Abstract
Drought stress, which is one of the most serious world environmental threats to crop production, might be compensated by some free living and symbiotic soil microorganisms. The physiological response of flax plants to inoculation with two species of arbuscular mycorrhizal (AM) fungi (Funneliformis mosseae or Rhizophagus intraradices) and a phosphate solubilizing bacterium (Pseudomonas putida P13; PSB) was evaluated under different irrigation regimes (irrigation after 60, 120, and 180 mm of evaporation from Class A pan as well-watered, mild, and severe stress, respectively). A factorial (three factors) experiment was conducted for 2 years (2014–2015) based on a randomized complete block design with three replications at Urmia University, Urmia, located at North-West of Iran (37° 39′ 24.82″ N44° 58′ 12.42″ E). Water deficit decreased biomass, showing that flax was sensitive to drought, and AM root colonization improved the performance of the plant within irrigation levels. In all inoculated and non-inoculated control plants, leaf chlorophyll decreased with increasing irrigation intervals. Water deficit-induced oxidative damage (hydrogen peroxide, malondialdehyde, and electrolyte leakage) were significantly reduced in dual colonized plants. All enzymatic (catalase, superoxide dismutase, glutathione reductase, and ascorbate peroxidase) and non-enzymatic (glutathione, ascorbic acid, total carotenoids) antioxidants were reduced by water-limiting irrigation. Dual inoculated plants with AM plus Pseudomonas accumulated more enzymatic and non-enzymatic antioxidants than plants with bacterial or fungal inoculation singly. Dual colonized plants significantly decreased the water deficit-induced glycine betaine and proline in flax leaves. These bacterial-fungal interactions in enzymatic and non-enzymatic defense of flax plants demonstrated equal synergism with both AM fungi species. In conclusion, increased activity of enzymatic antioxidants and higher production of non-enzymatic antioxidant compounds in symbiotic association with bacteria and mycorrhiza can alleviate reactive oxygen species damage resulting in improve water stress tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants growing in a detrimental environment, such as those occurring in arid and semiarid soils, undergo water shortage which is the most common stress affecting plant growth in such regions (Marulanda et al. 2008). With severe drought an all-too-common occurrence, some farmers turn to irrigation for a solution, but it may not be feasible or even desirable. Plants have evolved complex physiological and biochemical adaptations in order to adjust and adapt to drought stress. The physiological mechanisms (accumulation of compatible osmolytes like proline and soluble carbohydrates, overproduction of reactive oxygen species [ROS] and formation of free-radical scavenging compounds such as ascorbate and glutathione) associated with water-stress tolerance have been extensively studied (Gill and Tuteja 2010; Osakabe et al. 2014). Osmolytes contribute to the lowering of the osmotic potential and, in turn, of the leaf water potential which allow the plants to maintain high organ hydration and turgor (Ashraf and Foolad 2007). Several factors activate the resistance response of plants to water stress so that they can alleviate detrimental effects of drought (Ramoliya et al. 2004).
Sustainable systems require an understanding of interactions between plants and microorganisms (e.g., arbuscular mycorrhizal fungi [AMF] and phosphate solubilizing bacteria [PSB]), with a direct influence on plant growth and water deficit stress tolerance (Habibzadeh et al. 2015; Marulanda et al. 2009). Mycorrhizal plants often have greater tolerance to drought than non-mycorrhizal plants (Al-Karaki et al. 2004). Several non-nutritional mechanisms have been proposed for explaining host plant protection by AM symbiosis against drought-induced detrimental effects (Ruiz-Lozano et al. 2001). The osmotic stress normally caused by drought is counteracted by mycorrhizal plants through biochemical changes that mostly include increased biosynthesis of osmolytes (mainly proline, soluble carbohydrates and glycine betaine) (Ashraf and Foolad 2007). Furthermore, mycorrhizal plants withstand drought-induced oxidative stress by increased production of enzymatic (catalase, superoxide dismutase, glutathione reductase and ascorbate peroxidase) and non-enzymatic (ascorbic acid, total carotenoids and glutathione) antioxidant compounds (Pedranzani et al. 2016). Zhu et al. (2010) reported that the AM fungus (Glomus etunicatum) is capable of alleviating the damage caused by stress by reducing membrane lipid peroxidation and membrane permeability and increasing the accumulation of osmotic adjustment compounds and antioxidant enzyme activity.
Mycorrhizal colonized plants can interact with several soil microorganisms including phosphate solubilizing bacteria that are capable of making the plant more tolerant to drought stress conditions (Dimkpa et al. 2009). Inoculated seedlings with the exopolysaccharide (EPS) producing strain Pseudomonas putida showed improved soil aggregation, root-adhering soil, and higher relative water content (RWC) of leaves that imparted tolerance against drought stress to plants (Bensalim et al. 1998; Sandhya et al. 2009). Different arbuscular mycorrhizal fungi species differentially can affect both the populations and activity of Pseudomonas (Marschner and Crowley 1996), and bacteria (especially those producing plant growth regulators) can affect the development of mycorrhizas (Azcon et al. 1978). Pseudomonas putida strains, gram-negative rhizobacteria, have the capacity to adapt to diverse niches in the soil (Wu et al. 2010) and also can colonize a wide range of plants (Fernandez-Pinar et al. 2012). P. putida exhibited a high osmotic tolerance and also showed increased proline concentration, involved in osmotic cellular adaptation (Marulanda et al. 2009). Moreover, co-inoculation with PSB and mycorrhizal fungi has been proposed as an efficient procedure for increasing plant growth. Assessing the effects of plant-beneficial microorganisms (two Pseudomonas strains and a mixed mycorrhizal inoculum, alone or in combination) on the quality of tomato fruits of plants cultivated in the field, showed the benefit of dual colonization (Bona et al. 2017).
Flax or linseed (Linum usitatissimum, Linaceae), native of Europe and South Asia, is an economically important oilseed and a multi-purpose crop (the third largest natural fiber crop and one of the five major oil crops in the world), and its importance is shown by all parts of the plant having specific economic uses. The quality and utilization of flax oil is determined by its fatty acid composition (FAOSTAT 2014; Muir and Westcott 2003). Flax is highly dependent on AM fungi in order to meet its early phosphorus requirements (Thingstrup et al. 1998). It has been reported that inoculation of the AM fungus F. mosseae led to various increased morphological and physiological parameters of flax (Neetu et al. 2011).
