Abstract
Here we present a method for selectively and efficiently immobilizing antibodies to enhance the detection performance of surface plasmon resonance immune-sensors (SPRIs) for diagnostic applications. To improve the performance of antibody arrays, protein G was used as antibody-selective linkage layer with aldehyde functionalized poly-(para-xylylene) film. To estimate the efficiency of antibody immobilization, immunoglobulin G (IgG) was measured using the anti-IgG immobilized SPRIs. To demonstrate the proof-of-concept validation, the signal detected from the IgG using parylene-H film was compared with that of a combination of parylene-H and protein G in SPRIs. The results showed that the detection of IgG on the immobilized anti-IgG layer using the combination of parylene-H and protein G has a larger change of signal than that of using parylene-H layer. These results also imply that the anti-IgG was densely and efficiently immobilized on the modified surface with the linkage layer in a combination with parylene-H and protein G. Therefore, we believe that this combinatorial approach could selectively immobilize the antibodies, and also be applied for detection and diagnosis of immune diseases in the field of many SPRIs applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With increased interest in the early diagnosis, immunoassay based sensing technologies have been intensively researched. The immunosensor is a biosensor based on the specific antibody-antigen interaction and widely used to detect biomarkers for diagnosis since its high specificity (Tomizaki et al. 2010; Tsai et al. 2007; Marco and Barceló 1996). Various highly sensitive sensing methods are used as immunosensor including field-effect transistor (FET) (Wang 2006), quartz crystal microbalance (QCM) (Arlett et al. 2011), surface plasmon resonance (SPR) (Fan et al. 2008; Tsai and Pai 2009) and micro cantilever (Arlett et al. 2011; Fritz 2008). The SPR based immunosensor is a surface sensitive method and compatible for biomolecular analysis as it allows detection of small molecular interaction on the thin metal film, label-free and real-time monitoring (Pluchery et al. 2011; Thariani and Yager 2008).
In the immunoassay based sensor, efficient immobilization of the specific antibody on the sensor chip surface is necessary since the sensitivity of the immunosensor depends on the immobilization condition of antibodies (Tomizaki et al. 2010; Tsai et al. 2007; Hanash et al. 2008; Weiping et al. 1999). The antibody (Immunoglobulin) is a Y-shaped protein which recognizes the specific target antigen and composed of one Fc region and two fabricated regions which has the antigen binding site (Edelman 1973; Amit et al. 1986). Hence the Fab region exposed antibody immobilization can improve the antigen detection ability of immunoassay (Tsai and Pai 2009). The biochemical modification is a most typical method to create antibody immobilized surfaces (Hanash et al. 2008; Lin et al. 2010) such as self-assembled monolayer (SAM) (Weiping et al. 1999), polymer films (Liu 2007), protein G and protein A (Bae et al. 2005). Parylene is a trade name for a poly-(para-xylylene) series which has been received attention as a surface modification material. The parylene is a thin polymer film which made by chemical vapor deposition (Fortin and Lu 2002; Tan and Craighead 2010). For the biological application, the parylene film has many advantages including the chemical inertness, possibility of deposition at the room temperature, conformal coating and low intrinsic stress (Liu 2007; Fortin and Lu 2002; Tan and Craighead 2010). Also the surface modification method using parylene film can overcome the several disadvantages of biochemical surface modification techniques which are multi-step chemical process, time-consuming treatment and dependence of surface material (Prakash et al. 2009). And due to its chemical vapor deposition process, parylene film has high conformity and free of pinhole and stress. More importantly, various functional groups can be introduced into the parylene structure which can bind with desired biochemical molecules (Liu 2007; Tan and Craighead 2010). The basic structured parylene is called parylene-N and up to four functional groups can be introduced. The newly synthesized parylene materials introduced the desired chemical group are actively studied. The parylene-H is a recently developed parylene polymer and has a aldehyde group which can make a covalent bond with the amine group of proteins without additional chemical process (Ko et al. 2011). The specific structure of aldehyde group introduced parylene-H is presented in Fig. 1a. The deposition process of parylene can be separated into the following steps: (1) the dimers are sublimated into the gas phase at temperature above 100 °C, (2) the vaporized parylene-dimers are cracked into the monomers at the temperature above 600 °C, (3) the parylene monomers are absorbed onto the substrate in the deposition chamber at the room temperature. The polymerized monomers deposits a conformal and uniform parylene film (Fortin and Lu 2002).
