Abstract
This study evaluates antioxidant responses and jasmonate regulation in Digitaria eriantha cv. Sudafricana plants inoculated (AM) and non-inoculated (non-AM) with Rhizophagus irregularis and subjected to drought, cold, or salinity. Stomatal conductance, photosynthetic efficiency, biomass production, hydrogen peroxide accumulation, lipid peroxidation, antioxidants enzymes activities, and jasmonate levels were determined. Stomatal conductance and photosynthetic efficiency decreased in AM and non-AM plants under all stress conditions. However, AM plants subjected to drought, salinity, or non-stress conditions showed significantly higher stomatal conductance values. AM plants subjected to drought or non-stress conditions increased their shoot/root biomass ratios, whereas salinity and cold caused a decrease in these ratios. Hydrogen peroxide accumulation, which was high in non-AM plant roots under all treatments, increased significantly in non-AM plant shoots under cold stress and in AM plants under non-stress and drought conditions. Lipid peroxidation increased in the roots of all plants under drought conditions. In shoots, although lipid peroxidation decreased in AM plants under non-stress and cold conditions, it increased under drought and salinity. AM plants consistently showed high catalase (CAT) and ascorbate peroxidase (APX) activity under all treatments. By contrast, the glutathione reductase (GR) and superoxide dismutase (SOD) activity of AM roots was lower than that of non-AM plants and increased in shoots. The endogenous levels of cis-12-oxophytodienoc acid (OPDA), jasmonic acid (JA), and 12-OH-JA showed a significant increase in AM plants as compared to non-AM plants. 11-OH-JA content only increased in AM plants subjected to drought. Results show that D. eriantha is sensitive to drought, salinity, and cold stresses and that inoculation with AM fungi regulates its physiology and performance under such conditions, with antioxidants and jasmonates being involved in this process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants growing under natural conditions are exposed to a variety of biotic and abiotic stresses, which limit their development and productivity due to inhibition of a number of physiological and metabolic processes (Seki et al. 2003). Drought, salinity, and cold are important plant growth constraints in many parts of the world. The soils of central Argentina are semiarid with a variable moisture regime and humidity restricted to part of the year. This region is generally characterized by poorly developed soils, with little horizon differentiation which are highly drained, with low water-holding capacity and organic matter content (Colazo et al. 2010). Due to its poor structure, this soil is highly susceptible to wind and water erosion in vast areas of the region (Peña Zubiate and D’hiriart 2005; Colazo et al. 2010). Salinity affects approximately 2.5 million hectares in central Argentina, 20 % of which are permanent and semipermanent saline lakes. This problem is compounded by drainage practices and the expansion of irrigated agriculture in arid areas with high evapotranspiration rates (Malpassi et al. 2004). In this region of Argentina, recent records show about 60 days of frost per year (Collado 2003). Therefore, it is necessary to use species, especially those producing forage, which can tolerate abiotic stress conditions such as drought, salinity, and cold.
The different plant responses produced to cope with environmental stresses are regulated by crosstalk between hormones and signal molecules. The role of abscisic acid (ABA) in plant responses to abiotic stresses is well known (Wilkinson and Davies 2002; Hirayama and Shinozaki 2007). In contrast, the role of other plant hormones such as jasmonic acid (JA) is less well known (Sanchez-Romera et al. 2014). Jasmonic acid, a plant hormone belonging to the octadecanoid family, is not only involved in the plant’s response to biotic and abiotic stresses but also in the regulation of plant development (Wasternack and Hause 2013). JA is synthesized by the oxygenation of α-linolenic acid through a series of reactions within the chloroplast and the peroxisome, with cis-12-oxophytodienoc acid (OPDA) being the main intermediate of JA biosynthesis (Wasternack 2007). JA can be converted into numerous conjugates and derivatives, some of which have a well-described biological activity, such as the JA-methyl ester (MeJA), cis-jasmon, and JA-isoleucine conjugate (JA-Ile, Wasternack and Hause 2013).
On the other hand, during abiotic stress, different metabolic pathways are uncoupled and electrons are transferred to molecular oxygen to form reactive oxygen species (ROS) such as superoxide radicals (O2•−), hydrogen peroxide (H2O2), or hydroxyl radicals (OH•) (Noctor et al. 2014). These ROS are toxic molecules capable of causing oxidative damage to proteins, DNA, and lipids (Miller et al. 2010). It has been estimated that, under CO2 scarcity conditions due to abiotic stress-induced stomatal closure, up to 50 % of the entire photosynthetic electron flow may end up as O2•− (Biehler and Fock 1996). Antioxidant systems eliminate excess ROS produced under such stress conditions (Gill and Tuteja 2010). The scavenging of ROS is achieved through the action of different enzymatic and non-enzymatic compounds, including superoxide dismutase (SOD), glutathione reductase (GR), catalase (CAT), ascorbate- or thiol-dependent peroxidases, and the enzymes of the ascorbate-glutathione pathway. Non-enzymatic mechanisms include compounds, such as ascorbic acid, glutathione, and α-tocopherol, capable of directly scavenging several ROS (Scheibe and Beck 2011).
Arbuscular mycorrhizal (AM) fungi have been used and studied for its influence on the antioxidative responses in plants of agronomic importance, such as grape (Alarcon 2001), soybean (Porcel and Ruiz-Lozano 2004), rice (Ruiz-Sánchez et al. 2010), wheat (Abdel Latef 2010), tomato (Dell’Amico et al. 2002; Abdel Latef and Chaoxing 2011), or lettuce (Ruiz-Lozano et al. 1996; Aroca et al. 2008; Baslam and Goicoechea 2012) subjected to abiotic stress. In tomato plants, inoculation with AM fungi caused an increase in SOD, CAT, peroxidase (POD), and ascorbate peroxidase (APX) activity in salt-affected leaves and reduced oxidative damage to lipids as indicated by malondialdehyde (MDA) content (Abdel Latef and Chaoxing 2011). At the molecular level, Aroca et al. (2007) found that AM symbiosis regulates root hydraulic properties and enhances Phaseolus vulgaris tolerance to drought, cold, and salt stress. Such regulation closely correlated with the regulation of PIP2 protein levels and phosphorylation state. In addition, depending on the presence of AM fungi, differential expression of PIP genes studied under each stress was observed.