Over the last two decades, the area of flax cultivation has decreased, resulting in a worldwide gap between production and consumption. It is necessary to increase flax productivity per unit area in water deficit conditions, because drought has played a significant role in this reduction. One possible way to enhance production is tolerance to stresses by means of rhizosphere microbial manipulation. This study aimed at investigating directly in the field, the interaction effects of two AMF species, and a Pseudomans strain on the water deficit-induced physiological responses of flax plants including antioxidant defense machinery (enzymatic and non-enzymatic) against drought stress damage.
Materials and Methods
Experimental design and plant culture
A 2-year field experiment was conducted in the research field of Urmia University, Urmia, Iran, located in the northwest of Iran (37° 39′ 24.82″ N latitude, 44° 58′ 12.42″ E longitude, 1338 m altitude). Environmental conditions of the experimental site from April to September for the 2 years include the monthly highest (27.6 °C) and lowest (11.3 °C) temperature, sum of sunny hours (323.8 h) and rainfall (0.5 mm) during 2014 and 2015. For each year, the experiment was laid out in a factorial arrangement based on a randomized complete block design with three replications. Factors were various irrigation regimes (irrigation after 60 (I1), 120 (I2), or 180 (I3) mm of evaporation from Class A pan), AMF inocula (non-inoculated, F. mosseae, or R. intraradices) and bacterial strain (non-inoculated or P. putida strain P13).
Irrigation water needed before irrigation (VN) is the amount of water needed during irrigation to replenish the soil moisture deficit, thereby restoring the soil to field capacity. The value of VN was calculated according to Benami and Ofen (1984):
where VN is the irrigation water needed before irrigation (m3), FC is the field capacity (%), WP is the wilting point (%), BD is the bulk density (g cm−3), D is the root zone depth (m), ASM is the available soil moisture before irrigation (a fraction), and A is the area of the field (m2).
The mycorrhizal inoculum (initially isolated from the endemic AMF community of a maize farm) was a mixture of sterile sand, mycorrhizal hyphae, and spores (20 spores g−1 inoculum), and colonized root fragments which were produced on maize (Zea mays L.) host plants by Dr. Younes Rezaee Danesh at Urmia University. Inoculum (250 g m−2) was banded in the rows below the flax seeds and lightly covered with soil. For the non-mycorrhizal control treatment, seeds were sown without inoculation. Inoculum potentials were 3400 and 3360 propagules per plant for F. mosseae and R. intraradices, respectively.
Pseudomonas putida strain P13 (abbreviated P. putida), was provided by Dr. Malboobi, Green Biotech, Iran. This strain, isolated by screening soil samples collected from different region of Iran, was able to withstand temperature as high as 42 °C, high concentrations of NaCl up to 5%, and a broad range of initial pH from 5 to 11. Such criteria make it a superior candidate for biofertilizers that are able to utilize both organic and mineral phosphate substrates to release absorbable phosphate ion for plants (Malboobi et al. 2009a). Seeds were inoculated with the microbial inoculum (P. putida) before sowing. Wet seeds were rolled into the bacterial suspension (108 cfu ml−1) until they were uniformly coated. For the non-bacterial control plants, seeds were sown without inoculation.
Prior to sowing, the land was harrowed, plowed and rolled. Seeds (brown flax seeds with 99% germination and 5 g 1000-seed weight) were provided by Agricultural Research, Education and Extension Organization (AREEO) West Azarbaijan, Urmia, Iran. They were sown on 19 April 2014 and 14 April 2015 into a loamy soil by hand at a depth of 2 cm in plots of 1.5-by-2 m size, with plant spacing of 20 by 2 cm. Then they were covered with only a thin layer of soil and the soil was kept moist until they sprouted. After sowing, all plots were given a pre-emergence irrigation. Irrigation treatments were applied after 5 May for 2 years. By this time, flax plants were at the 4-leaf stage.
The soil texture was loamy (32% silt, 24% clay, and 44% sand) with 24% field capacity, 1.2 g cm−3 soil density, 1.34% organic matter (OM), 0.9 dS m−1 electrical conductivity (EC), 8.1 pH, 0.09% nitrogen, 25 mg kg−1 available phosphorus, and 166 mg kg−1 available potassium. Soil pH, EC, and concentration of K (using atomic absorption spectrometry), were determined in saturated soil extracts (Tandon 1993), total organic matter by di-chromate oxidation (Nelson and Sommers 1986), and available phosphorus (Pava) by sodium bicarbonate extraction (Olsen and Sommers 1986). Soil texture was determined according to Gee and Bauder (1986). The number of native AMF spores (30 per 10 g soil) (Gerdemann and Nicolson 1963) and P. putida strain P13 population (2 × 102 cfu g−1 soil) (Minaxi et al. 2013) were determined in the field soil before planting.
Parameters measured
Evaluation of AM fungal colonization
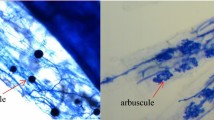
The percentage of flax root length colonized was determined for four plants per treatment. Root colonization was measured in fresh roots (washed with distilled water over a sieve to remove soil) cleared by 10% KOH for 10 min at 90 °C and stained in 0.05% lactic acid-glycerol-Trypan Blue (Phillips and Hayman 1970). The gridline intersection method (Giovannetti and Mosse 1980) was used. Root samples from each plant were evaluated for 100 intersections per sample at ×100 magnification. Total colonization was the number of intersections assessed minus intersections lacking fungal structures divided by the total number of intersections assessed.
Biomass
At the end of the growing season, when the plants had produced mature seeds (on 6, 20, and 27 August in both years for I3, I2, and I1, respectively, with different times of harvesting because of different dates of seed maturity under the three irrigation regimes), all the plants were harvested and the root systems were separated from the shoots. Aerial parts of the plants were dried in a forced-air oven at 70 °C for 2 days and their dry weights were obtained as biomass.
