotic sporulation of R. irregularis DAOM 197198
A glass Petri plate (9 cm in diameter) was used to incubate spores in vitro. In each plate, approximately 100 spores were inoculated onto MSR medium without hairy roots. Ten inoculated plates were prepared for the observation of asymbiotic sporulation. Efforts were made to separate each spore from others during inoculation, but, in most cases, several spores were attached to each other with the subtending hyphae entangled. Therefore, they were inoculated onto the medium as clustered spores (CS); single spores (SS), although limited in number, also were inoculated onto the medium. All plates were incubated in the dark at 25 °C for 4 weeks at which time daughter spores appeared.
Experiment 2: Effects of exogenous ABA on asymbiotic sporulation of R. irregularis DAOM 197198
MSR medium was prepared with the addition of ABA (final concentration of 1 × 10−7 M) or not for the evaluation of any ABA effect on asymbiotic sporulation. ABA was first dissolved in 1 M NaOH to produce a 1 M ABA solution, and then, the solution was diluted with ddH2O to produce 1 × 10−2 M ABA solution which was further sterilized with 0.22-μm pore-sized membrane filtration. Finally, the sterilized ABA solution was added to the autoclaved MSR medium so that the final concentration of ABA was 1 × 10−7 M. For the control without ABA application, the solution without ABA addition was used. Spores were inoculated onto MSR medium as described in Experiment 1. Two treatments (−ABA and +ABA) were established without hairy roots present. A total of 12 plates, with 6 replicates for each treatment, were incubated in the dark at 25 °C for 12 weeks. Then, the daughter spores in each plate were counted and recorded.
Experiment 3: Effects of exogenous ABA and root exudates on pre-symbiotic sporulation of R. irregularis DAOM 197198
To distinguish the effects of ABA versus hairy root exudates, each plate was divided into two equal compartments with two types of barriers, aluminum sheet or 0.45-μm pore-sized cellulose membrane, thus producing a root compartment (RC) and a spore compartment (SC) (Fig. 1). Any diffusion of root exudates between the two compartments and physical contact of roots and germ tubes were prevented by the aluminum sheet, while only physical contact of roots and germ tubes was prevented by the cellulose membrane. Moreover, neither barrier type could prevent the diffusion of root volatiles. In each RC, 5 hairy root fragments of ~3 cm length were incubated, while approximately 100 spores were incubated in each SC as described previously. For each type of barrier, three treatments were set depending on ABA application, including no application, application to the RC, or application to the SC. A total of 36 plates, with 6 replicates for each treatment, were incubated in the dark at 25 °C for 12 weeks.
The experimental setup used in Experiment 3. a Plate was divided into two equal compartments with aluminum sheet sealed with silicon sealant. b Plate was divided into two equal compartments with 0.45-μm pore cellulose membrane sealed with silicon sealant. c Diagram showing the growth of hairy roots and spore germination in the root compartment (RC) and spore compartment (SC). d Picture showing the real status corresponding to (c)
During the experiments, we observed that the size of the pre-symbiotically produced spores was much smaller than that of symbiotically produced spores (namely the mother spores). In order to compare the diameters of newly produced spores with pre-symbiotic and symbiotic status, 6 plates inoculated with hairy roots and spores were prepared to establish symbiosis for symbiotic sporulation. The plates were incubated in the dark at 25 °C for 12 weeks.
Microscopic observation and measurements of spores
AM fungal sporulation in asymbiotic and pre-symbiotic status was observed and recorded under microscopy (Olympus BX53). Daughter spores in each SC were counted at 4, 8, and 12 weeks after inoculation (WAI). For the measurement of spore diameter, an open-access software, ImageJ (https://imagej.nih.gov/ij/), was used. Fifty spores were randomly selected for the daughter spores produced from the CS in pre-symbiotic status or for those produced in symbiotic status, while only 25 spores were measured for those produced from SS in pre-symbiotic status due to their limited number as mentioned previously.