AM also affects the above-ground part of plants, leading frequently to higher tolerance to abiotic stresses than that of non-mycorrhizal (non-AM) plants. For instance, AM symbiosis increased photosynthetic efficiency by over 40 %, induced the accumulation of the antioxidant molecule glutathione, and reduced the accumulation of hydrogen peroxide and oxidative damage to lipids in rice plants subjected to drought stress (Ruiz-Sánchez et al. 2010). Increases in photosynthetic activity and water use efficiency have been reported in AM plants growing under drought (Birhane et al. 2012; Liu et al. 2015) or under salt stress conditions (Sheng et al. 2008; Hajiboland et al. 2010). The alleviation of metabolic inhibitions of photosynthesis by AM symbiosis has been found to be related to the stimulation of carbohydrate transport and metabolism between source and sink tissues (Kaschuk et al. 2009). AM fungi modulate source-sink relations and can stimulate photosynthesis rates sufficiently to compensate for fungal carbon requirements (Kaschuk et al. 2009; Dodd and Pérez-Alfocea 2012).
Digitaria eriantha Steudel, subspecies eriantha, is a native component of grasslands in eastern and southern Africa. It has been adapted and grown in the semiarid central region of Argentina as a perennial grass, with biomass values of 3200 kg dry matter ha−1 (Veneciano 2006). Nowadays, D. eriantha, a source of forage, is available throughout the year. Indeed, it is an economically and nutritionally important resource for cattle, particularly cows in calf in winter months, and for weaning calves in the summer (Frasinelli and Martínez Ferrer 1999). With regard to its adaptation to specific climatic conditions, some studies have provided evidence that D. eriantha plants respond to abiotic stress at different stages of their life cycle. For example, Di Giambatista et al. (2010) found that during germination at 25–30 ° C, although seeds tolerated an osmotic potential of −1 MPa, the germination rate decreased significantly at lower levels of osmotic potential. Additionally, Garbero et al. (2010; 2012) found that exposure of D. eriantha cv. Sudafricana plants to cold caused an increase in MDA content, affected antioxidant defense and chlorophyll biosynthesis, and resulted in serious anatomical damage to leaves. This negative effect of cold on growth, anatomy, ABA and JA levels, and antioxidant defense was obvious only in D. eriantha cv. Sudafricana plants but not in cv. Mejorada INTA, thus indicating that cv. Sudafricana is more sensitive to cold stress.
In order to expand the use of D. eriantha in Argentina, it is necessary to improve its tolerance to environmental stresses such as cold, drought, and salinity. To achieve this, the use of AM fungi could be a worthwhile strategy. Elucidation of a plant’s biochemical and physiological responses common to different abiotic stresses when associated with AM fungi will help us to improve the characteristics of this species for its growth in semiarid habitats.
Materials and methods
Experimental design
The experiment consisted of a randomized complete block design with ten replicates per treatment. The experiment had two factors: (1) inoculation treatment, with non-inoculated (non-AM) control plants and plants inoculated with the AM fungus Rhizophagus irregularis (AM) and (2) abiotic stress applications. This means that one group of plants was cultivated under optimal conditions during the entire experiment and the other groups of plants were subjected to either drought, cold, or salt stress. Thus, there were eight treatments, each with ten replicates, giving a total of 80 pots.
Soil and biological material
A loamy soil was collected from the grounds at the Zaidin Experimental Station (Granada, Spain). The soil had a pH of 8.1 (measured in water, 1:5 w/v); 1.5 % organic matter; nutrient concentrations (g Kg−1): total N, 1.9; total P, 1 (NaHCO3-extractable P); and total K, 6.9. The available P in the soil was 27 mg Kg−1. The soil was sieved (5 mm), diluted with quartz-sand (<2 mm) (1:1, soil/sand, v/v), and sterilized by steaming (100 °C for 1 h on 3 consecutive days).
D. eriantha Steudel cv. Sudafricana seeds were washed for 3 min in pure ethanol and rinsed three times with distilled water. Ten seeds were then sown in 1000-ml pots containing a sterilized mixture of soil/sand (1:1, v/v) and thinned to 5 seedlings per pot after emergence. Mycorrhizal inoculum of R. irregularis (Schenck and Smith), strain EEZ 58 (Ri), was prepared as described by Porcel et al. (2006), and 10 g of the inoculum were added to half of the pots at sowing time, just below seeds. Non-inoculated pots received the same amount of autoclaved mycorrhizal inoculum together with 2 ml of AM inoculum filtrate in order to provide a general microbial population free of AM propagules.
Growing conditions
Inoculated (AM) and non-inoculated (non-AM) plants were cultivated in a greenhouse at 24:20 °C (day/night), with 16:8 photoperiod, a relative humidity of 50–60 %, and an average photosynthetic photon flux density of 800 μmol m−2 s−1, as measured with a light meter (LICOR, Lincoln, NE, USA, model LI-188B). The plants were watered to field capacity and maintained under optimal conditions for 6 weeks. After that period, the AM and non-AM plants were divided into four groups. One group was kept as control at 24 °C with soil at field capacity (under non-stress conditions). The remaining three groups were subjected to the following treatments: (1) 24 °C and 60 % field capacity for 1 week (drought stress), (2) 4 °C for 72 h (cold stress), and (3) 24 °C and soil irrigated with 200 mM NaCl for 2 weeks (salt stress). The duration and levels of stress imposed were based on bibliographical references to these stresses (Pedranzani et al. 2005; Aroca et al. 2007; Di Giambatista et al. 2010; Garbero et al. 2010, 2012). The plants were harvested after the stress treatments.
Parameters measured
Mycorrhizal development and determination of plant biomass production
Mycorrhizal colonization was estimated by visual inspection of fungal structures after clearing the roots in 10 % KOH and staining with 0.05 % (w/v) trypan blue in lactic acid as described by Philips and Hayman (1970). The percentage of mycorrhizal colonization was calculated according to the gridline intersect method (Giovannetti and Mosse 1980).