Leaf phosphorus
To measure leaf P, dried leaves were milled, digested, and analyzed as described by Watanabe and Olsen (1965) and Ohnishi et al. (1975) with combustion (4 h at 500 °C) of the leaf sample. The plant ashes (5 mg) were digested in 1 ml of concentrated HCl. The samples were then filtered, and total P was quantified as PO4 − using the ascorbic acid method (Watanabe and Olsen, 1965). The amount of PO4 − in solution was determined colorimetrically at 882 nm.
Physiological parameters
Ten plants were used from each replicate plot, and samples of leaves were collected on 6 July in each of the 2 years. Fresh leaf samples were covered with aluminum foil and frozen in liquid nitrogen before being stored in plastic envelopes at −80 °C. These samples were used for determinations of physiological parameters. Fresh leaves were used to measure electrolyte leakage (EL).
Total soluble sugars (TSS)
Leaf total soluble sugars were determined based on the phenol sulfuric acid method (Dubois et al. 1956). In this method, 0.5 g of fresh leaves was homogenized with ethanol. The extract was filtered and treated with 5% phenol and 98% sulfuric acid. This mixture was left for 1 h and its absorption was measured by spectrophotometer at 485 nm.
Proline
Leaf proline colorimetric determination proceeded according to Bates et al. (1973) based on proline’s reaction with ninhydrin. Leaf tissue (0.5 g) was crushed in a mortar and was homogenized in 10 ml 3% sulfosalicylic acid and the extract was centrifuged to eliminate the leaf tissue. For proline colorimetric determinations, a 1:1:1 solution of proline, ninhydrin acid, and glacial acetic acid was incubated at 100 °C for 1 h. The reaction was arrested in an ice bath, the chromophore was extracted with 4 ml of toluene, and its absorbance was determined in a spectrophotometer at 520 nm. The proline concentration was determined by a standard curve prepared with proline.
Glycine betaine (GB)
The amount of GB was assessed based on the method of Grieve and Grattan (1983). Dry plant material (0.5 g) was mechanically shaken with 20 ml of deionized water for 48 h at 25 °C before filtering. Thawed extracts were diluted 1:1 with 2 N sulfuric acid. The aliquots were kept in centrifuge tubes and cooled in ice water for 1 h. Cold potassium iodide-iodine reagent (0.2 ml) was added. The samples were stored at 0–4 °C for 16 h and were centrifuged at 10,000g for 15 min. The supernatant was aspirated. Periodite crystals were dissolved in 9 ml of 1, 2-dichloroethane (reagent grade). After 2–2.5 h, the absorbance was measured at 365 nm with a spectrophotometer.
Evaluation of enzymatic defense activity (catalase, CAT; superoxide dismutase, SOD; ascorbate peroxidase, APX; glutathione reductase, GR)
Frozen leaf samples (0.25 g) were crushed with liquid nitrogen and extracted with a pestle in an ice-cold mortar with 4 ml of 0.05 M Na2HPO4/NaH2PO4 (pH 7.0) buffer containing 0.2 mM ethylene-diamine tetracetic acid (EDTA) and 1% polyvinylpyrrolidone (PVP). The homogenates were centrifuged at 4 °C for 20 min at 15000 g. The supernatants were collected and used for enzyme activity assays.
CAT activity was assayed by measuring the rate of disappearance of hydrogen peroxide using the method of Maehly and Chance (1959). The reaction mixture contained 2.5 ml of 50 mM phosphate buffer (pH 7.4), 0.1 ml of 1% hydrogen peroxide, and 50 ml enzyme extract diluted to keep measurements within the linear range of the analysis. The decrease in hydrogen peroxide was followed by a decline in absorbance at 240 nm.
APX activity was determined according to the method of Chen and Asada (1989) with minor modifications. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbate, 1.54 mM hydrogen peroxide, and 50 ml enzyme extract. The oxidation of ascorbate was followed by decrease in the absorbance at 240 nm.
Superoxide dismutase activity was measured using the method described by Dhindsa et al. (1981). SOD activity was assayed by its ability to inhibit photochemical reduction of nitroblue tetrazolium. The test tubes containing the assay mixture (1.5 ml reaction buffer, 0.2 ml of methionine, 0.1 ml enzyme extract with an equal amount of NaCO3, NBT solution, riboflavin, EDTA and 1.0 ml DDW) were incubated in light under 15 W fluorescent lamps for 15 min. Illuminated and non-illuminated reactions without supernatant served as calibration standards. Absorbance of samples and controls (blank) was read at 560 nm wavelength.
Glutathione reductase activity was determined according to Sgherri et al. (1994). Extraction of GR was performed in 1.5 ml of solution containing 100 mM potassium phosphate buffer (pH 7.0), 1 mM Na2EDTA, and 2% PVP. The homogenates were filtered and centrifuged at 18,000 g for 20 min at 4 °C. The assay mixture comprised 200 mM potassium phosphate buffer (pH 7.5), 0.2 mM Na2EDTA, 1.5 mM MgCl2, 0.5 mM GSSG, 50 μM NADPH, and enzyme extract containing 100 μg protein in a final volume of 1 ml. Correction was made for the non-enzymatic oxidation of NADPH by recording the decrease in absorbance at 340 nm.
Chlorophylls and carotenoids
Chlorophyll a, chlorophyll b, and carotenoids were estimated by extracting the leaves using 80% acetone as explained by Lichtenthaler (1987). The chlorophyll concentration was calculated based on the following formulas:
In this formula, C a , C b , and CX+C indicate the concentrations of chlorophyll a, chlorophyll b, and carotenoids, respectively. “A” indicates the absorbance at the designated wavelength.
Glutathione (GSH) and ascorbic acid (AsA)
Glutathione concentration was measured as described by Smith (1985). Five hundred milligrams of the leaves was homogenized in a cold mortar with 5 ml 5% (w/v) sulfosalicylic acid and the homogenate was filtered and centrifuged at 1000g for 10 min. One milliliter of supernatant was neutralized by 1.5 ml of 0.5 mM K-phosphate buffer (pH 7.5). The standard incubation medium was a mixture of: 0.5 ml 0.1 M sodium phosphate buffer (pH 7.5) containing 5 mM EDTA, 0.2 ml 6 mM 5, 5-dithiobis-(2-nitrobenzoic acid), 0.1 ml of 2 mM NADPH, and 0.1 ml (1 unit) glutathione reductase. The reaction was initiated by adding 0.1 ml glutathione standard or extract. The change in absorbance was recorded at 412 nm for 9 min.
