Abstract
It is generally assumed that recruitment and expansion of alien species along elevation gradients are constrained by climate. But, if plants are not fully constrained by climate, their expansion could be facilitated or hindered by other factors such as biotic interactions. Here, we assessed the composition of arbuscular mycorrhizal fungi (AMF) in soils along an elevation gradient (i.e. 900 m, 1600 m, 2200 m and 2700 m a.s.l.) through a fungal DNA meta-barcoding approach. In addition, we studied in the greenhouse the effects of AMF on growth and phosphorous (P) nutrition of seedlings of the alien trees Gleditsia triacanthos, Ligustrum lucidum and Pyracantha angustifolia cultivated in soils from those elevations, spanning the elevation at which they already form monospecific stands (below 1450 m a.s.l.) and higher elevations, above their current range of distribution in montane ecosystems of Central Argentina. For comparison, we also included in the experiment the dominant native tree Lithraea molleoides that historically occurs below 1300 m a.s.l. Arbuscular mycorrhizal fungal community composition showed strong community turnover with increasing elevation. The effects of these AMF communities on plant growth and nutrition differed among native and alien trees. While P nutrition in alien species’ seedlings was generally enhanced by AMF along the whole gradient, the native species benefited only from AMF that occur in soils from the elevation corresponding to its current altitudinal range of distribution. These results suggest that AMF might foster upper range expansion of these invasive trees over non-invaded higher elevations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is generally assumed that recruitment and expansion of alien species along elevation gradients are constrained by climate (Alexander et al. 2011). But, if plants are not fully constrained by climate, their expansion could be facilitated or constrained by other factors, such as competition, and/or biotic interactions (Pellissier et al. 2013; Brown and Vellend 2014; Tecco et al. 2016; Marcora et al. 2018).
The interactions of alien plants with soil biota are widely studied (e.g. Reinhart and Callaway 2006; Nuñez and Dickie 2014; Dickie et al. 2017). The net effects of soil biota on plant growth are the result of antagonists (e.g. pathogens) and mutualists (e.g. mycorrhizal fungi). Lack of effects of antagonists and positive effects of mutualists on alien plants in their invasive range is frequently observed (but see Jeschke et al. 2012). This often results in positive effects of soil biota on alien plant growth (Klironomos 2002; Reinhart and Callaway 2006; Callaway et al. 2011).
Among soil mutualists, arbuscular mycorrhizal fungi (AMF) are the most important, widespread and generalist plant root symbionts in terrestrial ecosystems (Smith and Read 2008). They colonise plant roots and provide plants access to limited nutrients such as phosphorous, among other benefits. Together with available mineral nutrients in soils (e.g. Hoeksema et al. 2010), the outcome of the mycorrhizal symbiosis on plant performance depends on the identity and growth form of the plant host and on the composition of the local mycorrhizal fungal community (Klironomos 2003; Öpik et al. 2009; Hoeksema et al. 2018).
Most studies on the benefits provided by AMF to alien plants focus on ecosystems that already have been invaded (e.g. Klironomos 2003; Callaway et al. 2011). Whether those benefits also extend to non-invaded ecosystems with different AMF communities remains almost unstudied (Tomiolo and Ward 2018). Indeed, there is no study on the role of soil biota on alien plant expansion over upper elevations in montane ecosystems. This information is highly relevant in the context of global environmental changes in which expansion of organisms into novel environments is expected, especially those involving abrupt climatic gradients such as mountains.
The Sierras de Córdoba mountain ranges of Central Argentina include a wide elevational gradient (500–2790 m a.s.l.) that is subject to an incipient spread of alien tree species from lower elevations (i.e. 500–1500 m a.s.l.) (Giorgis et al. 2011a, 2016). Together with Pinus elliottii (Urcelay et al. 2017), the alien tree species Gleditsia triacanthos, Ligustrum lucidum and Pyracantha angustifolia are the most successful invaders of low-elevation ecosystems in the region (Furey et al. 2014; Giorgis et al. 2011b, 2017; Tecco et al. 2013; Zeballos et al. 2014). Although some isolated individuals of P. angustifolia occur at 1750 m a.s.l. (Giorgis et al. 2011a), populations of these three species are absent above 1500 m a.s.l. Recent experimental evidence, however, shows that germination and establishment of seedlings of these alien species occur above their current ranges of distribution (Tecco et al. 2016).
Some studies have shown that important changes in fungal community composition occur across elevation gradients (Kivlin et al. 2017; Geml 2017). In particular, abundance and richness of AMF decrease with increasing elevation. Moreover, it recently has been shown that dissimilarity among fungal communities increases with increasing elevation in the tropical Andes (Nottingham et al. 2018). Nonetheless, there is a dearth of relevant empirical data for many geographic regions (Geml 2017). Whether alien plants can establish the symbiosis and benefit from AMF at high elevations, where different fungal communities are expected, is unknown.
According to this framework, we asked: (1) does AMF community composition at different elevations along the Sierras de Córdoba elevation gradient differ and (2) might potential differences in AMF species presence influence the growth of three aliens and one native tree species?