After treatments, AM and non-AM plants were harvested and the fresh weight (FW) of roots and shoots was determined separately. Shoot and root dry weight (DW) was measured after being dried in a forced hot-air oven at 70 °C for 2 days. Shoot dry matter content was calculated as 1-(FW-DW)/FW (Marulanda et al. 2007), and expressed as gram dry weight per gram FW.
Stomatal conductance and photosynthetic efficiency
Stomatal conductance was measured 2 h after the onset of light using a porometer system (Porometer AP4, Delta-T Devices Ltd., Cambridge, UK) following the user manual instructions. Measurements were taken at the first true leaf from five different plants per treatment.
The efficiency of photosystem II (Qy) was measured using Fluor Pen FP100 (Photon Systems Instruments, Brno, Czech Republic) which enables a non-invasive assessment of plant photosynthetic performance to be carried out by measuring chlorophyll a fluorescence. Fluor Pen quantifies the quantum yield of photosystem II as the ratio between actual fluorescence yield in the light-adapted state (F′V) and maximum fluorescence yield in the light-adapted state (FM), according to the method described by Oxborough and Baker (1997). Measurements were taken in the first true leaf of five different plants for each treatment.
Hydrogen peroxide accumulation and determination of oxidative damage to lipids
The accumulation of hydrogen peroxide in leaves was determined using Patterson’s method (Patterson et al. 1984) with slight modifications as described by Aroca et al. (2003). Briefly, 250 mg FW of shoots were homogenized with 5 ml 5 % (w/v) trichloroacetic acid (TCA) containing 0.1 g activated charcoal and 1 % (w/v) polyvinylpolypyrrolidone (PVPP). The homogenate was centrifuged at 18,000g for 10 min. The supernatant was filtered through a Millipore filter (0.22 μm). A 1.2-ml volume of 100 mM potassium phosphate buffer (pH 8.4) and 0.6 ml of colorimetric reagent were added to 130 μl of the supernatant. Fresh colorimetric reagent was made by mixing 1:1 (v/v) 0.6 mM potassium titanium oxalate and 0.6 mM 4–2 (2-pyridylazo) resorcinol (disodium salt). The samples were incubated at 45 °C for 1 h and absorbance at 508 nm was recorded. Blanks were made by replacing leaf extract with 5 % TCA. Concentration values were expressed as nmol H2O2 g−1 FW.
Lipid peroxides were extracted by grinding 500 mg of leaves and 6 ml of 100 mM potassium phosphate buffer (pH 7) using an ice-cold mortar. Homogenates were filtered through one layer of Miracloth and centrifuged at 15,000g for 20 min. The chromogen was formed by mixing 200 μL of supernatants with 1 ml of a reaction mixture containing 15 % (w/v) TCA, 0.375 % (w/v) 2-thiobarbituric acid (TBA), 0.1 % (w/v) butyl hydroxytoluene, 0.25 N HCl, and by incubating the mixture at 100 °C for 30 min (Minotti and Aust 1987). After cooling at room temperature, the tubes were centrifuged at 800g for 5 min, and the supernatant was used for spectrophotometric measurement at 532 nm. Lipid peroxidation was estimated as the content of 2-thiobarbituric acid-reactive substances (TBARS) and expressed as equivalents of malondialdehyde (MDA) according to Halliwell and Gutteridge (1989). The calibration curve was made using MDA in a range of 0.1–10 nmol. A blank was prepared by replacing the sample with extraction medium, and controls for each sample were prepared by replacing TBA with 0.25 N HCl. In all cases, 0.1 % (w/v) butyl hydroxytoluene was included in the reaction mixtures to prevent artifactual formation of TBARS during the acid-heating step of the assay.
Determination of antioxidant enzymatic activities
Enzymes were extracted at 0–4 °C from 1 g FW of shoots with 50 mg PVPP and 10 ml of 50 mM K-phosphate buffer (pH 7.8) containing 0.1 mM EDTA for superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX). For extraction of glutathione reductase (GR), the buffer was supplied with 10 mM β-mercaptoethanol (Porcel et al. 2003). The extracts were filtered through four layers of nylon cloth and centrifuged at 20,000×g at 0–4 °C for 20 min. The supernatants were kept at −70 °C for subsequent enzymatic assays.
Total SOD activity (EC 1.15.1.1) was measured according to the method described by Beyer and Fridovich (1987) based on the ability of SOD to inhibit the reduction of nitroblue tetrafolium (NBT) by superoxide radicals generated photochemically. One unit of SOD was defined as the amount of enzyme required to inhibit the reduction rate of NBT by 50 % at 25 °C. CAT activity (EC 1.16.1.6) was measured by the disappearance of H2O2 (Aebi 1984). The reaction mixture (3 ml) contained 10.6 mM H2O2. The reaction was initiated by adding 25 μl of the extract and monitoring the change in absorbance at 240 nm and 25 °C for 3 min. APX activity (EC 1.11.1.11) was measured in a 1-ml reaction volume containing 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM hydrogen peroxide, and 0.5 mM ascorbate. The addition of the H2O2 initiated the reaction, and the decrease in absorbance at 290 nm was recorded for 1 min to determine the oxidation rate of ascorbate (Amako et al. 1994). Finally, GR activity (EC 1.6.4.2) was determined by the procedure described by Carlberg and Mannervik (1985). The reaction mixture (1 ml) contained 0.1 M HEPES pH 7.8, 1 mM EDTA, 3 mM MgCl2, 0.5 mM oxidized glutathione, 0.2 mM NADPH, and 150 μl of the enzyme extract. The rate of NADPH oxidation was monitored by the decrease in absorbance at 340 nm for 2 min. Two blanks, one without the enzyme extract and the other without oxidized glutathione, were used as controls.
Extraction, purification, and determination of jasmonates
12-cis-oxophytodienoic acid (OPDA), JA, 11-hydroxy-JA (11-OH-JA), and 12-OH-JA were extracted, pre-purified, and determined by GC-MS according to Miersch et al. (2008) using 0.5 g (FW) of leaf material. As internal standards, (2H6)JA, (2H5)OPDA, 11-(2H3)OAc-JA, and 12-(2H3)OAc-JA were added in the appropriate amounts.