Data analysis and statistics
All the presented data were the means of 4~6 replicates due to contamination in several plates, although 6 replicates were prepared for each treatment at the beginning. An independent sample t test, two-way analysis of variance (ANOVA), and post hoc test (Tukey HSD) were performed with SPSS v.21. To compare the effects of ABA and root exudates (e.g., with hairy roots present) on sporulation, variance partitioning analysis (VPA) was performed using the “varpart” function in R vegan package (Peres-Neto et al., 2006; Oksanen et al., 2016, Chen et al., 2017).
Results
Rhizophagus irregularis DAOM 197198 sporulated in asymbiotic status (Experiment 1)
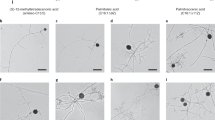
In Experiment 1, we observed that the spores of R. irregularis DAOM 197198 germinated on the medium within 2 days without hairy roots present, and these germinated spores (both clustered spores and single spores) asymbiotically sporulated within 4 WAI (Fig. 2). The germ tubes produced both daughter spores and highly branched hyphal structures (HBHS) (Fig. 2a). Daughter spores at the proximal position were more mature (dark yellow to brown in color) than those at the distal position (transparent to white-yellow in color) (Fig. 2b). The daughter spores were produced at the branched hyphal tip singly or in pairs with the single pattern dominating (Fig. 2c).
The sporulation of R. irregularis DAOM 197198 in asymbiotic status on MSR medium within 4 weeks after inoculation (Experiment 1). Each panel is a composite picture of several original pictures. a Clustered mother spores germinated with one highly branched hyphal structure (HBHS) and one daughter spore singly produced at the tip of a hypha. b Clustered mother spores germinated with 3 daughter spores singly produced at the tips of hyphae. c Single mother spore germinated with 4 daughter spores produced in pairs at the tips of hyphae. CS, clustered mother spores; SS, single mother spore; GT, germ tube; HBHS, highly branched hyphal structure. Arrow heads indicate asymbiotically produced daughter spores without hairy roots present. Bar indicates 500 μm
Exogenous ABA promoted the asymbiotic sporulation of Rhizophagus irregularis DAOM 197198 (Experiment 2)
In Experiment 2, the asymbiotic sporulation of R. irregularis DAOM 197198 without hairy roots present occurred on MSR medium, which was consistent with that in Experiment 1. Intriguingly, the numbers of the daughter spores were 0.5, 3.2, and 4.7 per plate at 4, 8, and 12 WAI with no application of exogenous ABA, in contrast to those of 3.4, 7.6, and 8.8 per plate with application of exogenous ABA (Fig. 3), which clearly indicates the significant promotion of asymbiotic sporulation (P = 0.018, 0.007 at 4 and 8 WAI, respectively) by application of exogenous ABA at two out of the three sampling times.
The promotion of AM fungus asymbiotic sporulation by exogenous ABA applied to the spore compartment without hairy roots present (Experiment 2). −ABA, no ABA application to medium; +ABA, application of 1.0 × 107 M ABA to medium; WAI, weeks after inoculation. t tests for each sampling time were performed to evaluate the effects of exogenous ABA applied to the medium. The upper and lower edges of each box indicate the 75% and 25% quantiles, and the upper and lower whiskers indicate the maximum and minimum values
Exogenous ABA promoted the pre-symbiotic sporulation of Rhizophagus irregularis DAOM 197198 (Experiment 3)
In Experiment 3 with compartmented plates, hairy roots were present but spatially separated from spores. Two-way ANOVA did not indicate a significant difference between the aluminum sheet barrier and the membrane barrier (P = 0.184, 0.628, and 0.126 at 4, 8, and 12 WAI, respectively), but does indicate a significant difference among the three ABA treatments (P = 0.009 and < 0.001 at 8 and 12 WAI, respectively) (Table 1). In detail, according to Tukey’s post hoc test, it seems that the exogenous ABA applied to the spores significantly promoted pre-symbiotic sporulation especially at 12 WAI (Table 1).