Ascorbate measurement was based on the reduction of ferric to ferrous ion with ascorbate in acid solution followed by the formation of a pink complex between ferrous ion and bipyridyl that absorbs at 525 nm. Fresh tissue (0.1 g) was homogenized in 1.5 ml of 5% ice-cold metaphosphoric acid and centrifuged at 10,000g for 10 min. Supernatant was collected and used for the estimation of ascorbate according to Law et al. (1983).
Electrolyte leakage (EL)
Leaf samples (freshly harvested, not frozen) were washed with three changes of deionized water to eliminate surface-adhered electrolytes. Leaf samples (0.1 g) were cut into discs of uniform size and incubated in deionized water at room temperature. After 1 h, the electrical conductivity (L1) of the immersion solution was measured by using a conductivity meter. The immersion solution was then placed in a boiling water bath at 100 °C for 10 min, and the electrical conductivity (L2) was measured after cooling. Membrane relative permeability was calculated by the formula L1/L2 × 100% (Bai et al. 1996).
Malondialdehyde (MDA)
Malondialdehyde was measured according to the thiobarbituric acid (TBA) reaction as described by Zhang and Qu (2004). Fresh leaf tissues (0.5 g) were frozen in liquid nitrogen and homogenized in 1 ml of 5% trichloroacetic acid (TCA). The homogenates were transferred into tubes and centrifuged at 4000 g for 10 min at room temperature. Two milliliters of extract was added to 2 ml 0.6% TBA and placed in a boiling water bath for 10 min. Absorbance of the supernatant was determined spectrophotometrically at 532, 600, and 450 nm (A532, A600, and A450). The concentration of MDA was calculated based on the formula:
Hydrogen peroxide (H2O2)
The concentrations of H2O2 were assayed by using 0.5 g of leaf tissue which was homogenized in an ice bath with 5 ml 0.1% (w/v) TCA. The homogenate was centrifuged at 12,000 g for 15 min, and then 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI (potassium iodide). The absorbance was measured at 390 nm. H2O2 was quantified versus a calibration curve using solutions with known H2O2 concentrations (Velikova et al. 2000).
Statistical analysis
To determine the individual and interactive effects, four-way, factorial analysis of variance (ANOVA) including the data collected for both years (with year as a factor) was performed using SAS software. All data were analyzed using a general linear model (GLM). The model was subsequently validated by checking normality and homoscedasticity of residuals. No transformations were used. The mean values of levels within factors were compared by Student–Neuman-Keul (SNK) test at (P ≤ 0.05).
Results
The combined ANOVA of 2-year data revealed a significant interaction effect of irrigation regimes × bacteria × mycorrhiza on dry weight, total soluble sugars, glycine betaine, glutathione reductase, total chlorophyll, carotenoids, glutathione, ascorbic acid, H2O2 (P ≤ 0.01), root colonization, catalase, malondialdehyde, protein and leaf phosphorus concentration (P ≤ 0.05; Table 1). Furthermore, there were significant interactions of irrigation regimes × mycorrhiza on electrolyte leakage (P ≤ 0.05), year × irrigation regimes × bacteria × mycorrhiza on proline and ascorbate peroxidase (P ≤ 0.01), irrigation regimes × mycorrhiza, irrigation regimes × bacteria and bacteria × mycorrhiza on superoxide dismutase (Table 1).
Biomass
The dry weight of the plants that had been subjected to drought treatment was significantly lower compared to the well-watered plants. Plants cultivated under mild stress conditions that had received inoculation, especially dual-colonized (PSB and AMF) plants, attained a higher dry weight than non-inoculated plants. The great reduction of biomass (33%) in severe stress could not be compensated by any single or dual inoculation treatment. In well-watered plants, biomass was increased by single AMF and PSB inoculation similarly to dual colonization (Table 2).
AM fungal root colonization
The highest root colonization belonged to R. intraradices (83%) and F. mosseae (77%) in dual-colonized plants irrigated after 120 mm of pan evaporation. The synergistic effect of P. putida on root colonization was observed only in the mild-stress condition, but there was no superiority of dual colonization on root colonization in well-irrigated or severe-stressed plants. Non-inoculated plants (control) had the lowest colonization for all irrigation regimes similar to PSB-only plants (Table 2).
Leaf phosphorus concentration
Leaf phosphorus was diluted by limited irrigation, so the minimum leaf P was observed in severe stressed plants (irrigated after 180 mm of evaporation). Ion phosphorus accumulation in leaves of inoculated plants of both fungal species was higher than those of the leaves of the control and bacteria-only inoculated plants. A positive synergistic effect of test organisms resulted in the highest leaf P in dual-colonized plants in all irrigation regimes (Table 2).
Proline
Leaf proline concentration decreased in all mycorrhizal (single and dual inoculation) plants as a result of water stress. The maximum leaf proline concentration (41.50 and 45.30 mg/ g FW in 2014 and 2015, respectively) was obtained under the most restrictive irrigation regime (I3) with a decreasing trend in well-watered conditions (I1). The reduction was more pronounced in co-inoculated plants than non-inoculated control plants or plants singly inoculated with PSB or AMF. Leaf proline concentration in mycorrhizal plants (single and dual colonization) was respectively reduced by 20, 25, and 38% for I3, I2, and I1 (Table 3).
Total soluble sugars
Well-watered plants contained higher total soluble sugars (18.92 μmol/g FW) than those with limited irrigation. The lowest leaf TSS (9.93 μmol/g FW) was obtained in non-inoculated control plants under severe drought conditions being 50% less than well-watered plants. In dual colonization, TSS accumulated in high amounts for all irrigation regimes followed by AMF- and PSB-alone treatments. The superiority of well-watered AMF plants in terms of TSS accumulation changed to similarity with PSB in limited irrigation regimes (Table 2). Leaf TSS was higher in the second than in the first year (Table 4).