Statistical analysis
All data were subjected to two-way analysis of variance (ANOVA) with inoculation treatment and abiotic stress as sources of variation. Post hoc comparisons with Duncan’s Multiple Range Test (Duncan 1955) were used to determine differences between the groups with the aid of the Statistical Analytical Software (SAS) program, version 3.5 (1991).
Results
Mycorrhizal development
In this study, no mycorrhizal colonization was observed in D. eriantha plants not provided with AM inoculum. The inoculated plants showed between 68 and 72 % of mycorrhizal root colonization under the different treatments (data not shown). However, no significant differences were detected following the application of abiotic stresses.
Stomatal conductance, photosynthetic efficiency, and plant biomass production
AM symbiosis might result in altered rates of water movement into, through, and out of host plants, with consequent effects on tissue hydration and leaf physiology (Augé 2001). Therefore, parameters reflecting water-related leaf physiology were determined in order to elucidate the impact of AM on stress responses in D. eriantha.
Stomatal conductance showed a significant decrease under all stress conditions in AM as well as in non-AM plants as compared to control conditions. Under non-stress, drought, and salinity conditions, however, the AM plants exhibited higher stomatal conductance as compared to non-AM plants. The lowest values for stomatal conductance were observed under cold stress, with both AM and non-AM plants showing similar values (Table 1). The efficiency of Photosystem II in non-AM plants was similar under non-stress conditions and drought treatments but decreased under salinity and cold stress conditions. In AM plants, photosynthetic efficiency decreased significantly under drought conditions, while, under cold stress, AM plants exhibited increased photosynthetic efficiency as compared to non-AM plants (Table 1).
The shoot/root biomass ratio was enhanced by AM symbiosis under non-stressful and drought stress conditions, but was lower in AM plants than in non-AM plants under salinity and cold stress conditions (Table 1). However, under all growing conditions, AM plants enhanced shoot dry matter content except under salinity stress, when no significant differences between AM and non-AM plants were found (Table 1).
Hydrogen peroxide, oxidative damage to lipids, and antioxidant enzyme activity
Salt, drought, and cold stresses are well known to induce oxidative stress in plants (Abdel Latef 2010; Aroca et al. 2003; Garbero et al. 2010). Plant cells contain an array of protection mechanisms and repair systems that can minimize the occurrence of oxidative damage caused by reactive oxygen species (ROS) (Abdel Latef 2010). Moreover, the antioxidant capacity of the host plant can be activated by AM symbiosis (Abdel Latef and Chaoxing 2011). To elucidate these effects of AM, ROS levels, oxidative damage to lipids, and corresponding enzyme activity were determined in non-AM and AM plants subjected to stress.
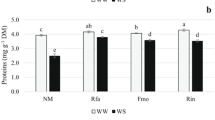
The level of hydrogen peroxide accumulated in roots was significantly higher in the non-AM plants than in AM plants in all treatments, reaching maximum differences of up to 75 % under drought stress (Fig. 1), while in AM plants no differences were observed among treatments. In the shoots of AM plants, H2O2 increased by 95 % under non-stress and drought conditions, while, in AM plants subjected to salinity or cold, it decreased by 40 and 68 %, respectively. H2O2 accumulation increased in non-AM plants by up to 40 % under cold as compared to non-stress conditions.
Root and shoot hydrogen peroxide accumulation in D. eriantha plants non-inoculated (non-AM) or inoculated (AM) with the AM fungus R. irregularis and cultivated under either optimal, drought, salt, or cold stress conditions. Bars represent means ± standard error. Values showing different letters are significantly different (P ≤ 0.05), as determined by Duncan’s multiple range test (n = 5)
In roots, oxidative damage to lipids increased significantly by 115 % only as consequence of drought in both non-AM and AM plants as compared to non-stressed plants (Fig. 2). Under salinity, stress significantly decreased the levels of MDA in both AM and non-AM plants. In shoots, MDA levels decreased in AM plants under non-stress and cold stress conditions. By contrast, under drought and salinity treatments, the levels of MDA were significantly higher in AM plants as compared to non-AM plants.
Oxidative damage of lipids in roots and shoots of D. eriantha plants non-inoculated (non-AM) or inoculated (AM) with the AM fungus R. irregularis and cultivated under either optimal, drought, salt, or cold stress conditions. Bars represent means ± standard error. Values showing different letters are significantly different (P ≤ 0.05), as determined by Duncan’s multiple range test (n = 5)
Among the enzymes known to be involved in ROS detoxification, SOD, CAT, APX, and GR were selected and their activity was determined in the shoots and roots of all plants (Table 2). In roots, SOD activity was significantly lower in AM plants than in non-AM plants under non-stress, drought, and salinity conditions. Moreover, drought and salinity increased SOD activity in both non-AM and AM plants. By contrast, cold stress drastically decreased SOD activity in non-AM plants and increased this activity in AM plants to levels similar to those in non-stressed non-AM plants. In shoots, SOD activity levels were lower under stress conditions as compared to non-stressed treatments. However, AM plants showed significantly higher values than non-AM plants under the different conditions assayed (Table 2).
CAT activity appeared to be consistently enhanced in both the roots and shoots of AM plants under non-stressed and stressed conditions (Table 2). This increase was significantly higher in roots subjected to cold stress and in shoots subjected to drought stress. In general, non-AM plants did not show differences in CAT activity among the different stress treatments as compared to non-stress conditions.
APX activity was always significantly higher in AM plants than in non-AM plants, in both root and shoot tissues under all the treatments (Table 2), especially under drought and cold conditions.
GR activity was always significantly higher in both the roots and shoots of non-AM plants as compared to AM plants. GR activity in the shoots of non-AM plants decreased by about 43 % under drought and salinity conditions as compared to non-stress and cold conditions (Table 2).
Jasmonate levels
The roots of AM plants are known to contain higher levels of jasmonates (Wasternack and Hause 2013). Alterations in the JA levels of shoots upon AM, combined with abiotic stresses are, however, less understood. Therefore, jasmonate levels in the shoots of non-AM and AM plants subjected to stress were determined. The levels of OPDA (JA precursor) were significantly higher in AM plants than in non-AM plants for all treatments except under cold stress conditions, where the increase was not significant (Fig. 3a). In non-AM plants, only salt stress caused significant differences in OPDA content with respect to non-stress conditions (Fig. 3a). This is reminiscent of the role played by OPDA in the salt stress response of plants (Hazman et al. 2015). Drought and cold stress did not show differences as compared to the non-AM treatment under non-stress conditions.