To answer these questions, we assessed the compositional turnover of the AMF community along the elevation gradient through a fungal DNA meta-barcoding approach. In addition, we studied the effects of AMF from different elevations on growth and phosphorous nutrition of seedlings of the aliens Gleditsia, Ligustrum and Pyracantha grown in the greenhouse. This was performed in soils from low-elevation areas where they have already invaded and reach high densities (i.e. 900 m a.s.l.) and in soils from three other elevations (1600 m, 2200 m and 2700 m a.s.l.) above their current ranges of distribution. In addition, we assessed mycorrhizal colonisation. For comparison, we also included in the experiment the dominant native tree Lithraea molleoides that historically dominated in the same vegetation belt in which the invaders currently occur, i.e. up to 1300 m a.s.l., but which does not expand to higher elevations (Giorgis et al. 2017).
Materials and methods
Study site
In this study, we collected soil from plots established in the Sierras Grandes, which is the central mountain range of the Sierras de Córdoba in Central Argentina. The montane system comprises the following vegetation belts described by Cabrera (1976) for the Mountain Chaco District: (1) the upper portion of Chaco mountain woodlands together with secondary grasslands that are distributed from 400 to 1400 m a.s.l., (2) an intermediate belt of mountain grasslands and shrublands (1300 m to 1700 m a.s.l.) and (3) a mosaic of high mountain grasslands and Polylepis australis (Rosaceae) woodlands (above 1700 m a.s.l.). The experimental plots were placed along an elevational gradient ranging from 900 to 2700 m a.s.l. (Linderos Road, 32° 50′ S, 64° 90′ W), near the highest peak of the mountain range. All sites were short grasslands, established on hillsides with similar gentle slopes and high solar insolation (see Marcora et al. (2008) and Tecco et al. (2016) for further details). The plots were devoid of trees, either aliens or natives. The mean annual temperature at the lower end of the gradient (900 m a.s.l.) is 15.7 °C and drops to 7.4 °C near the summit at 2700 m a.s.l. (Marcora et al. 2008). There is no frost-free period over 1800 m a.s.l. The mean annual precipitation varies between 750 and 970 mm, with most rainfall concentrated in the warmest months, from October to April. Consistent environmental changes have been recorded along the elevation gradient with significant decreases in soil temperature and increasing soil moisture towards the upper portion of the gradient (Tecco et al. 2016).
Arbuscular mycorrhizal fungal composition in the soil at the sampled elevations
In order to characterise the richness and abundance of AMF taxa (Glomeromycota) present in the soil at the experimental sites, soil samples were taken in November 2014 for fungal DNA meta-barcoding using deep sequencing as follows: ten soil cores, each ca. 4 cm in diameter and 10–15 cm deep, were randomly collected more than 1 m from each other and were pooled to form a composite sample for each of the three plots at each of the four sites. Genomic DNA was extracted from 0.5 g of dry soil using the NucleoSpin® Soil kit (Macherey-Nagel GmbH & Co., Düren, Germany), according to the manufacturer’s protocol. The ITS2 region (ca. 250 bp) of the nuclear ribosomal DNA (rDNA) repeat was amplified using PCR. One microliters of DNA template was used for the 40 μl PCR reaction containing 25.6 μl of Milli-Q water, 4 μl of 10× buffer, 1.5 μl dNTP (2.5 mM), 1.5 μl of reverse and forward primers (10 mM), 4 μl MgCl2 (50 mM), 0.5 μl BSA (10 mg/ml) and 0.4 μl BIOTAQ polymerase (5 U/μl). Primers fITS7 (Ihrmark et al. 2012) and ITS4 (White et al. 1990) and Ion Torrent adapters were used to amplify the ITS2 region (ca. 250 bp) of the nuclear ribosomal rDNA repeat, using the following PCR conditions: 1 cycle of 95 °C for 5 min, then 37 cycles of 95 °C for 20 s, 56 °C for 30 s and 72 °C for 1.5 min, ending with 1 cycle of 72 °C for 7 min. A 250-μl aliquot of the sample was used for emulsion PCR according to the Ion PGM™ 200 Xpress™ Template Kit manual and was sequenced by an Ion Torrent Personal Genome Machine (PGM; Life Technologies, Guilford, CT, USA) at the Naturalis Biodiversity Center, Leiden.
The initial clean-up of the raw sequence data (1,306,069 sequence reads) was carried out using the online platform Galaxy (https://main.g2.bx.psu.edu/root), in which the sequences were sorted according to samples. Adapters (identification tags) were removed. The primers were removed, and poor-quality ends were trimmed based on 0.02 error probability limits using Geneious Pro 5.6.1 (Biomatters, New Zealand). Subsequently, sequences were filtered using USEARCH v.8.0 (Edgar 2010) based on the following settings: all sequences were truncated to 200 bp, and sequences with an expected error > 0.5 were discarded. The resulting 551,691 high-quality sequences were grouped into 6990 operational taxonomic units (OTUs) with USEARCH at 97% sequence similarity, following other fungal meta-barcoding studies (e.g. Bjorbækmo et al. 2010; Geml et al. 2010; Bellemain et al. 2013), while simultaneously excluding 6396 putative chimeric sequences. We assigned sequences to taxonomic groups based on pairwise similarity searches against the curated UNITE fungal ITS sequence database containing identified fungal sequences with assignments to Species Hypothesis groups (Kõljalg et al. 2013). After discarding global singletons and OTUs that did not have at least 80% similarity to any fungal sequence in UNITE, the final dataset contained 3024 OTUs. For this paper, 145 OTUs belonging to Glomeromycota were detected (see Table 1 in the Supplementary information).