Comparison of the sporulation of Rhizophagus irregularis DAOM 197198 among asymbiotic, pre-symbiotic, and symbiotic statuses
It is interesting to compare the sporulation between asymbiotic (without hair roots present, Experiment 2) and pre-symbiotic status (with hairy roots present but no colonization, Experiment 3). Regardless of the application of exogenous ABA, the overall mean spore numbers in asymbiotic status were 1.8, 5.2, and 6.5 per plate at 4, 8, and 12 WAI respectively, while those in pre-symbiotic status were 6.9, 25.3, and 41.8 per plate at 4, 8, and 12 WAI, respectively. The difference between asymbiotic and pre-symbiotic status was significant (P = 0.002, < 0.001, and < 0.001 at 4, 8, and 12 WAI respectively) across all three sampling times. Moreover, VPA analysis indicates that the contribution of hairy roots (67% and 54% at 8 and 12 WAI, respectively) to the promoted sporulation was much higher than that of ABA (23% and 21% at 8 and 12 WAI, respectively).
The average diameter of the daughter spores was further compared between the pre-symbiotic status and the symbiotic status. It is notable that the average diameter of spores produced symbiotically was 89.2 μm, significantly larger than that (43.1 μm) produced pre-symbiotically (P < 0.001) (Fig. 4). Moreover, in the pre-symbiotic status, the average diameter of daughter spores produced from clustered mother spores was 48.6 μm, significantly larger than that (32.1 μm) produced from single mother spores (P < 0.001).
The diameter (μm) of spores produced from colonized hairy roots or from pre-symbiotic spores (Experiment 3). For the daughter spores produced from clustered mother spores in pre-symbiotic status or those produced in symbiotic status, 50 spores were randomly selected, while only 25 spores were measured for those produced from single mother spores in pre-symbiotic status due to limited spore numbers. symbiotic, spores produced from colonized hairy roots; pre-symbiotic-single, spores produced from single mother spores in pre-symbiotic status; pre-symbiotic-clustered, spores produced from clustered mother spores in pre-symbiotic status. The means accompanied by the same lowercase letter do not differ significantly by the Tukey HSD (P = 0.05). The upper and lower edges of each box indicate the 75% and 25% quantiles, and the upper and lower whiskers indicate the maximum and minimum values
Discussion
AM fungi are obligately symbiotic fungi; however, R. irregularis DAOM 197198 sporulated in asymbiotic and pre-symbiotic status without the establishment of symbiosis with roots in this study (experiment 1). To our knowledge, this is not the sole report of asymbiotic or pre-symbiotic sporulation in AM fungi. Recently, Kokkoris et al. (2019) found that an isolate (9A2) of Rhizoglomus irregulare (also known as Rhizophagus irregularis) sporulated in asymbiotic status as in this study, although other 13 isolates of this species did not. Similarly, Müller et al. (2017) reported that dead roots supported the sporulation of Funneliformis mosseae, indicating the possibility of an abiotrophic nature. Sometimes, asymbiotic sporulation by Glomus intraradices was induced by the presence of particular bacterial taxa, such as Paenibacillus validus (Hildebrandt et al. 2002, 2005). Therefore, it is likely that asymbiotic and pre-symbiotic sporulation does exist but is not common across all AM fungal species and is confined only to some species or isolates, controlled by unknown mechanisms. Hildebrandt et al. (2005), however, demonstrated the normal functions (e.g., germination, colonizing capacity) of those daughter spores derived from asymbiotic culture. Functions of the daughter spores from our study need further investigation.
In this study, exogenous ABA was able to promote sporulation in both asymbiotic (Experiment 2) and pre-symbiotic (Experiment 3) status, highlighting the direct effect of ABA on spores or germ tubes. Moreover, in the promotion of pre-symbiotic sporulation, the root volatile effect was greater than that of ABA as revealed by VPA. To the best of our knowledge, this is the first report demonstrating the direct and promotive effect of ABA on AM fungal sporulation. Although roots and root-associated microbes produce and release ABA into surrounding soils (Egamberdieva et al. 2017), ABA concentration in natural soils is only 0.6~2.8 nM (Hartung et al. 1996) which is a hundredfold lower than that applied in this study. Previous work suggested that either geminating spores or germ tubes can sense substrates, where root exudates (strigolactones), biocides (triclosan), and even seed exudates affect spore germination and germ tube branching (Akiyama et al. 2005; Twanabasu et al. 2013; Coelho et al. 2019). Therefore, we infer that germinating spores or germ tubes perceived ABA in the medium which in turn promoted the subsequent sporulation.