Glycine betaine
Glycine betaine was in higher concentration in 2014 than in 2015 (Table 4). In all treatments, accumulation of GB was significantly increased (from 17.84 to 30.21 μmol/g) with drought caused by increasing irrigation intervals. In mycorrhizal plants inoculated with F. mosseae or R. intraradices, GB accumulation was significantly reduced similarly to bacterial colonized plants. Simultaneous use of fungi and bacteria showed the highest reduction in glycine betaine concentration (Table 2).
Catalase
In both 2014 and 2015, catalase activity was significantly decreased by extensive irrigation intervals, so the highest and lowest catalase activity belonged to irrigation after 60 and 180 mm of evaporation, respectively. CAT activity of AM and PSB inoculations was higher in I1 and I2 than for the control plants. At all irrigation levels, the CAT activity increases were higher for dual colonized plants than those of other treatments (Table 2).
Superoxide dismutase
Increasing irrigation intervals led to a reduction of SOD activity in non-inoculated control flax to less than that in PSB and AMF inoculated plants (Fig. 1a, b). The decreasing trend of SOD activity with increasing irrigation interval was steeper in 2015 than in 2014 (not illustrated). The SOD activity in co-inoculated plants was greater than in plants singly inoculated with an AM fungus or P. putida (Fig. 1c).
Glutathione reductase
Co-inoculated plants (AMF + PSB) showed the highest activity of glutathione reductase under all irrigation regimes. We observed a descending trend for GR activity by severity of water deficit stress from 60 to 180 mm of evaporation, although, these reductions were different for AMF and PSB colonized plants (Table 2).
Ascorbate peroxidase
The activity of APX in well-watered plants demonstrated a significant increase in dual-inoculated similar to PSB- and AMF- (R. intraradices, only) inoculated plants. There were no significant differences, however, in APX activity of severely stressed plants (I3). But, APX activity was higher in dual-colonized plants irrigated after 120 mm of evaporation. All changes in APX activity were similar between the 2 years (Table 3).
Glutathione and ascorbic acid
Mycorrhizal plants cultivated under well-watered conditions contained the highest concentrations of non-enzymatic defense compounds (glutathione and ascorbic acid) in their leaves. When the plants were subjected to drought stress (I2 and I3), the concentrations of glutathione and ascorbic acid were reduced in all treatments. Although, the leaf glutathione and ascorbic acid concentrations were higher in AM-colonized plants (alone or in combination with P. putida) than in the controls, that was to a greater extent in dual colonized plants with R. intraradices than F. mosseae (Table 2).
Photosynthetic pigments
The highest leaf chlorophyll concentration was obtained in dual-inoculated plants under well-watered conditions, and it was significantly reduced under stress conditions (I2 and I3). This declining trend of chlorophyll was observed for PSB-inoculated plants as well as mycorrhizal flax. The superiority of dual colonization in higher leaf chlorophyll was not noticeable under mild stress (Table 2).
Carotenoid, a non-enzymatic defense of plants against water deficit stress, increased in dual-inoculated plants as much as for single-inoculated mycorrhizal plants and PSB inoculation alone. This effectiveness of co-inoculation was in the order of I2 > I3 > I1 (Table 2). Total carotenoids were higher in 2015 than in 2014 (Table 4).
Malondialdehyde
As an indicator of lipid peroxidation, the concentration of malondialdehyde was higher in the leaves of plants exposed to drought stress versus those of the controls. The concentration of MDA in I2 and I3 increased 25 and 42% more than in plants that were well irrigated (I1), respectively (Table 2). The use of microorganisms had a significant role in modifying this increase. Therefore, in I2 and I3, concentrations of MDA in mycorrhizal plants were lower than in control plants. The combination of P. putida with two types of AM fungi resulted in 50, 38, and 14% reductions in MDA for I1, I2, and I3, respectively. Between AMF species, there was no superiority with respect to MDA concentration reductions (Table 2).
Electrolyte leakage
In flax leaves, increasing irrigation intervals caused major damage to the cell membrane in non-mycorrhizal control plants as revealed by electrolyte leakage. In AM-colonized plants, EL significantly dropped to the same extent with both species of inoculant fungi, thus indicating compensation of cell membrane damage (Fig. 2a). We also observed a 22% reduction of EL in plants colonized with the PSB compared to the controls (Fig. 2b). The highest electrolyte leakage was observed in 2015 (Table 4).
Hydrogen peroxide
Leaf H2O2, a non-free-radical which is involved in a number of signaling cascades in plants was increased along with increasing water deficit stress from I1 to I3. Both AMF species reduced the leaf H2O2 in each irrigation regime, although R. intraradices was more effective than F. mosseae. This reduction was greater in plants inoculated with P. putida than for mycorrhizal plants. Dual-inoculated plants, however, exhibited the greatest reduction (45% for I2 and 24% for I3) of H2O2 compared to non-mycorrhizal control plants (Table 2). Nevertheless, the leaf hydrogen peroxide concentration was in greater in 2015 than in 2014 (Table 4).
Protein concentration
The highest concentration of protein was achieved with well-watered dual inoculation as well as bacterial inoculation alone. Protein concentration significantly decreased with increasing irrigation interval. The single- and dual-inoculated plants showed similar concentrations of leaf protein in I2 and I3. In spite of small alterations of leaf protein concentrations under stressed conditions, AM-colonized plants (R. intraradices in I1 and F. mosseae in I3) produced higher protein concentrations than non-inoculated control plants (Table 2). The highest concentration of leaf protein over all treatments was observed in 2015 (Table 4).
Discussion
Despite there being native AMF in the field soil, mycorrhizal colonization was enhanced by AMF inoculation both without and with PSB and likely contributed to all the results. The yield (biomass) improvement in AM and PSB treatments for well-watered plants and in the dual-inoculated plants with R. intraradices for I2 were consequences of plant-microorganism interactions in response to drought.
Because of high solubility of phosphorus in well-irrigated soil, leaf phosphorus concentration was reduced by greater irrigation intervals with which there likely was diminished solubility, mobility (mass flow or diffusivity), transport between roots and shoots, and thus P uptake under drought conditions (Sawers et al., 2008; He and Dijkstra, 2014). The greater P uptake by plants co-inoculated with PSB and AM fungus can be attributed to transport of P by the AM fungus after solubilization by PSB (Minaxi et al., 2013) reflecting access to a large soil volume which facilitates absorption and dissolution of relatively-insoluble P (Neetu et al. 2011).