Jasmonate content in D. eriantha plants non-inoculated (non-AM) or inoculated (AM) with the AM fungus R irregularis and cultivated under either optimal, drought, salt, or cold stress conditions. a 12-cis-oxophytodienoic acid (OPDA). b Jasmonic acid (JA). c 11-hydroxy-jasmonic acid (11-OH-JA). d 12-hydroxy-jasmonic acid (12-OH-JA). Bars represent means ± standard error. Values showing different letters are significantly different (P ≤ 0.05), as determined by Duncan’s multiple range test (n = 5)
The level of JA in the shoots of non-AM plants did not differ among stress treatments as compared to non-stress conditions (Fig. 3b). However, the shoots of non-stressed AM plants exhibited higher JA levels than the shoots of non-AM plants, with D. eriantha showing an increase in JA upon mycorrhization. Under salt stress conditions, AM plants showed JA content similar to that under non-stress conditions, while drought and cold stress decreased JA levels in AM plants to those of non-stressed, non-AM plants.
The levels of 11-OH-JA did not differ among treatments in the non-AM plants, while, in AM plants an increase was observed under drought stress conditions as compared to non-stressed AM plants and to non-AM plants (Fig. 3c). No differences in the 12-OH-JA levels of non-AM plants were observed, except under cold condition, where there was a significant decrease with respect to non-stress conditions. In AM plants, drought and salinity showed a significant increase in 12-OH-JA levels as compared to non-stress conditions and non-AM plants (Fig. 3d).
Discussion
AM fungi have been shown to improve plant tolerance to abiotic stresses (Ruiz-Lozano and Aroca 2010). Therefore, the effects of AM on the stress tolerance of D. eriantha were elucidated in terms of mycorrhization, biomass production as well as, accumulation of ROS and jasmonates. To obtain an overview of these processes, treatments were applied resulting in three different stresses: drought, salt, and cold stress.
For several species of agronomic interest, information concerning the link between abiotic stress and mycorrhiza is abundant. To date, however, no studies have linked the regulation by the AM symbiosis of plant physiology and performance under abiotic stresses with the alteration of antioxidants and jasmonates levels in the host plant, and few studies have investigated common mechanisms underlying AM-induced tolerance to different abiotic stresses (Aroca et al. 2007). To gain an insight into these mechanisms, D. eriantha plants were inoculated with Rhizophagus intraradices and showed a colonization rate of about 70 % colonization in their roots. Given this finding, the values for colonization observed in Digitaria are greater than those recorded for tomato plants (34 %, Dell’Amico et al. 2002 and 55 % Abdel Latef and Chaoxing 2011), soybean (50 %, Porcel and Ruiz-Lozano 2004), and rice (45 %, Ruiz-Sánchez et al. 2010) which were not subjected to stress and were inoculated with the same fungus. In other studies using different species of Rhizophagus, colonization of grape roots did not exceed 41 % (Alarcón et al. 2001). Colonization percentages in the roots of D. eriantha were not affected by drought, salt, or cold stress treatments as compared to the non-stressed control group. This differs from other plant species, such as tomato, soybean, rice, and lettuce, which showed a significant decrease in colonization rates when plants were subjected to water stress (Dell’Amico et al. 2002; Porcel and Ruiz-Lozano 2004; Ruiz-Sánchez et al. 2010) or salt stress (Abdel Latef and Chaoxing 2011; Aroca et al. 2013). These results indicate that R. irregularis is highly efficient in terms of associating with D. eriantha cv. Sudafricana, even in the abiotic stress situations studied.
In the present study, the shoot/root biomass ratios of non-stressed AM plants and those subjected to drought increased. In addition, AM plants also enhanced shoot dry matter content as compared to non-AM plants under all the conditions tested except for salinity. Nevertheless, salinity and cold temperatures caused a decrease in the shoot/root biomass ratios of AM plants. This may be due to the decrease in stomatal conductance and efficiency of Photosystem II (PSII), given the inability of plants to counteract the toxic effect of NaCl (Evelin et al. 2009), representing a dehydration that causes a common physiological disorder to water, salt, and cold stresses (Solanke and Sharme 2008). However, under saline conditions, AM symbiosis can stimulate root development in the host plant as a strategy to cope with soil salinity. Indeed, in a previous study comparing the symbiotic efficiency of two Glomus strains with differential adaptation to salinity, the mechanism used by Glomus sp. to protect lettuce plants from the detrimental effects of salt was found to be based on the stimulation of root development, while Glomus deserticola led to an improvement in plant nutrition (Ruiz-Lozano and Azcón 2000). Thus, in this study of D. eriantha and R. irregularis, the fungus also stimulated root development in the host plant under saline conditions (data not shown), which resulted in a significant reduction in the shoot/root ratio of these plants.
The mycorrhizal colonization of roots has a marked effect on the stomatal behavior of the host plant’s leaves which also favors the exchange of CO2 (Goicoechea et al. 1997; Augé et al. 2007; Ruiz-Lozano and Aroca 2010). When the stomatal conductance of AM plants differs from that of non-AM plants, symbiosis in roots causes a fundamental change in the physiology of the leaf, including alterations in its intrinsic biochemical and hydraulic properties (Augé 2000). Plants colonized by R. irregularis presented higher stomatal conductance values than non-AM plants (Augé 2001). In this study, non-stressed AM plants and AM plants subjected to drought and salinity showed significantly higher stomatal conductance values (30 % on average) than non-AM control plants, a result similar to that reported by Augé et al. (2014) in a meta-analysis of the effect of mycorrhization on stomatal conductance. By contrast, the values obtained under cold stress conditions did not differ between treatments, indicating that mycorrhization did not mitigate the effect of low temperatures.