Seed collection
We collected fruits of at least 20 mature individuals of Ligustrum lucidum W.T.Aiton (Oleaceae), Gleditsia triacanthos L. (Fabaceae) and Pyracantha angustifolia (Franch.) C.K.Schneid. (Rosaceae) and from the dominant native Lithraea molleoides (Vell.) Engl. (Anacardiaceae) in different areas of the lower montane belt. Gleditsia is a deciduous tree while the others are evergreen species (Tecco et al. 2013). The fleshy mesocarp of Pyracantha and Ligustrum fruits was removed, and seeds of Gleditsia were individually scarified to overcome their physical dormancy. The seed weight (g) (mean ± SD, n = 6) is 0.205 ± 0.03 for Gleditsia, 0.018 ± 0.0008 for Ligustrum, 0.004 ± 0.0003 for Pyracantha and 0.064 ± 0.0047 for Lithraea. Seeds of each species were pooled, in order to standardise any differences associated with local seed origin as well as to incorporate genetic variability. Seeds were surface sterilised with 10% bleach (sodium hypochlorite) for 10 min. They were then germinated in a greenhouse in an autoclaved mix of sand and native soil (2:1 v/v−1). After 30 days, these seedlings were used for the greenhouse experiment.
Greenhouse experiment
Seedlings of all four species were transplanted at the same time to pots (500 cm3) in November 2011. They were grown in soils collected from plots at the four elevations, spanning the whole elevation range of the region (900 m, 1600 m, 2200 m and 2700 m a.s.l.). These were the same plots from which we collected the soil for molecular analyses. The dominant plant species at each elevation were (expressed in % cover) as follows: at 900 m a.s.l., Cuphea glutinosa (25%), Piptochaetium montevidense (20%) and Eryngium elegans (20%); at 1600 m a.s.l., Piptochaetium montevidense (40%), Setaria parviflora (15%) and Paspalum quadrifarium (15%); at 2200 m a.s.l., Deyeuxia hieronymi (60%) and Poa stuckertii (30%); and at 2700 m a.s.l., Deyeuxia hieronymi (50%), Poa stuckertii (10%) and Festuca dissitiflora (10%). Soil properties in these plots are shown in Table 1. The soil samples were sieved (2-mm mesh), pooled within elevation and stored for 5 weeks at 4 °C for the subsequent experiment in the greenhouse. These soils also were used as the source of inoculum (see below).
All pots contained 450 ml of an autoclaved (1.5 atm for 1 h) mix of silicon sand and native soil (2:1 v/v−1). The sand is infertile and mainly composed of feldspar and quartz. Accordingly, macronutrients from native soils were diluted in the sand. This nutrient dilution may exacerbate mycorrhizal functioning but also compensate for the lack of plant competition in the pots. The native soil in each elevational treatment belongs to the corresponding elevation. Three different soil biota treatments were applied for each elevation: (a) sterile soil, (b) sterile soil + microorganisms excluding arbuscular mycorrhizal fungi and (c) sterile soil + microorganisms + AMF, all from the pertinent elevation. These treatments were prepared as follows:
Sterile soil (S)
Twenty-five cubic centimeters of autoclaved soil and 30 ml of water were added to each pot.
Microorganisms without AMF (M)
Twenty-five cubic centimeters of autoclaved soil and 30 ml of microbial slurry were added. The slurry was prepared for each elevation by filtering a soil suspension in water (1:5 v/v−1) through a 36-μm mesh to remove AMF spores and mycorrhizal root fragments, but allowing other microorganisms, including soil pathogens (such as bacteria and fungi) to pass through the mesh (Koide and Li 1989; Perez and Urcelay 2009).
Microorganisms with AMF (M + AMF)
Twenty-five cubic centimeters of non-sterile soil and 30 ml of water were added to each pot (Perez and Urcelay 2009).
These three soil treatments from each of the four elevations were applied to each of the four plant species with six individuals per species (N = 288 pots).
Plants were grown in a greenhouse with temperatures ranging from 15 to 25 °C under well-watered conditions, without water stress (daily watering with tap water). The pots were randomised on the bench and repositioned weekly to avoid any potential biases related to their position in the greenhouse. After 90 days, plants were harvested in order to avoid pot volume limitation for roots, then washed and separated into shoots and roots. They were dried at 60 °C for 72 h and weighed.
Phosphorous concentration in tissues
It is widely known that AMF provide access to mineral nutrients, mainly P (Smith and Read 2008). To assess P nutrition, dried aboveground tissues were ground with a vibratory micromill and used to measure P concentration following the US EPA 365.4 method. Some seedlings of Ligustrum and Lithraea grown in soils from upper elevations had to be pooled because of insufficient biomass for the analyses. For this reason, in these analyses, the n for Ligustrum at 2700 m a.s.l. was 4, 4 and 3 for M + AMF, M and S, respectively. In the case of Lithraea, the n was 2, 3 and 2 for M + AMF, M and S at 2700 m a.s.l., respectively. Also, at 2200 m a.s.l., it was 3, 4 and 3 for M + AMF, M and S, respectively.