Silva-Flores et al. (2019) demonstrated the association between some edaphic factors and AM fungal sporulation. For instance, clay content, electrical conductivity, and total N showed positive correlations while available P and organic matter showed negative correlations with spore density. Promoted sporulation under drought condition (rainy season vs. dry season) has been reported previously (Leal et al. 2016). More intriguingly, a period of drought stress before harvest is frequently practiced to promote sporulation in AM fungal propagation systems (Selvakumar et al. 2018). However, whether these factors affect AM fungal sporulation directly or via host passage is yet to be elucidated.
Our study indicates a direct effect of ABA on AM fungal sporulation with asymbiotic and pre-symbiotic culture systems. Sporulation in fungi is one of the mechanisms by which fungi respond to adverse conditions, in many cases producing chlamydospores (Spraker et al. 2016; Dijksterhuis 2018). Optimal osmotic stress facilitates the sporulation of Monilinia fructicola (Hong and Michailides 1999). Although ABA biosynthesis by fungi has been documented and reviewed, ABA functionality in fungi is less investigated than its biosynthesis (Hartung 2010).
AM fungi are symbiotic fungi, whose response to stresses is strongly associated with hosts. Thus, AM fungi and hosts might develop some common mechanism to co-adapt to stresses, e.g., ABA signaling. When encountering stresses, ABA content in plants normally increases and regulates a series of physiological processes which enhance host tolerance (Kuromori et al. 2018). In this scenario, we speculate that the increased ABA in plants in response to stresses can be transferred to AM fungi and probably can contribute to triggering the events involved in sporulation. Moreover, AM fungi began to form symbiosis with plants more than 450 million years ago (Redecker et al. 2000); therefore, it is possible that AM fungi have evolved to sense and respond to ABA signaling. The response of sporulation to ABA might be vertically inherited so that germinating spores can respond to exogenously applied ABA, as in this study. However, we have little knowledge on how ABA regulates AM fungal sporulation in symbiotic, asymbiotic, or pre-symbiotic status thus far. We searched the published AM fungal genome (Tisserant et al. 2013) for the information on ABA receptors, but no relevant information was available. On the other hand, ABA overproduction mutants could be employed to test the results obtained in this study, but no such mutant has been reported. Presently, only some ABA-deficient mutants are available (Groot and Karssen 1992; Nitsch et al. 2012).
In this study, we prevented the establishment of symbiosis with two types of barriers (aluminum plate vs. membrane). Although root exudates could pass membranes, they did not affect pre-symbiotic sporulation. We propose two explanations: (1) root exudates really were not capable of affecting pre-symbiotic sporulation, although they can affect other pre-symbiotic bioprocesses such as spore germination and hyphal branching (Gemma and Koske 1988; Suriyapperuma and Koske 1995; Akiyama et al. 2005) or (2) root exudates did not reach spores due to restricted diffusion in agar. In contrast, root volatiles could reach spores regardless of barrier type. The comparison between experiment 2 and experiment 3 suggested that hairy root volatiles promoted pre-symbiotic sporulation. The spore density in experiment 3 doubled that in experiment 2 and higher spore density may inhibit spore germination in fungi (Louis et al. 1988; Page et al. 2017). It might also be inhibitory to subsequent hyphal growth and sporulation. This suggests that root volatiles might be particularly potent in promoting pre-symbiotic sporulation of AM fungi. Although many studies have investigated the effects of root exudates on pre-symbiotic behavior (Gemma and Koske 1988; Suriyapperuma and Koske 1995; Akiyama et al. 2005; Besserer et al. 2008), fewer studies have focused on root volatiles. According to early studies (Bécard et al. 1992; Ishii et al. 1996), CO2 and ethylene, both of which can be derived from roots, greatly increased germ tube growth in pre-symbiotic status of AM fungi. Our study demonstrates for the first time that the root volatiles from tomato hairy roots were potent in promoting the pre-symbiotic sporulation of R. irregularis DAOM 197198. However, we did not identify the root volatiles and could not tell whether they were CO2 or ethylene. Much work is required to provide insights into this phenomenon, e.g., identification of the functional volatile components and their effects on other AM fungal processes.