The reported results of 2-year increase in leaf proline concentration in consequence of increasing water deficit stress suggest that the production of proline probably is a common response of flax under drought conditions as an osmotic adjustment. A lower concentration of proline may be attributed to either greater drought resistance or less injury of colonized plants under drought stress conditions (Wu and Xia 2006), and proline concentration was lower in dual-inoculation treatments than in single-inoculant treatments.
We also observed a reduction in the total soluble sugars concentration when flax plants were subjected to drought. Although previous studies (Masoudi-Sadaghiani et al. 2011; Vijayalakshmi et al. 2012) have demonstrated that water deficit can increase concentrations of TSS in leaves, other studies indicated no change or even diminished concentrations in stressed plants such as we found. Jamil Marur et al. (1996) reported a 55% decrease of TSS in stressed plants versus controls. The decrease in TSS in response to drought might be related to limited carbohydrate availability, as a result of reduced photosynthesis (Goicoechea et al. 2005; Gogorcena et al. 1997). Although damage to cell membranes by water stress probably limits osmotic adjustment, high leaf water content under drought stress may prevent the accumulation of osmolytes such as total soluble sugars. Nevertheless, we found synergistic effects of dual inoculation (mycorrhizal fungi plus P. putida) on TSS concentrations likely because of enhanced photosynthesis.
Increased glycine betaine with water stress conditions (I2 and I3) was reduced by the application of AM fungi and PSB. This indicates that AM fungus and PSB inoculation alleviated the water stress. Accumulation of GB was reported in cotton (Lv et al. 2007) as an effective compatible solute enhanced by drought stress. The major role of GB might be protection of the integrity of the cell membrane from drought stress damage and involvement in osmotic adjustment (Lv et al. 2007).
In our study, all enzyme activities diminished with increasing irrigation interval. Increased enzyme activities in mycorrhizal plants, PSB-inoculated plants, and dual-inoculated plants indicated that the microorganisms alleviated the oxidative damage from water shortage. Similar to our results, Sandhya et al. (2010) found that inoculation with Pseudomonas augmented antioxidants under severe drought conditions, suggesting that they can alleviate the drought-induced oxidative damage. Additionally, symbiosis with mycorrhizal fungi helps plants to cope with drought stress, probably by maintaining photosynthetic processes intact or little altered as a result of a rise in antioxidant activities (Ruiz-lozano et al. 1996). Detoxification of cellular H2O2 through the activity of the Asada-Halliwell scavenging cycle, which involves the oxidation and re-reduction of ascorbate and glutathione through the action of APX and GR, among other enzymes, is an important element of plant defense against ROS (Donahue et al. 1997). The reduction in CAT activity is regarded as a general response to water stress (Pan et al. 2006; Liu et al. 2008) as a result of the inhibition of enzyme synthesis or change in the assembly of enzyme subunits under stress conditions. It also may be associated with degradation caused by induced peroxisomal proteases or may be a consequence of the photo-inactivation of the enzyme (Liu et al. 2008). Under stress conditions, the modest increase of leaf CAT activity in P. putida-treated and mycorrhizal plants that we observed suggests that inoculated plants might have a potential to activate this enzyme to counteract oxidative, water deficit-induced damage (Ghorbanpour et al. 2013; Wu and Zou 2009). Thus, the inoculants were able to regulate oxidative reactions and antioxidant defense (Ortiz et al. 2015). Effects of each of the mycorrhizal and bacterial inoculants on the water content of plants were obvious, whereas a maximized positive effect on water content was observed in dual-inoculated plants (Armada et al. 2014; Marulanda et al. 2009). Notwithstanding, plants in drought conditions may show different trends (increase, decrease or no change) in antioxidant enzyme activities (Masoudi-Sadaghiani et al. 2011; Armada et al. 2014).
In our experiment, drought stress tended to diminish leaf chlorophyll concentrations (Kpyoarissis et al. 1995). Under well-watered conditions, however, the increased chlorophyll concentrations that we observed with dual inoculation of AMF and PSB might have contributed to an increased rate of photosynthesis (Vafadar et al. 2014) possibly related to a large number of chloroplasts in bundle sheaths in the leaves (Krishna and Bagyaraj 1984).
Overexpression of glutathione reductase in chloroplasts doubled the concentrations of ascorbate and glutathione in leaves and conferred increased resistance to oxidative stress. The decrease in glutathione concentration under drought stress (Foyer et al. 1997) resulted in enhanced lipid peroxidation. Inoculation with AMF increased ascorbate and glutathione as protective compounds to cope with the harmful effects of water shortage. Flax plants benefited not only from the AM symbiosis but also from P. putida irrespective of the watering level, similar to the results of Ruiz-Sanchez et al. (2011). Both AMF inoculation and inoculation with PSB alone significantly increased ascorbate concentration versus the control treatment, and they had an additive effect after co-inoculation. Under severe drought, higher MDA concentrations in leaves may be associated with higher accumulation of H2O2 in stressed plants which could reveal the degree of membrane lipids peroxidation (Gill and Tuteja 2010). Our inoculated plants, however, showed less MDA than their respective controls suggesting the involvement of both types of microorganisms in ROS metabolism (Mo et al. 2016).
Water deficit-induced electrolyte leakage was reduced by mycorrhizal inoculation as well as by P. putida inoculation. The low value of EL in addition to the low MDA concentration with AM symbiosis provides evidence of reduced cell membrane damage (Fouad et al. 2014). Such increased membrane stability has been attributed to mycorrhiza-mediated enhanced P uptake and increased antioxidant production (Feng et al. 2002).
The protection of host plants against oxidative stress by increasing antioxidant enzyme activities is responsible for the elimination of ROS, as evidenced by the lower accumulation of H2O2 (Fouad et al. 2014). AM formation contributes to the production of scavenging peroxyl radicals, buffering cellular free-radicals and producing a powerful ROS-scavenging system (Ashraf and Foolad 2007).