Mycorrhizal colonisation in plant roots
The complete root system of each plant was stained using the hot staining technique (Grace and Stribley 1991). Potassium hydroxide (KOH; 10%) was used to clear roots for a period of approximately 20 min (± 10 min depending on the species) at 90 °C. Then, the roots were washed with tap water and acidified with 10% hydrochloric acid (HCl) for 10 min (± 3 min depending on the species) at the same temperature. Finally, they were rinsed and stained with 0.025% aniline blue for 15 min (± 5 min depending on the species) at 90 °C. Randomly selected roots below 2 mm diameter were mounted on semi-permanent slides in polyvinyl-lactic acid-glycerol (one slide per plant). Ten to twelve root pieces were mounted on each slide. AMF colonisation rates were determined following the magnified intersection method of McGonigle et al. (1990) using a compound microscope (Nikon Optical, model E200) at × 200 magnification. One hundred intersections per sample were counted to assess hyphae, vesicles and arbuscules. The number of intersections with mycorrhizal fungal structures was used to calculate percentages of colonised root by every AMF structure (total mycorrhizal colonisation) and by arbuscules for all individuals.
Statistical analyses
Community composition of AMF along the elevation gradient was compared using permutation-based non-parametric MANOVA (PERMANOVA, Anderson 2001) and non-metric multidimensional scaling (NMDS) in PC-Ord v. 6.0 (McCune and Grace 2002). Both were applied to primary presence/absence and abundance matrices. Data were subjected to 500 iterations per run using the Bray-Curtis distance measure.
Dry mass and P concentration were compared using generalised linear models (GLMs). “Elevation” (900 m, 1600 m, 2200 m and 2700 m a.s.l.) and “Soil biota” (S, M and M + AMF) were used as fixed factors. Interaction terms (“Elevation” × “Soil biota”) were tested and included in the analysis. Tukey’s tests were applied a posteriori to identify differences among treatment means. Total mycorrhizal colonisation and colonisation by arbuscules were compared among elevations only for the mycorrhizal treatment because non-mycorrhizal treatments were almost not colonised (see below). Because root colonisation data did not meet the assumptions of normal distribution and/or homogenous variance, we used non-parametric Kruskal-Wallis analyses. Statistical analyses were performed in R (version 3.0.2; 2013 September 25) (R Development Core Team) through the interface implemented in Infostat (version 2013) (Di Rienzo et al. 2013).
Results
Composition of AMF along the elevation gradient
AMF community composition in soils differed among elevations (PERMANOVA; presence/absence: pseudo-F = 3.77, P < 0.001; relative abundance: pseudo-F = 2.37, P < 0.001). NMDS analyses resulted in two-dimensional solutions for the presence/absence (Fig. 1) as well as the abundance (not shown) data with final stress values of 0.06 and 0.086, respectively, and final instability < 0.00001. The NMDS ordination plots not only showed a strong elevational turnover of AMF but also revealed that the community composition in intermediate elevations represented gradual transitions between sites at the two extremes of the gradient (see also supplementary data, Table 1S).
Plant growth, P concentration and mycorrhizal colonisation in the greenhouse
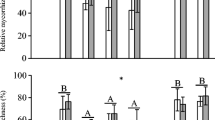
Alien seedlings were generally benefited by AMF communities from all elevations, particularly reflected by enhanced P concentrations in aboveground tissues. In contrast, P concentration in tissues of the native seedlings was enhanced only by AMF from its current range of distribution (i.e. lowest elevation). Plant biomass showed fewer differences among treatments and varied among species (Table 2, Fig. 2a–h). Phosphorous concentration and plant biomass never differed significantly between M and S treatments. In turn, AMF colonisation did not significantly differ among soils from different elevations in the aliens (except for Gleditsia at 1600 m a.s.l.) but decreased with soil inocula from upper elevations in the native (Table 3, Fig. 3a–h). In non-mycorrhizal treatments, the roots practically were not colonised. However, some individuals showed some stained hyphae that were counted. In these roots, the average colonisation never exceeded 0.36% and arbuscules were never found, so the absence of functional symbiosis can be assumed.
Dry mass (g) and aboveground tissue P concentration (mg/g) of the aliens Gleditsia (a, b), Ligustrum (c, d) and Pyracantha (e, f) and of the native Lithraea (g, h) grown in soils from different elevations (m a.s.l.) under different treatments: white, sterile soil; grey, microorganisms without AMF; and black, microorganisms with AMF. Bars correspond to means + 1 SE. Bars with the same letter are not significantly different (Tukey’s HSD test: P < 0.05)
Total mycorrhizal colonisation (% root length colonised) and colonisation by arbuscules (% root length colonised) of the aliens Gleditsia (a, b), Ligustrum (c, d) and Pyracantha (e, f) and of the native Lithraea (g, h) grown in soils with AMF from different elevations (m a.s.l.). Bars correspond to means + 1 SE. Bars with the same letter are not significantly different (pairwise multiple comparison test: P < 0.05)
The dry mass of Gleditsia was higher for individuals inoculated with AMF than for non-inoculated individuals only at 2200 m a.s.l. (Fig. 2a). In the mycorrhizal treatment, AMF positively affected P concentration in aboveground tissues in most soils except for that from 1600 m a.s.l. (Fig. 2b). In the 1600-m treatment plants, total colonisation showed the lowest values and arbuscules were absent (Fig. 3a, b).