References
Akiyama K, Matsuzaki KI, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Al-Babili S, Bouwmeester HJ (2015) Strigolactones, a novel carotenoid-derived plant hormone. Ann Rev Plant Biol 66:161–186
Bécard G, Piché Y (1992) Establishment of vesicular-arbuscular mycorrhiza in root organ culture: review and proposed methodology. In: Norris JR, Read DJ, Varma AK (eds) Methods in microbiology, vol 24. Academic Press, Cambridge, pp 89–108
Bécard G, Douds DD, Pfeffer PE (1992) Extensive in vitro hyphal growth of vesicular-arbuscular mycorrhizal fungi in the presence of CO2 and flavonols. Appl Environ Microbiol 58:821–825
Besserer A, Bécard G, Jauneau A, Roux C, Séjalon-Delmas N (2008) GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol 148:402–413
Cao FY, DeFalco TA, Moeder W, Li B, Gong Y, Liu XM, Taniguchi M, Lumba S, Toh S, Shan L, Ellis B, Desveaux D, Yoshioka K (2018) Arabidopsis ETHYLENE RESPONSE FACTOR 8 (ERF8) has dual functions in ABA signaling and immunity. BMC Plant Biol 18:211
Charpentier M, Sun J, Wen J, Mysore KS, Oldroyd GE (2014) Abscisic acid promotion of arbuscular mycorrhizal colonization requires a component of the PROTEIN PHOSPHATASE 2A complex. Plant Physiol 166:2077–2090
Chen W, Li J, Zhu H, Xu P, Chen J, Yao Q (2017) The differential and interactive effects of arbuscular mycorrhizalfungus and phosphorus on the lateral root formation in Poncirus trifoliata (L.). Sci Hortic 217:258–265
Coelho L, Mignoni DS, Silva FS, Braga MR (2019) Seed exudates of Sesbania virgata (Cav.) Pers. stimulate the asymbiotic phase of the arbuscular mycorrhizal fungus Gigaspora albida Becker Hall. Hoehnea 46:e272018
Declerck S, Strullu DG, Plenchette C (1998) Monoxenic culture of the intraradical forms of Glomus sp. isolated from a tropical ecosystem: a proposed methodology for germplasm collection. Mycologia 90:579–585
Dijksterhuis J (2018) Fungal spores: highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol 81:2–11
Egamberdieva D, Wirth SJ, Alqarawi AA, Abd_Allah EF, Hashem A (2017) Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol 8:2104
Etemadi M, Gutjahr C, Couzigou JM, Zouine M, Lauressergues D, Timmers A, Audran C, Bouzayen M, Bécard G, Combier JP (2014) Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol 166:281–292
Fousia S, Tsafouros A, Roussos PA, Tjamos SE (2018) Increased resistance to Verticillium dahliae in Arabidopsis plants defective in auxin signalling. Plant Pathol 67:1749–1757
Gemma JN, Koske RE (1988) Pre-infection interactions between roots and the mycorrhizal fungus Gigaspora gigantea: chemotropism of germ-tubes and root growth response. Trans Bri Mycol Soc 91:123–132
Graham JH (1982) Effect of citrus root exudates on germination of chlamydospores of the vesicular-arbuscular mycorrhizal fungus, Glomus epigaeum. Mycologia 74:831–835
Groot SP, Karssen CM (1992) Dormancy and germination of abscisic acid-deficient tomato seeds: studies with the sitiens mutant. Plant Physiol 99:952–958
Hartung W (2010) The evolution of abscisic acid (ABA) and ABA function in lower plants, fungi and lichen. Funct Plant Biol 37:806–812
Hartung W, Sauter A, Turner NC, Fillery I, Heilmeier H (1996) Abscisic acid in soils: what is its function and which factors and mechanisms influence its concentration? Plant Soil 184:105–110