D. eriantha’s Photosystem II efficiency was evaluated using chlorophyll a fluorescence. It has previously been reported that mycorrhizal plants show higher values under drought (Ruiz-Sánchez et al. 2010; Birhane et al. 2012) and salt stress (Ruiz-Lozano et al. 1996; Dell’Amico et al. 2002; Sheng et al. 2008) treatments. In the present study, the photosynthetic efficiency values for AM and non-AM plants decreased significantly in relation to non-stressed plants when subjected to stress. However, drought caused a reduction in the photosynthetic efficiency of AM plants as compared to non-AM plants, while cold temperatures resulted in a significant increase in the photosynthetic efficiency of AM plants as compared to non-AM plants. These results suggest that the abiotic stresses tested affected the photosynthetic system by reducing the photosynthetic efficiency of AM and non-AM plants and that damage to Photosystem II was more severe in plants subjected to drought and salinity.
A positive correlation between tolerance to abiotic stresses in AM plants and maintenance of Photosystem II efficiency has been demonstrated which, in turn, maintains (Porcel and Ruiz-Lozano 2004) or even increases (Ruiz-Sánchez et al. 2010; Evelin et al. 2009) the productivity of the plant.
Drought, salinity, extreme temperatures, and oxidative stresses are accompanied by the formation of ROS such as superoxide radicals (O2•−) and H2O2 that damage membranes and macromolecules (Miller et al. 2010; Noctor et al. 2014). Plants have developed various antioxidative strategies to flush out these toxic components. The enhancement of antioxidant defenses increases tolerance to different abiotic factors (Wang et al. 2003). AM symbiosis positively affects plants through nutrient acquisition or tolerance to environmental stresses (Fuentealba 2014). In this study, AM plants reduced hydrogen peroxide levels under all stress treatments, thus demonstrating their ability to counteract damage, which is in line with that observed in mycorrhizal soybean and ryegrass plants subjected to drought (Porcel and Ruiz-Lozano 2004; Lee et al. 2012) and in two tomato cultivars under salt stress conditions (Hajiboland et al. 2010).
MDA levels in roots were similar in AM and non-AM plants for each stress treatment. Drought increased MDA accumulation both in AM and non-AM plants, while salinity reduced its accumulation in relation to non-stressed control plants. The reduction in H2O2 levels under the different stress conditions could be explained by the significant increase in the CAT and APX enzyme activity of AM plants as compared to non-AM plants with respect to all treatments, as reported by Porcel and Ruiz-Lozano (2004). However, the values for SOD and GR activity were lower in AM plants as compared to non-AM plants. Nevertheless, these two enzymes are not directly involved in the removal of H2O2. SOD dismutates superoxide radicals into hydrogen peroxide, which is then converted into water and molecular oxygen by CATs in peroxisomes, while GR reduces dehydroascorbate to ascorbate in the ascorbate-glutathione cycle (Estrada et al. 2013). H2O2 content in the leaves of non-AM plants was only increased by cold stress, while AM symbiosis significantly decreased H2O2 under salinity and cold stress, similar to that observed in tomato plants under saline conditions by Hajiboland et al. (2010). MDA content also decreased in AM plants when subjected to cold temperatures, which is in line with that observed in tomato plants subjected to salinity (Abdel Latef and Chaoxing 2011) and in soybean and rice plants under drought stress conditions (Porcel and Ruíz-Lozano 2004; Ruiz-Sánchez et al. 2010). However, under drought and saline conditions, MDA levels remained high as compared to non-AM plants. The erratic behavior of oxidative damage to lipids observed in D. eriantha could be explained by the findings of Porcel and Ruiz-Lozano (2004)) who point out that H2O2 is involved in almost all areas of the plant’s aerobic biochemistry, such as electron transport in respiration and photosynthesis as well as glucose oxidation, and is produced in large quantities by various enzymatic systems even under optimal conditions. Moreover, under certain stress conditions, H2O2 can be used by plants as a defense mechanism (Quan et al. 2008).
With regard to enzymatic activity in leaves, CAT, APX, and SOD showed significant increases in AM plants as compared to non-AM plants, while GR activity decreased in AM plants under all the stress conditions studied. Previous research has also reported increases in SOD, CAT, and APX activity in AM tomato plants subjected to saline stress (Abdel Latef and Chaoxing 2011). Moreover, decreases in GR activity have been reported in the roots and stems of AM soybean plants subjected to drought stress (Porcel and Ruiz-Lozano 2004). CAT and APX activities are both involved in the scavenging of hydrogen peroxide, although APX has a much higher affinity for H2O2 than CAT (Estrada et al. 2013). CAT activity is lower in the shoots of plants subjected to salinity than in control plants not subjected to stress, which may indicate that, in D. eriantha plants subjected to salinity, hydrogen peroxide could be preferentially scavenged by APX activity. Indeed, in these plants, APX activity is significantly higher than in control plants not subjected to stress.
The involvement of jasmonates in the formation and development of mycorrhizal symbiosis is widely accepted (Wasternack and Hause 2013; Bucher et al. 2014). Increases in JA have been shown to correlate with the activation of genes for enzymes of the biosynthesis of this hormone (Hause et al. 2007). Moreover, tomato plants defective in JA synthesis have been found to exhibit a lower rate of AM root colonization than wild-type plants (Leon-Morcillo et al. 2012). It was also found that the application of JA either reduces (Ludwig-Muller et al. 2002; Herrera-Medina et al. 2008) or increases AM root colonization (Landgraf et al. 2012). Thus, the role of JA in AM symbiosis is still a matter of debate. In addition, there is no clear information on the precise role played by this phytohormone and its family of compounds in abiotic stress responses in plants which are associated with AM fungi. Our results show that JA levels in the shoots of non-AM plants subjected to different abiotic stresses did not differ from those in unstressed control plants. However, in AM plants, endogenous levels of JA increased significantly when subjected to drought and salt stresses, while, under cold stress conditions, their behavior was more irregular. Levels of JA and its precursor OPDA in control plants were significantly higher in AM plants than in non-AM plants, which is in line with the results obtained for the mycorrhizal roots of most plants by Hause and Schaarschmidt (2009). The higher values for OPDA in relation to JA in all AM plant treatments could be explained by the specific role played by OPDA in the expression of some genes involved in stress responses (Wasternack and Hause 2013). Cold stress behaved differently from the other stress treatments applied at all the jasmonate levels measured. Following cold treatment, the levels of JA, OPDA, and 11-OH-JA did not differ between non-AM and AM plants, while 12-OH-JA levels slightly increased in AM plants as compared to non-AM plants. Whether the reduced level of JA in cold-stressed plants contributes to increased or reduced stress responses remains to be determined.