Conversely, the growth of Ligustrum decreased with increasing elevation (Fig. 2c). Arbuscular mycorrhizal fungi from all elevations significantly affected P concentration (Table 2), albeit these effects were not significant within each elevation (Fig. 2d). In turn, there was a significant effect of soil treatments on growth (Table 2), but differences were not significant within elevations (Fig. 2c). Mycorrhizal colonisation did not significantly change with elevation (Fig. 3c, d).
The growth of Pyracantha was lower in soil of the highest elevation than in soils from the other three elevations (Fig. 2e). AMF increased P concentration at all elevations but had no effect on plant growth (Fig. 2e, f). Mycorrhizal colonisation did not significantly differ with elevation (Fig. 3e, f).
In the case of Lithraea, the growth was lower in soils from upper elevations but was not affected by AMF (Fig. 2g). There was a positive effect of AMF from the lowest elevation on P concentration in plant tissues. This effect disappeared in soils from upper elevations (Fig. 2h). Total mycorrhizal colonisation and arbuscular colonisation significantly decreased with elevation (Fig. 3g, h).
Discussion
We have shown strong AMF community turnover with increasing elevation. In addition, we have provided novel evidence on the role of AMF from non-invaded ecosystems on alien tree species growth and nutrition.
Despite the reported widespread distribution of AMF (Kivlin et al. 2011; Davison et al. 2015), changes in AMF communities at the landscape level are generally observed (e.g. Hazard et al. 2013; Jansa et al. 2014; Xu et al. 2017). A recent meta-analysis revealed that AMF community composition changes considerably along elevation gradients (Kivlin et al. 2017). In line with those findings, our results reveal a strong turnover in AMF community composition with increasing elevation (Fig. 1). These shifts are generally attributed to changes in climate, edaphic variables and plant hosts (Kivlin et al. 2017). In our study region, vegetation, temperature, humidity, organic matter and available nitrogen differ with elevation (Table 1) (Tecco et al. 2016; Giorgis et al. 2017), but available data cannot distinguish among them or determine their relative contributions to the patterns we observed.
As reported by studies on AMF communities in other regions (e.g. Berruti et al. 2017; Lekberg et al. 2018), Glomeraceae dominated in these soils. It is worth mentioning that the ITS2 marker used here to assess AMF composition may show some slight differences when compared with other widely employed markers such as the SSU. Nonetheless, they show comparable results when assessing AMF community structure and response to environmental variables (Berruti et al. 2017; Lekberg et al. 2018). Importantly, most glomeromycotan clades were recovered in this study.
With some nuances, the growth and nutrition of the studied plant species were similar in treatments that excluded AMF, either with or without microorganisms. These results suggest a minor role of soil pathogens either in constraining or releasing the expansion of the studied tree species. It is worth mentioning that soil microorganisms could have included potentially beneficial microorganisms (e.g. P-solubilising bacteria) (Kucey 1983) that can counteract the effects of antagonists.
The most important result that we found for alien plants was their response to AMF in terms of P nutrition. In general, P concentrations in aboveground tissues of aliens were notably higher when plants were grown with AMF than without them, regardless of the elevation provenance of the soil biota. The exception was Gleditsia in soils from 1600 m a.s.l., which was mirrored by a lack of arbuscular colonisation. In contrast, P concentration in the native tree was enhanced only by AMF from soil corresponding to 900 m a.s.l. This elevation corresponds to its current and historical range of distribution (Giorgis et al. 2017). Moreover, the native Lithraea showed higher mycorrhizal colonisation with inocula belonging to this elevation than in the other three studied elevations. There are 22 OTUs from different lineages that occur in soils from this elevation (see Table S1 in the supplementary material) and are absent from the others. In addition, some OTUs, such as no. 1392 (Glomeraceae) and no. 2927 (Diversisporaceae), are abundant at the lower elevation but not in the others. Alien species, instead, were able to form mycorrhizal symbiosis even when AMF communities in soils changed, suggesting that they have no major incompatibilities with different AMF symbionts. Alternatively, it is possible that they were colonised by generalist AMF occurring at the four elevations. Although we cannot exclude this possibility, it might be unlikely because only one OTU (no. 1392; Table S1) was present at all four elevations. Because the soil DNA meta-barcoding analysis and the greenhouse experiment were not carried out on simultaneously collected soils, however, comparisons between them should be interpreted with caution. Nonetheless, even when seasonal and inter-annual changes in fungal composition are observed (e.g. Siles et al. 2017), the trend of decreasing AMF richness and abundance and changes in composition with elevation along elevation gradients are consistent across continents and biomes (Kivlin et al. 2017).