Herrera-Medina MJ, Steinkellner S, Vierheilig H, Ocampo Bote JA, García Garrido JM (2007) Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol 175:554–564
Hildebrandt U, Janetta K, Bothe H (2002) Towards growth of arbuscular mycorrhizal fungi independent of a plant host. Appl Environ Microbiol 68:1919–1924
Hildebrandt U, Ouziad F, Marner FJ, Bothe H (2005) The bacterium Paenibacillus validus stimulates growth of the arbuscular mycorrhizal fungus Glomus intraradices up to the formation of fertile spores. FEMS Microbiol Lett 254:258–267
Hong C, Michailides TJ (1999) Mycelial growth, sporulation, and survival of Monilinia fructicola in relation to osmotic potential and temperature. Mycologia 91:871–876
Ishii T, Shrestha YH, Matsumoto I, Kadoya K (1996) Effect of ethylene on the growth of vesicular-arbuscular mycorrhizal fungi and on the mycorrhizal formation of trifoliate orange roots. J Jap Soc Hort Sci 65:525–529
Kokkoris V, Miles T, Hart MM (2019) The role of in vitro cultivation on asymbiotic trait variation in a single species of arbuscular mycorrhizal fungus. Fungal Biol 123:307–317
Kuromori T, Seo M, Shinozaki K (2018) ABA transport and plant water stress responses. Trend Plant Sci 23:513–522
Leal PL, Varón-López M, de Oliveira Prado IG, dos Santos JV, Soares CRFS, Siqueira JO, de Souza Moreira FM (2016) Enrichment of arbuscular mycorrhizal fungi in a contaminated soil after rehabilitation. Braz J Microbiol 47:853–862
Liao D, Wang S, Cui M, Liu J, Chen A, Xu G (2018) Phytohormones regulate the development of arbuscular mycorrhizal symbiosis. Int J Mol Sci 19:3146
Louis I, Chew A, Lim G (1988) Influence of spore density and extracellular conidial matrix on spore germination in Colletotrichum capsici. Trans Bri Mycol Soc 91:694–697
Martín-Rodríguez JÁ, León-Morcillo R, Vierheilig H, Ocampo JA, Ludwig-Müller J, García-Garrido JM (2011) Ethylene-dependent/ethylene-independent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytol 190:193–205
Müller A, Ngwene B, Peiter E, George E (2017) Quantity and distribution of arbuscular mycorrhizal fungal storage organs within dead roots. Mycorrhiza 27:201–210
Nair MG, Safir GR, Siqueira JO (1991) Isolation and identification of vesicular-arbuscular mycorrhiza-stimulatory compounds from clover (Trifolium repens) roots. Appl Environ Microbiol 57:434–439
Nitsch L, Kohlen W, Oplaat C, Charnikhova T, Cristescu S, Michieli P, Rieu I (2012) ABA-deficiency results in reduced plant and fruit size in tomato. J Plant Physiol 169:878–883
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PRO, Hara RB,Simpson GL, Solymos P, Stevens MHH, Wagner H (2016) Vegan: Community Ecology Package. R Package Vegan, Vers 2. 3–5, https://cran.r-project. org/web/packages/vegan/index.html
Page DE, Glen M, Ratkowsky DA, Beadle CL, Rimbawanto A, Mohammed CL (2017) Ganoderma basidiospore germination responses as affected by spore density, temperature and nutrient media. Trop Plant Pathol 42:328–338
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Powell JR, Rillig MC (2018) Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol 220:1059–1075
Redecker D, Kodner R, Graham LE (2000) Glomalean fungi from the Ordovician. Science 289:1920–1921
Scervino JM, Ponce MA, Erra-Bassells R, Vierheilig H, Ocampo JA, Godeas A (2005) Arbuscular mycorrhizal colonization of tomato by Gigaspora and Glomus species in the presence of root flavonoids. J Plant Physiol 162:625–633