Elevated leaf protein concentrations occurred in plants inoculated with AMF and PSB alone as well as in dual-inoculated plants depending upon drought stress. High concentrations of protein might be attributable to improved efficiency of the osmotic regulation mechanism, which in turn prevents protein catabolism under stress (Kumar et al. 2010) and induces the synthesis of osmotically active proteins. The protein increase can lead to membrane stabilization and, related to MDA and EL, helps plants to grow under stress conditions (Goudarzi and Pakniyat 2009). Protein synthesis can be dramatically reduced or even stopped in stressed plant leaves and roots because of decreased photosynthesis (Mohammadkhani and Heidari 2008).
Conclusions
Based on these results, increased osmolytes (proline and glycine betaine) under drought conditions support their potential to protect flax plant cells via osmoregulation. Drought-induced oxidative damage (H2O2, MDA and EL) were reduced in AMF-inoculated plants as well as PSB-inoculated plants, and was lowest after dual inoculation. Dual inoculation increased enzymatic and non-enzymatic antioxidants more than in plants inoculated only with AMF or PSB. The microorganisms maintained an appropriate plant water status under drought through improved osmotic adjustment owing to the enhancement of compatible solutes and the regulation of antioxidant systems. Therefore, we have demonstrated that the use of the biofertilizers (AM fungi and PSB) in an open field trial can mitigate water stress damage by alleviating ROS and can improve water stress tolerance.
References
Al-Karaki G, McMichael B, Zak J (2004) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14:263–269
Armada E, Portela G, Roldán A, Azcón R (2014) Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 232-234:640–648
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Azcon R, Azcon Aguilar C, Barea JM (1978) Effects of plant hormones present in bacterial cultures on the formation and responses to VA endomycorrhiza. New Phytol 80:359–364
Bai BZ, Yu SQ, Tian WX, Zhao JY (1996) Plant physiology. China Agricultural Science, Beijing
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benami A, Ofen A (1984) Irrigation engineering—sprinkler, trickle and surface irrigation: principles, design and agricultural practices. Irrigation Engineering Scientific Publications, Haifa
Bensalim S, Nowak J, Asiedu SK (1998) A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am J Potato Res 75:145–152
Bona E, Cantamessa S, Massa N, Manassero P, Marsano F, Copetta A, Lingua G, D’Agostino G, Gamalero E, Berta G (2017) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonas improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza 27:1–11
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Dhindsa RH, Plumb-Dhindsa R, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Dimkpa CO, Merten D, Svatos A, Buechel G, Kothe E (2009) Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Biol Biochem 41:154–162
Donahue JL, Okpodu CM, Cramer CL, Grabau EA, Alscher RG (1997) Responses of antioxidants to paraquat in pea leaves (relationship to resistance). Plant Physiol 113:249–257
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
FAOSTAT (2014) [online] available at http://faostat.fao.org.
Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhizal is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12:185–190
Fernandez Pinar R, Espinosa Urgel M, Dubern JF, Heeb S, Ramos JL, Camara M (2012) Fatty acid-mediated signaling between two Pseudomonas species. Environ Microb Rep 4:417–423
Fouad MO, Essahibi A, Benhiba A, Qaddoury A (2014) Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Span J Agric Res 12:763–771
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signaling. Physiol Plant 100:241–254
Gee GW, Bauder JW (1986) Particle size analysis. In: Klute A (ed) Methods of soil analysis part 2nd ed. Agronomy. Monograph no. 9. American Society of Agronomy and Soil Science Society of America, Madison, pp 383–411
Gerdemann JW, Nicolson TH (1963) Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc 46:235–244
Ghorbanpour M, Hatami M, Khavazi K (2013) Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloid production of Hyoscyamus niger under water deficit stress. Turk J Biol 37:350–360
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Gogorcena Y, Gordon AJ, Escuredo PR, Mincin FR, Witty JF, Moran JF, Becana M (1997) N fixation, carbon metabolism, and oxidative damage in nodules of dark-stressed Common Bean plants. Plant Physiol 113:1193–1201
Goicoechea N, Merino S, Sanchez Diaz M (2005) Arbuscular mycorrhizal fungi can contribute to maintain antioxidant and carbon metabolism in nodules of Anthyllis cytisoides L. subjected to drought. J Plant Physiol 162:27–35
Goudarzi M, Pakniyat H (2009) Salinity causes increase in proline and protein contents and peroxidase activity in wheat cultivars. J App Sci 9:348–354
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Habibzadeh Y, Jalilian J, Zardashti MR, Pirzad A, Eini O (2015) Some morpho-physiological characteristics of mung bean mycorrhizal plants under different irrigation regimes in field condition. J Plant Nutr 38:1754–1767
He M, Dijkstra FA (2014) Drought effect on plant nitrogen and phosphorus: a meta-analysis. New Phytol 204:924–993
Jamil Marur C, Mazzafera P, Celso Magalhaes A (1996) Carbon assimilation and export in leaves of cotton plants under water deficit. Braz J Plant Physiol 8:181–186
Kpyoarissis A, Petropoulou Y, Manetas Y (1995) Summer survival of leaves in a soft-leaved shrub (Phlomis fruticosa L., Labiatae) under Mediterranean field conditions: avoidance of photoinhibitory damage through decreased chlorophyll contents. J Exp Bot 46:1825–1831
Krishna KR, Bagyaraj DJ (1984) Growth and nutrient uptake of peanut inoculated with mycorrhizal fungus Glomus fasciculatum compared with uninoculated ones. Plant Soil 77:405–408
Kumar A, Sharma S, Mishra S (2010) Influence of arbuscular mycorrhizal (AM) fungi and salinity on seedling growth, solute accumulation and mycorrhizal dependency of Jatropha curcas L. J Plant Growth Regul 29:297–306
Law Y, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacea oleracea) chloroplasts. Biochem J 210:899–903
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu J, Xie X, Du J, Sun J, Bai X (2008) Effects of simultaneous drought and heat stress on Kentucky bluegrass. J Hortic Sci 115:190–195