The lower (and even lack of) AMF colonisation in roots of the native Lithraea with increases in elevation might be attributable to incompatibility because of partner selectivity in the symbiosis (Helgason et al. 2002; Öpik et al. 2009; Yang et al. 2012). Indeed, some evidence suggests that natives establish selective associations with AMF, while aliens are generalists (Moora et al. 2011; Anacker et al. 2014). Moreover, in a meta-analysis on the interactions between AMF and non-woody native/alien plants, Bunn et al. (2015) found a positive correlation between AMF colonisation and growth responses by natives but not by aliens.
Our results are in line with the idea that invasive alien plants rely on generalist symbiotic associations (Richardson et al. 2000; Moora et al. 2011; Anacker et al. 2014), so novel AMF communities do not constrain the formation of their mycorrhizal symbioses and may provide benefits that would facilitate their expansion into non-invaded ranges. Given the significant increases of organic matter with increasing elevation (Siles et al. 2017; this study), we cannot discard that AMF provide plants with mineral nutrients released from organic matter by decomposer microorganisms in soils at high elevations (e.g. see Aristizábal et al. 2004).
An alternative hypothesis to “partner selectivity” in the native Lithraea could be that it just had small stature and limited leaf area in the three upper-elevation soils, and so provided little carbon to AMF, hence was poorly colonised. An interesting test to this hypothesis could be to fertilise Lithraea seedlings growing in soils from the upper elevations to see if that could increase root colonisation. Moreover, further studies including analyses of AMF composition in roots of native and alien trees growing in soils from different ecosystems would shed light on the links between the generalist-selective character of the plant and the benefits received from AMF.
The overall trend of growth lessening in soils from higher elevation cannot be attributed to soil phosphorous availability because its decrease towards high elevation was not significant (Table 1). Instead, it is possible that other soil properties such as the high proportion of silt observed in soils of the upper belt may underlie the observed growth trends, albeit conclusive evidence is still lacking.
Finally, the results reported here correspond to the seedling stage that is the critical stage for plant establishment. Because seedlings were grown for 90 days, it cannot be ruled out that the differences would have increased over an extended experiment. Also, our results with plants grown in the greenhouse remain to be corroborated under field conditions.
Conclusions
The effects of soil biota from different elevations in montane soils differed between native and alien trees. While the alien plant species benefited from AMF along the entire gradient, mainly in terms of P nutrition, the native species benefited only from mutualists that occur at its current altitudinal range of distribution. These results suggest that AMF may foster upper range expansion of the studied alien trees over not yet-invaded high elevations.
References
Alexander JM, Kueffer C, Daehler CC, Edwards PJ, Pauchard A, Seipel T, MIREN Consortium (2011) Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc Natl Acad Sci 108:656–661
Anacker BL, Klironomos JN, Maherali H, Reinhart KO, Strauss SY (2014) Phylogenetic conservatism in plant -soil feedback and its implications for plant abundance. Ecol Lett 17:1613–1621
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Aristizábal C, Rivera EL, Janos DP (2004) Arbuscular mycorrhizal fungi colonize decomposing leaves of Myrica parvifolia, M. pubescens and Paepalanthus sp. Mycorrhiza 14:221–228
Bellemain E, Davey ML, Kauserud H, Epp LS, Boessenkool S, Coissac E, Geml J, Willerslev E, Gussarova G, Taberlet P, Brochman C (2013) High paleodiversity of fungi revealed using high-throughput metabarcoding of ancient DNA from arctic permafrost. Environ Microbiol 15:1176–1189
Berruti A, Desiro A, Visentin S, Zecca O, Bonfante P (2017) ITS fungal barcoding primers versus 18S AMF-specific primers reveal similar AMF-based diversity patterns in roots and soils of three mountain vineyards. Environ Microbiol Rep 9:658–667
Bjorbækmo MFM, Carlsen T, Brysting A, Vrålstad T, Høiland K, Ugland KI, Geml J, Schumacher T, Kauserud H (2010) High diversity of root associated fungi in both alpine and arctic Dryas octopetala. BMC Plant Biol 10:244
Brown C, Vellend M (2014) Non-climatic constraints on upper elevational plant range expansion under climate change. Proc R Soc B 281:20141779
Bunn RA, Ramsey PW, Lekberg Y (2015) Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. J Ecol 103:1547–1556
Cabrera A (1976) Regiones fitogeográficas argentinas. In: Enciclopedia Argentina de Agricultura y Jardinería, 2nd edn. ACME, Buenos Aires
Callaway RM, Bedmar EJ, Reinhart KO, Silvan CG, Klironomos J (2011) Effects of soil biota from different ranges on Robinia invasion: acquiring mutualists and escaping pathogens. Ecology 92:1027–1035