The immobilization of antibody using a parylene-H film and b a combination of protien G and parylene-H. Although the parylene-H includes a carboxyl group which can bind with a primary amine group of anti-body, the anti-IgG may have been randomly immobilized since the several regions of anti-IgG have amine group. The structure of antibody and combination of parylene-H and protein G used antibody immobilization method are presented in b. The protein G was formed densely and directionally on the parylene-H layer with the Fc binding site of the protein G facing away from the chip surface. Thus, the binding capacity of the anti-IgG to IgG is improved when using the combination of protein G and parylene-H
This surface modification methods using parylene-H demonstrated the potential of antibody immobilization, but have also magnified the need for methods which can maintain the activity of antibody using control the immobilization morphologies. The Fab region, antigen binding site of antibody has amine group which can bind with aldehyde group of the parylene-H. Hence the antigen specific region of antibody can be blocked by binding with parylene-H film surface. To improve the efficiency of antibody immobilization, compatible linkage molecule is suggested. Protein G, a cell surface protein produced by Streptococcus sp. is an immunoglobulin binding protein. The protein G consists of three domains including alpha, beta and gamma, and the beta domain of protein G can specifically bind with Fc domain of immunoglobulin G (IgG) subtypes. Thus, the protein G used for the immobilization of antibodies can lead efficient antibody immobilization that Fab region exposed (Sauer-Eriksson et al. 1995).
In this research, a novel linkage layer for the selective and efficient immobilization of antibody using the combination of protein G and parylene-H film was proposed as (Fig. 1b). The suggested linkage-layer was applied to the SPR-based biosensor for the detection of specific target molecules. The applicability of the combination of parylene-H film and protein G to a SPR biosensor was investigated by evaluating the changes in efficiency, sensitivity, and detection range according to the composition of linkage layer. The far more improved immobilization efficiency as well as the sensitivity and specificity of the SPR biosensor were demonstrated by the combination of parylene-H film and protein G.
2 Materials and methods
2.1 Immobilization materials
To immobilize the antibody on the gold chip surface (BK7, K-MAC, Korea), protein G was used and purchased from Sigma (MO, USA). For the modification of antibody and protein G, the parylene-H powder (4-formyl[2,2]paracyclophane) was purchased from Femto-Science Ltd. (Seoul, Korea). The phosphate buffered saline (PBS) which was used as buffer solution for sample proteins was purchased from Sigma (MO, USA). To verify the surface modification condition, anti-human IgG and human IgG were purchased from Sigma (MO, USA).
2.2 Deposition of parylene-H layer on a SPR sensor chip
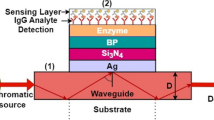
SPR sensor chip was sonicated in acetone, methanol, and DI-water for 5 min each for surface cleaning. Then the parylene film was deposited on a sensor chips by a parylene coater (NRPC-500, Nuri-Tech, Korea) through the following polymerization steps: (1) vaporization of parylene-H dimer at the temperature of 100 °C, (2) pyrolysis for the production of parylene-H monomer at the temperature of 670 °C, and (3) deposition on the chip surface at room temperature. The vacuum condition of parylene deposition chamber was maintained <0.01 Torr (Fig. 2a). The correlation between the parylene film thickness and the initial amount of parylene dimer depends on the deposition conditions as well as the structure of the parylene coater. The amount of used parylene-H dimer was 60 mg and the thickness of the deposited parylene-H film was 21 nm on average. The thickness of the parylene film was measured by surface profiler (Dektak XT, Bruker, Germany).