In summary, the results presented show that D. eriantha cv. Sudafricana is sensitive to drought, salinity, and cold stresses and that inoculation with AM fungi regulates its physiology and performance under such stress conditions. The effects of AM symbiosis on antioxidant plant responses and jasmonates accumulation depend on the intrinsic characteristics of the stress applied. In general, the level of JA and its precursors was higher in AM plants under the different stress conditions, which could help these plants to better cope with stressful conditions.
Abbreviations
- AM:
-
Arbuscular mycorrhiza (l)
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GR:
-
Glutathione reductase
- MDA:
-
Malondialdehyde
- Non-AM:
-
Non-inoculated plants
- SOD:
-
Superoxide dismutase
References
Abdel Latef A (2010) Changes of antioxidant enzymes activity in salinity tolerance among different wheat cultivars. Cereal Res Commun 38:43–55
Abdel Latef A, Chaoxing H (2011) Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci Hortic 127:228–233
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alarcón A, González-Chávez MC, Ferrera-Cerrato R, Villegas-Monter A (2001) Efectividad de Glomus fasciculatum y Glomus etunicatum en el crecimiento de plantulas de Vitis vinifera L. obtenidas por micropropagacion. TERRA 19:29–35
Amako K, Che GX, Asada K (1994) Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isoenzymes of ascorbate peroxidase in plants. Plant Cell Physiol 35:497–504
Aroca R, Irigoyen J, Sánchez-Díaz M (2003) Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol Plant 117:540–549
Aroca R, Porcel P, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173:808–816
Aroca R, Vernieri P, Ruiz-Lozano JM (2008) Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. J Exp Bot 59:2029–2041
Aroca R, Ruiz-Lozano JM, Zamarreño AM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55
Augé RM (2000) Stomatal behavior of arbuscular mycorrhizal plants. In: Kapulnik Y, Douds DD (eds) Arbuscular mycorrhizas: physiology and function. Kluwer Academic Publishers, Dordrecht, the Netherlands, pp 201–237
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Augé RM, Toler HD, Moore JL, Cho K, Saxton AM (2007) Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo. J Plant Physiol 164:1289–1299
Augé RM, Toler HD, Saxton AM (2014) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25:13–24
Baslam M, Goicoechea N (2012) Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 22:347–359
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Ann Biochem 161:559–566
Biehler K, Fock H (1996) Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol 112:265–272
Birhane E, Sterck F, Fetene M, Bongers F, Kuyper T (2012) Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 169:895–904
Bucher M, Hause B, Krajinski F, Küster H (2014) Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol 204:833–840
Carlberg I, Mannervik B (1985) Glutathione-reductase. Methods Enzymol 113:484–490
Colazo JC, Buschiazzo DE (2010) Soil dry aggregate stability y wind erodible fraction in a semiarid environment of Argentina. Geoderma 159:228–236
Collado AD (2003) Teledetección y estudios sobre desertificación en San Luis. In: Aguilera MO, Panigatti JL (Eds) Con las metas claras. La EEA San Luis: 40 años a favor del desarrollo sustentable. INTA pp. 159–179
Dell’Amico J, Rodríguez P, Torrecillas A, Morte A, Sánchez-Blanco M (2002) Influencia de la micorrización en el crecimiento y las relaciones hídricas de plantas de tomate sometidas a un ciclo de sequía y recuperación. Cultivos Tropicales 3:29–34
Di Giambatista G, Garbero M, Ruiz M, Giulietti A, Pedranzani H (2010) Germinación de Trichloris crinita y Digitaria eriantha en condiciones de estrés abiótico. Pastos y Forrajes 33:4. http://www.redalyc.org/articulo.oa?id=269119492002. Accessed 26 May 2013
Dodd IC, Pérez-Alfocea F (2012) Microbial amelioration of crop salinity stress. J Exp Bot 63:3415–3428
Duncan DB (1955) Multiple range and multiple F-tests. Biometrics 11:1–42
Estrada B, Aroca R, Barea JM, Ruíz-Lozano JM (2013) Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Sci 201:42–51
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Frasinelli C, Martínez Ferrer J (1999) Resultados preliminares en sistemas de cría e invernada. In INTA-Forrajeras Avanzadas S.A. 38° Jornada Técnica sobre Digigrass (Digitaria eriantha). Villa Mercedes (SL). Marzo 24:3–11
Fuentealba A (2014) El potencial de las micorrizas arbusculares en la agricultura desarrollada en zonas áridas y semiáridas. IDESIA 1:3–8
Garbero M, Pedranzani H, Zirulnik F, Molina A, Pérez-Chaca MV, Vigliocco A, Abdala G (2010) Antioxidant and jasmonate response under short-term cold stress in two cultivars of Digitaria eriantha. Acta Physiol Plant 14:635–644
Garbero M, Andrade A, Reinoso H, Fernández B, Cuesta C, Granda V, Escudero C, Abdala G, Pedranzani H (2012) Differential effect of short-term cold stress on growth, anatomy, and hormone levels in cold-sensitive versus resistant cultivars of Digitaria eriantha. Acta Physiol Plant 34:2079–2091
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Physiol 84:489–500
Goicoechea N, Antolin MC, Sánchez-Díaz M (1997) Gas exchange is related to the hormone balance in mycorrhizal or nitrogen-fixing alfalfa subjected to drought. Physiol Plant 100:989–997
Hajiboland R, Aliasgharzadeh N, Laiegh S, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327
Halliwell B, Gutteridge JM (1989) Free radicals in biology and medicine, 2nd edn. Clarendon, Oxford
Hause B, Schaarschmidt S (2009) The role of jasmonates in mutualistic symbioses between plants and soil-born microorganisms. Phytochemistry 70:1589–1599
Hause B, Mrosk C, Isayenkov S, Strack D (2007) Jasmonates in arbuscular mycorrhizal interactions. Phytochemistry 68:101–110
Hazman M, Hause B, Eiche E, Nick P, Riemann M (2015) Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J Exp Bot (In press). doi: 10.1093/jxb/erv142