Davison J, Moora M, Öpik M, Adholeya A, Ainsaar L, Bâ A, Burla S, Diedhiou AG, Hiiesalu I, Jairus T, Johnson NC, Kane A, Kooren K, Kochar M, Ndiaye C, Pärtel M, Reier Ü, Sacks Ü, Singh R, Vasar M, Zobel M (2015) Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349:970–973
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2013). Infostat. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina
Dickie IA, Bufford JL, Cobb RC, Desprez-Loustau ML, Grelet G, Hulme PE, Klironomos J, Makiola A, Nuñez M, Pringle A, Thrall PH, Tourtellot SG, Waller L, Williams NM (2017) The emerging science of linked plant-fungal invasions. New Phytol 215:1314–1332
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Furey C, Tecco P, Perez-Harguindeguy N, Giorgis MA, Grossi M (2014) The importance of native and exotic plant identity and dominance on decomposition patterns in mountain woodlands of Central Argentina. Acta Oecol 54:13–20
Geml J (2017) Altitudinal gradients in mycorrhizal symbioses—the current state of knowledge on how richness and community structure change with elevation. In: Tedersoo L (ed) Ecological studies: biogeography of mycorrhizal symbioses. Springer, Berlin, pp 107–123
Geml J, Laursen GA, Herriott I, McFarland JM, Booth MG, Lennon N, Nusbaum C, Taylor DL (2010) Phylogenetic and ecological analyses of soil and sporocarp DNA sequences reveal high diversity and strong habitat partitioning in the boreal ectomycorrhizal genus Russula Pers. (Russulales; Basidiomycota). New Phytol 187:494–507
Giorgis MA, Tecco PA, Cingolani AM, Renison D, Marcora P, Paiaro V (2011a) Factors associated with woody alien species distribution in a newly invaded mountain system of Central Argentina. Biol Invasions 13:1423–1434
Giorgis MA, Cingolani AM, Chiarini F, Chiapella J, Barboza G, Ariza Espinar L, Morero R, Gurvich DE, Tecco PA, Subils R, Cabido M (2011b) Composición florística del Bosque Chaqueño Serrano de la provincia de Córdoba, Argentina. Kurtziana 36:9–43
Giorgis MA, Cingolani AM, Tecco PA, Cabido M, Poca M, von Wehrden H (2016) Testing alien plant distribution and habitat invasibility in mountain ecosystems: growth form matters. Biol Invasions 18:2017–2028
Giorgis MA, Cingolani AM, Gurvich DE, Tecco PA, Chiapella J, Chiarini F, Cabido M (2017) Changes in floristic composition and physiognomy are decoupled along elevation gradients in Central Argentina. Appl Veg Sci 20:558–571
Grace C, Stribley DP (1991) A safer procedure for routine staining of vesicular-arbuscular mycorrhizal fungi. Mycol Res 95:1160–1162
Hazard C, Gosling P, van der Gast CJ, Mitchell DT, Doohan FN, Bending G (2013) The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J 7:498–508
Helgason T, Merryweather JW, Denison J, Wilson P, Young JPW, Fitter AH (2002) Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J Ecol 90:371–384
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Hoeksema JD, Bever JD, Chakraborty S, Chaudhary VB, Gardes M, Gehring CA, Hart MM, Housworth EA, Kaonongbua W, Klironomos JN, Lajeunesse MJ, Meadow J, Milligan BG, Piculell BJ, Pringle A, Rúa MA, Umbanhowar J, Viechtbauer W, Wang YW, Wilson GWT, Zee PC (2018) Evolutionary history of plant hosts and fungal symbionts predicts the strength of mycorrhizal mutualism. Commun Biol 1:116. https://doi.org/10.1038/s42003-018-0120-9
Ihrmark K, Bödeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandström-Durling M, Clemmensen KE, Lindahl BD (2012) New primers to amplify the fungal ITS2 region—evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677
Jansa J, Erb A, Oberholzer HR, Šmilauer P, Egli S (2014) Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol Ecol 23:2118–2135
Jeschke JM, Aparicio LG, Haider S, Heger T, Lortie CJ, Pyšek P, Strayer DL (2012) Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14:1-20
Kivlin SN, Hawkes CV, Treseder KK (2011) Global diversity and distribution of arbuscular mycorrhizal fungi. Soil Biol Biochem 43:2294–2303
Kivlin SN, Lynn JS, Kazenel MR, Beals KK, Rudgers JA (2017) Biogeography of plant-associated fungal symbionts in mountain ecosystems: a meta-analysis. Divers Distrib 23:1067–1077
Klironomos JN (2002) Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417:67–70
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Koide RT, Li M (1989) Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytol 111:35–44
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson KH (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Kucey RMN (1983) Phosphate solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can J Soil Sci 63:671–678
Lekberg Y, Vasar M, Bullington LS, Sepp SK, Antunes PM, Bunn RA, Larkin BG, Öpik M (2018) More bang for the buck? Can arbuscular mycorrhizal fungal communities be characterized adequately alongside other fungi using general fungal primers? New Phytol. https://doi.org/10.1111/nph.15035
Marcora P, Hensen I, Renison D, Seltmann P, Wesche K (2008) The performance of Polylepis australis trees along their entire altitudinal range: implications of climate change for their conservation. Divers Distrib 14:630–636