The Schematic of a parylene-H film deposition process and a structure of SPR based biosensor and flow channel. The parylene-H has a aldehyde group which can make a covalently bond with the amine group of proteins. Firstly, the powder formed parylene-H precursors are vaporized at the 100 °C. Then the produced parylene dimers are heated at over 670 °C for pyrolysis into the monomer. The parylene monomers polymerised into the parylene polymers at the room temperature and make a conformal parylene film on the substrate. In the case of the parylene-H used antibody immobilization method, an aldehyde group included parylene-H interacts with the amine group of the antibody. However, there is a possibility that various parts of the amine group on the antibody could have interacted with the aldehyde group of the parylene-H. The 2-channeled SPR sensor was used to record the bio-molecular interaction in real time. The PBS solution was flowed continuously on the reference channel while whole experimentation. After the parylene-H film deposition procedure, the sample proteins were injected into the sample flow channel subsequently. Then the weakly absorbed or unreacted proteins were washed out by a PBS solution between sample injections
2.3 Modification of protein G layer Immobilization of anti-IgG
To immobilize the anti-immunoglobulin G, the protein G was modified on the SPR sensor chip surface. The two-channeled SPR sensor (SPRmicro, K-MAC, Korea) with peristaltic micro pump (REGLO, ISMATEC, USA) and degasser (DGU-12A, SHIMADZU, Japan) was used to record the bio-molecular interaction in real time (Fig. 2b). The PBS solution was flowed continuously on the reference channel while whole experimentation. After the parylene-H film deposition procedure, the 150 μl of protein G solution at a concentration of 200 μg/ml was injected into the sample flow channel of SPR sensor system. Then the weakly absorbed or unreacted protein G was washed out by a PBS solution.
2.4 Immobilization of anti-IgG
After the protein G modification, the 150 μl of anti-IgG solution at a concentration of 150 μg/ml was injected into the sample channel and react with the protein G on the chip surface. Then the weakly absorbed or unreacted antibodies were washed out by a PBS solution. And the 150 μl of bovine serum albumin (BSA) at a concentration of 100 μg/ml was subsequently flowed into the sample channel to prevent the non-specific binding.
2.5 Detection of IgG using SPR sensor
After the immobilization of anti-IgG and blocking step, the 150 μl of human IgG solution was injected into the modified sensor chip surface. The non-specifically bounded or weakly absorbed analytes were removed by a PBS solution flow after detection process. The concentration of IgG solution were 1, 3, and 5 μg/ml and the experiments were repeated for 3 times each IgG concentration. To verify the detection efficiency of the antibody immobilized sensor system, the IgG detection signals from the SPR sensor were measured and compared.
3 Results
The IgG detection capabilities of immobilized anti-IgG using parylene-H film and a combination of protein G and parylene-H were investigated and compared by SPR immunosensor. For the SPR based detection, gold deposited glass chip was used as sensing surface (Fig. 3a). The parylene-H layer was formed on the bare sensor chip surface to introduce the carboxyl group at the beginning of each experiment as shown in Fig. 3b. As the SPR sensor used here had two flow channels, the PBS solution was allowed to flow into both channels first. In the experiment of combination of protein G and parylene-H, the protein G was injected into the sample channel and bound with parylene-H film modified on the sensor chip surface (Fig. 3c). After confirming a stable output signal from both channels, the anti-IgG at a concentration of 150 μg/ml was injected into the sample channel and immobilized on the linkage layer on the sensor chip surface (Fig. 3d). The BSA solution at a concentration of 100 μg/ml was flowed into the sample channel and bind on the extra space of antibody immobilized on sensor chip surface to block any non-specific binding as presented in Fig. 3e. Finally, various concentrations of IgG (1, 3 and 5 μg/ml) were allowed to flow across the sensor chip surface. The IgG was bound with Fc region of anti-IgG (Fig. 3f). Between all the protein injection steps, the sensor chip was washed with a sufficient volume of the PBS solution to remove the unreacted proteins. Throughout each experiment, the PBS solution flowed continuously through the reference channel. For the analysis of protein interactions, the accurate signal was calculated by subtracting the reference signal from the sample signal to exclude other interference effects including temperature. The SPR angle shift from the whole experimentation was demonstrated in Fig. 4. The output signal was successively increased after the injection of sample proteins. The quantity of SPR angle shift starting from the injection of sample solution and ending at the saturation of the protein binding was used to estimate the binding amount. The varied output signals were calculated from the mean of the signal during 100 s in the stable phase after sufficient flow of the PBS solution and the error range of the SPR system was 0.001°. The responses of the SPR sensor to the various concentrations of IgG are presented in Fig. 5. The error bars illustrate the standard deviation for the three replicates. The SPR detection signal of each concentration of IgG using parylene-H was 0.02303°, 0.00610°, and 0.00292° respectively. And the output signal from combination of parylene-H and protein G was 0.1017°, 0.0685°, and 0.00514°, respectively. The sensitivities may be determined from the ratio of the angle shift and detected IgG concentration. The mean sensitivity of IgG detection using parylene-H was 0.00319° per μg/ml, and the combination of protein G and parylene-H was 0.01610° per μg/ml which is five times higher than parylene-H.