Herrera-Medina MJ, Tamayo MI, Vierheilig H, Ocampo JA, García-Garrido JM (2008) The jasmonic acid signalling pathway restricts the development of the arbuscular mycorrhizal association in tomato. J Plant Growth Regul 27:221–230
Hirayama T, Shinozaki K (2007) Perception and transduction of abscisic acid signals: keys to the function of the versatile plant hormone ABA. Trends Plant Sci 12:343–351
Kaschuk G, Kuyper TW, Leffelaar PA, Hungria M, Giller KE (2009) Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol Biochem 41:1233–1244
Landgraf R, Schaarschmidt S, Hause B (2012) Repeated leaf wounding alters the colonization of Medicago truncatula roots by beneficial and pathogenic microorganisms. Plant Cell Environ 35:1344–1357
Lee BR, Muneer S, Jung WJ, Avice JC, Ourry A, Kim TH (2012) Mycorrhizal colonization alleviates drought-induced oxidative damage and lignification in the leaves of drought-stressed perennial ryegrass (Lolium perenne). Physiol Plant 145:440–449
Leon-Morcillo RJ, Martin-Rodriguez AJ, Vierheilig H, Ocampo JA, Garcia-Garrido JM (2012) Late activation of the 9-oxylipin pathway during arbuscular mycorrhiza formation in tomato and its regulation by jasmonate signalling. J Exp Bot 63:3545–3558
Liu T, Sheng M, Wang CY, Chen H, Li Z, Tang M (2015) Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 53:250–258
Ludwig-Muller J, Bennett RN, Garcia-Garrido JM, Piche Y, Vierheilig H (2002) Reduced arbuscular mycorrhizal root colonization in Tropaeolum majus and Carica papaya after jasmonic acid application can not be attributed to increased glucosinolate levels. J Plant Physiol 159:517–523
Malpassi R, Grosso M, Basconsuelo S (2004) Anatomía ecológica. Capítulo 7: In: Bianco CA, Kraus TA and Vegetti AC (Eds) La Hoja. Morfología externa y anatomía. p. 199
Marulanda A, Porcel R, Barea JM, Azcón R (2007) Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microb Ecol 54:543–552
Miersch O, Neumerkel J, Dippe M, Stenzel I, Wasternack C (2008) Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch-off in jasmonate signaling. New Phytol 177:114–127
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stress. Plant Cell Environ 33:453–467
Minotti G, Aust D (1987) The requirement for iron (III) in the initiation of lipid peroxidation by iron (II) and hydrogen peroxide. J Biol Chem 262:1098–1104
Noctor G, Mhamdi A, Foyer CH (2014) The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol 164:1636–1648
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: calculation of qP and F′v/F′m without measuring F’o. Photosynt Res 54:135–142
Patterson BD, Mac Rae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Pedranzani H, Ruiz M, Garbero M, Terenti O (2005) Effects of low temperature on different parameters of production in Digitaria eriantha cv. Mejorada INTA. Phyton 54:121–126
Peña Zubiate CA, D’hiriart A (2005) Carta de Suelos de la República Argentina: Provincia de San Luis. EEA San Luis-INTA-Gobierno de la Provincia de San Luis
Philips J, Hayman DS (1970) Improved procedure of clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans British Mycol Soc 55:158–160
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subject to drought stress. J Exp Bot 55:1743–1750
Porcel R, Barea JM, Ruiz-Lozano JM (2003) Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol 157:135–143
Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM (2006) PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol Biol 60:389–404
Quan LJ, Zhang B, Shi WW, Li HY (2008) Hydrogen peroxide in plants: a versatile molecule of the reactive oxygen species network. J Integr Plant Biol 50(1):2–18
Ruiz-Lozano JM, Aroca R (2010) Host response to osmotic stresses: stomatal behaviour and water use efficiency of arbuscular mycorrhizal plants. In: Kapulnik Y, Koltai H (eds) Arbuscular mycorrhizas: physiology and function”, 2nd edn. Springer, Dordrecht. The Netherlands, pp 239–256
Ruiz-Lozano JM, Azcón R (2000) Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 10:137–143
Ruiz-Lozano JM, Azcón R, Gómez M (1996) Alleviation of salt stress by arbuscular mycorrhizal Glomus species in Lactuca sativa plants. Physiol Plant 98:767–772
Ruiz-Sánchez M, Aroca R, Muñoz Y, Polón R, Ruiz-Lozano JM (2010) The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol 167:862–869
Sánchez-Romera B, Ruiz-Lozano JM, Li G, Luu DT, Martínez-Ballesta MC, Carvajal M, Zamarreno AM, Garcia-Mina JM, Maurel C, Aroca R (2014) Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant Cell Environ 37:995–1008
Scheibe R, Beck E (2011) Drought, desiccation, and oxidative stress. In: Plant desiccation tolerance, ecological studies 215. Lüttge U, Beck E, Bartels D (Eds) Springer-Verlag, Berlin, pp 209–231.
Seki M, Kamei A, Yamaguchi-Shinozaki K, Shinozaki K (2003) Molecular responses to drought, salinity and frost: common and different paths for plant protection. Curr Opin Biotechnol 14:194–199
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296
Solanke A, Sharma A (2008) Signal transduction during cold stress in plants. Physiol Mol Biol Plants 14:69–79
Veneciano JH (2006) Gramíneas perennes para ambientes semiáridos: Características y productividad. Inf. Técnica 171. EEA del INTA San Luis. Argentina pp.1-77. ISSN 0327-425X
Wang W, Vinocur B, Altman A (2003) Plant response to drought, salinity and extreme temperature: towards genetic engineering for salt stress tolerance. Planta 218:1–140
Wasternack C (2007) Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot 100:681–697
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058
Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25:195–210
Acknowledgments
This work was financed by a research project supported by Junta de Andalucía (Spain) (Project P11-CVI-7107) and by a cooperative project supported by CSIC (Project COOPB20121). We would like to thank Michael O’Shea for proofreading the document.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedranzani, H., Rodríguez-Rivera, M., Gutiérrez, M. et al. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 26, 141–152 (2016). https://doi.org/10.1007/s00572-015-0653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0653-4