Lv S, Yang A, Zhang K, Wang L, Zhang J (2007) Increase of glycinebetaine synthesis improves drought tolerance in cotton. Mol Breed 20:233–248
Maehly AC, Chance B (1959) The assay of catalase and peroxidase. In: Glick D (ed) Methods of biochemical analysis, Vol. 1. Interscience Publishers, New York, pp 357–425
Malboobi MA, Owlia P, Behbahani M, Sarokhani E, Moradi S, Yakhchali B, Deljou A, Morabbi Heravi K (2009) Solubilization of organic and inorganic phosphates by three highly efficient soil bacterial isolates. World J Microbiol Biotechnol 25:1471–1477
Marschner P, Crowley DE (1996) Physiological activity of a bioluminescent Pseudomonas fluorescens (strain 2-79) in the rhizosphere of mycorrhizal and non-mycorrhizal pepper (Capsicum annum L.) Soil Biol Biochem 28:869–876
Marulanda A, Azcon R, Ruız-Lozano JM, Aroca R (2008) Differential effects of a Bacillus megaterium strain on Lactuca sativa plant growth depending on the origin of the arbuscular mycorrhizal fungus co-inoculated: physiologic and biochemical traits. J Plant Growth Regul 27:10–18
Marulanda A, Barea JM, Azcon R (2009) Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: mechanisms related to bacterial effectiveness. J Plant Growth Regul 28:115–124
Masoudi Sadaghiani F, Abdollahi B, Zardoshti MR, Rasouli Sadaghiani H, Tavakoli A (2011) Response of proline, soluble sugars, photosynthetic pigments and antioxidant enzymes in potato (Solanum tuberosum L.) to different irrigation regimes in greenhouse condition. Aust J Crop Sci 5:55–60
Minaxi Saxena J, Chandra S, Nain L (2013) Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. J Soil Sci Plant Nutr 13:511–525
Mo Y, Wang Y, Yang R, Zheng J, Liu C, Li H, Ma J, Zhang Y, Wei C, Zhang X (2016) Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front Plant Sci 7:1–15
Mohammadkhani N, Heidari R (2008) Effects of drought stress on soluble proteins in two maize varieties. Turk J Biol 32:23–30
Muir AD, Westcott ND (2003) Flax - the genus Linum. Taylor and Francis, London
Neetu N, Tanwar A, Aggarwal A (2011) Impact of arbuscular mycorrhizal fungi and other bioinoculants on growth promotion in Linum usitatissimum L. J Indian Bot Soc 90:216–223
Nelson BW, Sommers LE (1986) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2nd ed. Agronomy. Monograph no. 9. American Society of Agronomy and Soil Science Society of America, Madison, WI, pp 539–577
Ohnishi T, Gall RS, Mayer ML (1975) An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem 69:261–267
Olsen SR, Sommers LE (1986) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2nd ed. Agronomy. Monograph no. 9. American Society of Agronomy and Soil Science Society of America, Madison, WI, pp 403–427
Ortiz N, Armada E, Duque E, Roldán A, Azcón R (2015) Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: effectiveness of autochthonous or allochthonous strains. J Plant Physiol 174:87–96
Osakabe Y, Osakabe K, Shinozaki K, Tran LP (2014) Response of plants to water stress. Front Plant Sci 5:86
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycorhiza uralensis Fisch). J Plant Growth Regul 49:157–165
Pedranzani H, Rodríguez-Rivera M, Gutierrez M, Porcel R, Hause B, Ruiz-Lozano JM (2016) Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 26:141–152
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Ramoliya PJ, Patel HM, Pandey AN (2004) Effect of salinization of soil on growth and macro-and micro-nutrient accumulation in seedlings of Salvadora persica (Salvadoraceae). For Ecol Manag 202:181–193
Ruiz-Lozano JM, Azcon R, Palma JM (1996) Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. New Phytol 134:327–333
Ruiz-Lozano JM, Collados C, Miguel Barea J, Azcon R (2001) Arbuscular mycorrhizal symbiosis can alleviate drought-induced nodule senescence in soybean plants. New Phytol 151:493–502
Ruiz-Sanchez M, Armada E, Munoz Y, Garcia de Salamone IE, Aroca R, Ruiz-Lozano JM, Azcon R (2011) Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol 168:1031–1037
Sandhya V, Ali SKZ, Minakshi G, Gopal R, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fertil Soils 46:17–26
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30
Sawers RJ, Gutjahr C, Paszkowski U (2008) Cereal mycorrhiza: an ancient symbiosis in modern agriculture. Trends Plant Sci 13:93–97
Sgherri CLM, Liggini B, Puliga S, Navari Izzo F (1994) Antioxidant system in Sporoblus stapfianus. Changes in response to desiccation and rehydration. Phytochemistry 35:561–565
Smith IK (1985) Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol 79:1044–1047
Tandon HLS (1993) Methods of analysis of soils, plants, waters and fertilizer (Ed). Fertilizer development and consultation organization, New Delhi, India.
Thingstrup I, Rubaek G, Sibbesen E, Jakobsen I (1998) Flax (Linum usitatissimum L.) depends on arbuscular mycorrhizal fungi for growth and P uptake at intermediate but not high soil P levels in the field. Plant Soil 203:37–46
Vafadar F, Amooaghaie R, Otroshy M (2014) Effects of plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungus on plant growth, stevioside, NPK, and chlorophyll content of Stevia rebaudiana. J Plant Interact 9:128–136
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Vijayalakshmi T, Varalaxmi Y, Jainender S, Yadav S, Vanaja M, Jyothilakshmi N, Maheswari M (2012) Physiological and biochemical basis of water-deficit stress tolerance in pearl millet hybrid and parents. Am J Plant Sci 3:1730–1740
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am Proc 29:677–678
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Wu QS, Zou YN (2009) Mycorrhiza has a direct effect on reactive oxygen metabolism of drought-stressed citrus. Plant Soil Environ 10:436–442
Wu X, Monchy S, Taghavi S, Zhu W, Ramos J, Van der Lelie D (2010) Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS Microbiol Rev 35:299–323
Zhang ZL, Qu W (2004) Experimental guidance of plant physiology. High Education Press, Beijing
Zhu X, Song F, Xu H (2010) Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 20:325–332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahimzadeh, S., Pirzad, A. Arbuscular mycorrhizal fungi and Pseudomonas in reduce drought stress damage in flax (Linum usitatissimum L.): a field study. Mycorrhiza 27, 537–552 (2017). https://doi.org/10.1007/s00572-017-0775-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-017-0775-y