The process of IgG detection using a combination of protein G and parylene-H. a The SPR sensor chip was fabricated with 50 nm of gold film on 0.1 mm of glass substrate. b The 20 nm thickness of parylene-H film was deposited on the sensor chip surface by CVD process. c The protein G was modified on the parylene-H film with amide bond. d The anti-IgG was immobilized on the combination of protein G and parylene-H layer modified sensor chip surface. e To prevent non-specific bindings, the BSA was modified on the sensor chip surface. f IgG in sample solution binds with the immobilized anti-IgG
The SPR output signal of IgG detection using a parylene-H film and b the combination of parylene-H film and protein G layer. Throughout each experiment, a PBS solution flowed continuously through the reference channel. The accurate signal was calculated by subtracting the reference signal from the sample signal to exclude other interference effects including temperature
The SPR output signal of IgG detection using parylene-H film and a combination of parylene-H film and protein G layer. After sufficient flow of the PBS solution, the varied output signals were calculated from the mean of the signal during 100 s in the stable phase. The responses of the SPR sensor to the various concentrations of IgG are presented. The error bars illustrate the standard deviation for the three replicates. The SPR sensor using combination of parylene-H and protein G as the linkage showed higher increased output signal, indicating that the sensing capability was improved when using protein G on the parylene-H layer
4 Discussion
The high efficient immunoassay using the combination of protein G and parylene-H was proposed and the antigen detection capability using SPR based sensor was compared with previous immobilization method using single parylene-H film. The anti-IgG was immobilized on the each linkage layer and detection of various concentration of IgG was investigated. To verify the protein absorption on the sensor chip surface, the SPR signals from sensor chip surface were measured in real-time. In the anti-IgG immobilization step, the single parylene-H layer consumed more time in signal stabilization than the protein G combined method as shown in Fig. 4a. Almost one-third of the increased signal of anti-IgG was decreased through the PBS washing step and it can be assumed that the non-specifically bound anti-IgG molecules fell away by flow of PBS solution. In contrast, the SPR output signal from anti-IgG binding on the combination of protein G and parylene-H layer was stably increased and quickly settled after interaction as shown in Fig. 4b. The protein G was modified on the parylene-H deposited sensor chip surface, and anti-IgG was immobilized on the protein G by specific interaction. Figure 5 shows and compares the average of IgG detection signals using different linkage layers. Although the similar amount of anti-IgG molecules were immobilized on the each linkage layer (Fig. 4), the SPR sensor system using combination of protein G and parylene-H layer presented the higher sensitivity of the IgG detection. These results clearly show that the IgG sensing capability of anti-IgG array was improved when using the combination of protein G and parylene-H film as linkage layer for immobilization of anti-IgG. The parylene-H has carboxyl group which can bind with amine group of anti-IgG at several region including antigen binding site, thus it can be supposed that the anti-IgG was randomly immobilized on the parylene-H film and specific antigen binding sites of some molecules were blocked. However in the combination of protein G and parylene-H used experimentation, among the three domains of protein G, the beta domain has a specific affinity with Fc domain of anti-IgG which is lower part of antibody. Therefore, it can be assumed that the anti-IgG can immobilized on the protein G layer with specific orientation which has opened upper antigen binding site to the outside from sensor chip surface as aforementioned in Fig. 1b. From these results, the SPR sensor using the combination of parylene-H and protein G as the linkage layer showed the higher IgG detection efficiency.