Marcora PI, Ferreras AE, Zeballos SR, Funes G, Longo S, Urcelay C, Tecco PA (2018) Context-dependent effects of fire and browsing on woody alien invasion in mountain ecosystems. Oecologia 188:479–490. https://doi.org/10.1007/s00442-018-4227-y
McCune BP, Grace J (2002) Analysis of ecological communities. MjM Software, Gleneden Beach
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol 115:495–501
Moora M, Berger S, Davison J, Öpik M, Bommarco R, Bruelheide H, Kühn I, Kunin WE, Metsis M, Rortais A, Vanatoa A, Vanatoa E, Stout JC, Truusa M, Westphal C, Zobel M, Walther GR (2011) Alien plants associate with widespread generalist arbuscular mycorrhizal fungal taxa: evidence from a continental-scale study using massively parallel 454 sequencing. J Biogeogr 38:1305–1317
Nottingham AT, Fierer N, Turner BL, Whitaker J, Ostle NJ, McNamara NP, Bardgett RD, Leff JW, Salinas N, Silman M, Kruuk L, Meir P (2018) Microbes follow Humboldt: temperature drives plant and soil microbial diversity patterns from the Amazon to the Andes. Ecology. Accepted author manuscript 99:2455–2466. https://doi.org/10.1002/ecy.2482
Nuñez MA, Dickie IA (2014) Invasive belowground mutualists of woody plants. Biol Invasions 16:645–661
Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M (2009) Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol 184:424–437
Pellissier L, Pinto-Figueroa E, Niculita-Hirzel H, Moora M, Villard L, Goudét J, Guex N, Pagni M, Xenarios L, Sanders I, Guisan A (2013) Plant species distributions along environmental gradients: do belowground interactions with fungi matter? Front Plant Sci 500. https://doi.org/10.3389/fpls.2013.00500
Perez M, Urcelay C (2009) Differential growth response to arbuscular mycorrhizal fungi and plant density in two wild plants belonging to contrasting plant functional types. Mycorrhiza 19:517–523
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-roject.org
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Siles JA, Cajthaml T, Filipová A, Minerbi S, Margesin R (2017) Altitudinal, seasonal and interannual shifts in microbial communities and chemical composition of soil organic matter in Alpine forest soils. Soil Biol Biochem 112:1–13
Smith SE, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic, Cambridge
Tecco PA, Urcelay C, Díaz S, Cabido M, Perez-Harguindeguy N (2013) Contrasting functional trait syndromes underlay woody alien success in the same ecosystem. Aust Ecol 38:443–451
Tecco PA, Pais-Bosch AI, Funes G, Marcora P, Zeballos SR, Cabido M, Urcelay C (2016) Mountain invasions on the way: are there climatic constraints for the expansion of alien woody species along an elevation gradient in Argentina? J Plant Ecol 9:380–392
Tomiolo S, Ward D (2018) Species migrations and range shifts: a synthesis of causes and consequences. Persp Plant Ecol Evol Syst 33:62–77
Urcelay C, Longo S, Geml J, Tecco PA, Nouhra E (2017) Co-invasive exotic pines and their ectomycorrhizal symbionts show capabilities for wide distance and altitudinal range expansion. Fungal Ecol 25:50–58
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Xu X, Chen C, Zhang Z, Sun Z, Chen Y, Jiang J, Shen Z (2017) The influence of environmental factors on communities of arbuscular mycorrhizal fungi associated with Chenopodium ambrosioides revealed by MiSeq sequencing investigation. Sci Rep 7:45134. https://doi.org/10.1038/srep45134
Yang H, Zang Y, Yuan Y, Tang J, Chen X (2012) Selectivity by host plants affects the distribution of arbuscular mycorrhizal fungi: evidence from ITS rDNA sequence metadata. BMC Evol Biol 12:50
Zeballos SR, Tecco PA, Cabido M, Gurvich DE (2014) Composición de especies leñosas en comunidades invadidas en montañas del centro de Argentina: su relación con factores ambientales locales. Rev Biol Trop 62:1673–1681
Acknowledgements
The authors wish to acknowledge the assistance of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Universidad Nacional de Córdoba (U.N.C.), both of which have provided facilities used for this study. The soil DNA meta-barcoding work was sponsored by a Naturalis Research Initiative grant awarded to J. Geml. We also thank the land owners (J. Astrada, J.C., M. Chuit and R. Olguín) who generously provided access to the study site and allowed us to establish long-term exclosures in their properties and M. Cabido and M. Giorgis for the floristic relevés. C.U., S.L. and P.A.T. are the researchers of CONICET and professors at the U.N.C. David Janos and the two anonymous reviewers provided critical comments and suggestions that improved the quality of this manuscript.
Funding
This research program is funded by the Secretaría de Ciencia y Tecnología - Universidad Nacional de Córdoba (Secyt) (Universidad Nacional de Córdoba) and the Ministerio de Ciencia y Tecnología de Córdoba.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 67 kb)
Rights and permissions
About this article
Cite this article
Urcelay, C., Longo, S., Geml, J. et al. Can arbuscular mycorrhizal fungi from non-invaded montane ecosystems facilitate the growth of alien trees?. Mycorrhiza 29, 39–49 (2019). https://doi.org/10.1007/s00572-018-0874-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-018-0874-4