Scervino JM, Ponce MA, Erra-Bassells R, Bompadre MJ, Vierheilig H, Ocampo JA, Godeas A (2006) Glycosidation of apigenin results in a loss of its activity on different growth parameters of arbuscular mycorrhizal fungi from the genus Glomus and Gigaspora. Soil Biol Biochem 38:2919–2922
Selvakumar G, Shagol CC, Kang Y, Chung BN, Han SG, Sa TM (2018) Arbuscular mycorrhizal fungi spore propagation using single spore as starter inoculum and a plant host. J Appl Microbiol 124:1556–1565
Silva-Flores P, Bueno CG, Neira J, Palfner G (2019) Factors affecting arbuscular mycorrhizal fungi spore density in the Chilean mediterranean-type ecosystem. J Soil Sci Plant Nutrit 19:42–50
Spatafora JW, Chang Y, Benny GL, Lazarus K, Smith ME, Berbee ML, Bonito G, Corradi N, Grigoriev I, Gryganskyi A, James TY, O’Donnell K, Roberson RW, Taylor TN, Uehling J, Vilgalys R, White MM, Stajich JE (2016) A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 108:1028–1046
Spraker JE, Sanchez LM, Lowe TM, Dorrestein PC, Keller NP (2016) Ralstonia solanacearum lipopeptide induces chlamydospore development in fungi and facilitates bacterial entry into fungal tissues. ISME J 10:2317–2330
Suriyapperuma SP, Koske RE (1995) Attraction of germ tubes and germination of spores of the arbuscular mycorrhizal fungus Gigaspora gigantea in the presence of roots of maize exposed to different concentrations of phosphorus. Mycologia 87:772–778
Takeda N, Handa Y, Tsuzuki S, Kojima M, Sakakibara H, Kawaguchi M (2015) Gibberellins interfere with symbiosis signaling and gene expression and alter colonization by arbuscular mycorrhizal fungi in Lotus japonicus. Plant Physiol 167:545–557
Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, dit Frey NF, Gianinazzi-Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, Kawaguchi M, Krajinski F, Lammers PJ, Masclaux FG, Murat C, Morin E, Ndikumana S, Pagni M, Petitpierre D, Requena N, Rosikiewicz P, Riley R, Saito K, San Clemente H, Shapiro H, van Tuinen D, Bécard G, Bonfante P, Paszkowski U, Shachar-Hill YY, Tuskan GA, Young JPW, Sanders IR, Henrissat B, Rensing SA, Grigoriev IV, Corradi N, Roux C, Martin F (2013) Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. PNAS 110:20117–20122
Twanabasu BR, Stevens KJ, Venables BJ (2013) The effects of triclosan on spore germination and hyphal growth of the arbuscular mycorrhizal fungus Glomus intraradices. Sci Total Environ 454:51–60
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2017) Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep 36(12):1971–1984
Zhang W, Sun K, Shi RH, Yuan J, Wang XJ, Dai CC (2018) Auxin signalling of Arachis hypogaea activated by colonization of mutualistic fungus Phomopsis liquidambari enhances nodulation and N2-fixation. Plant Cell Environ 41:2093–2108
Funding
This study was financially supported by the Guangdong Province Science and Technology Innovation Strategy Special Fund (No. 2018B020205001) and the National Natural Science Foundation of China (31570395).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Feng, Z., Zhu, H. et al. Exogenous abscisic acid and root volatiles increase sporulation of Rhizophagus irregularis DAOM 197198 in asymbiotic and pre-symbiotic status. Mycorrhiza 29, 581–589 (2019). https://doi.org/10.1007/s00572-019-00916-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-019-00916-z