5 Conclusions
The antigen detection capability of the antibody on the SPR gold chip surface using a combination of protein G and parylene-H film was investigated and compared with single parylene-H used method. The protein G was immobilized on the parylene-H film deposited SPR sensor chip surface and the anti-IgG was immobilized on the protein G layer for detection of IgG. Thus, using the protein G on parylene-H layer, higher density of anti-IgG has higher probability to interact with IgG. Therefore, the present experimental results demonstrated that the proposed SPR sensor systems with sensor chips modified by the combination of protein G and parylene-H established the efficient immobilization of antibody and more sensitive detection of target antigens. In addition, the proposed combination of protein G and the parylene-H film can improve the sensitivity of the immunosensor system which can be used for the various biosensing applications.
References
Amit A et al (1986) Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science 233(4765):747–753
Arlett JL, Myers EB, Roukes ML (2011) Comparative advantages of mechanical biosensors. Nat Nanotechnol 6(4):203–215
Bae YM et al (2005) Study on orientation of immunoglobulin G on protein G layer. Biosens Bioelectron 21(1):103–110
Edelman GM (1973) Antibody structure and molecular immunology. Science 180(4088):830–840
Fan X et al (2008) Sensitive optical biosensors for unlabeled targets: a review. Anal Chim Acta 620(1–2):8–26
Fortin JB, Lu TM (2002) A model for the chemical vapor deposition of poly(para-xylylene) (parylene) thin films. Chem Mater 14(5):1945–1949
Fritz J (2008) Cantilever biosensors. Analyst 133(7):855–863
Hanash SM, Pitteri SJ, Faca VM (2008) Mining the plasma proteome for cancer biomarkers. Nature 452(7187):571–579
Ko H et al (2011) One step immobilization of peptides and proteins by using modified parylene with formyl groups. Biosens Bioelectron 30(1):56–60
Lin P-C, Weinrich D, Waldmann H (2010) Protein biochips: oriented surface immobilization of proteins. Macromol Chem Phys 211(2):136–144
Liu C (2007) Recent developments in polymer MEMS. Adv Mater 19(22):3783–3790
Marco M-P, Barceló D (1996) Environmental applications of analytical biosensors. Meas Sci Technol 7(11):1547
Pluchery O, Vayron R, Van K-M (2011) Laboratory experiments for exploring the surface plasmon resonance. Eur J Phys 32(2):585
Prakash S, Karacor MB, Banerjee S (2009) Surface modification in microsystems and nanosystems. Surf Sci Rep 64(7):233–254
Sauer-Eriksson AE et al (1995) Crystal structure of the C2 fragment of streptococcal protein G in complex with the Fc domain of human IgG. Structure 3(3):265–278
Tan CP, Craighead HG (2010) Surface engineering and patterning using parylene for biological applications. Materials 3(3):1803–1832
Thariani R, Yager P (2008) Novel, high-quality surface plasmon resonance microscopy. Sens Actuators B: Chem 130(2):765–770
Tomizaki K-Y, Usui K, Mihara H (2010) Protein–protein interactions and selection: array-based techniques for screening disease-associated biomarkers in predictive/early diagnosis. FEBS J 277(9):1996–2005
Tsai W-C, Pai P-J (2009) Surface plasmon resonance-based immunosensor with oriented immobilized antibody fragments on a mixed self-assembled monolayer for the determination of staphylococcal enterotoxin B. Microchim Acta 166(1–2):115–122
Tsai HY et al (2007) Detection of C-reactive protein based on immunoassay using antibody-conjugated magnetic nanoparticles. Anal Chem 79(21):8416–8419
Wang J (2006) Electrochemical biosensors: towards point-of-care cancer diagnostics. Biosens Bioelectron 21(10):1887–1892
Weiping Q et al (1999) Orientation of antibodies on a 3-aminopropyltriethoxylsilane-modified silicon wafer surface. J Incl Phenom Macrocycl Chem 35(1–2):419–429
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MEST) [NRF-2011-0014622, A High-throughput Label-free Virus Enumeration and Cryopreservation Technique using Localized Surface Plasmon (LSPR)].
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shin, E.J., Lee, W.G. & Moon, S.J. Characterization of a novel antibody immobilization combining protein G with parylene-H for surface plasmon resonance immunosensors. Microsyst Technol 22, 2093–2099 (2016). https://doi.org/10.1007/s00542-015-2534-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-015-2534-3