Abstract
Remimazolam is a short-acting benzodiazepine that was approved for clinical use in 2020. We report three patients who underwent surgery for cerebral and spinal cord tumors, in whom transcranial electrical stimulation-motor-evoked potential (TES-MEP) was successfully monitored under general anesthesia with remimazolam. During total intravenous anesthesia with propofol at a target concentration of 2.7 − 3.5 µg/mL and 0.1 − 0.35 µg/kg/min of remifentanil, delayed awakening, bradycardia, and hypotension during propofol anesthesia were expected in all three cases. With patient safety as the top priority, we considered changing the anesthetic agent. Propofol was replaced with remimazolam at a loading dose of 12 mg/kg/h for a few seconds (case 3), followed by 1 mg/kg/h for maintenance (cases 1–3). TES-MEP was recorded during propofol and remimazolam administration in all three patients. Amplitudes of TES-MEP during anesthesia with propofol and remimazolam were 461.5 ± 150 µV and 590.5 ± 100.9 µV, 1542 ± 127 µV and 1698 ± 211 µV, and 581.5 ± 91.3 µV and 634 ± 82.7 µV sequentially from Case 1. Our findings suggest that intraoperative TES-MEP could be measured when anesthesia was managed with remimazolam at 1 mg/kg/h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Intraoperative neurophysiological monitoring (IONM) using motor-evoked potentials (MEPs) with transcranial electrical stimulation (TES) or direct cortical stimulation has been widely used in neurosurgery [1,2,3]. Previously, stable intraoperative MEP recording was difficult to achieve [4]. However, the development of pulsed MEP stimulation techniques has led to successful IONM of the corticospinal tract under general anesthesia [1]. Currently, intraoperative total intravenous anesthesia (TIVA) using propofol and opioids is the standard [5, 6]. Remimazolam, a benzodiazepine with an ester bond, was approved for clinical use in Japan in 2020. However, it is currently unclear whether remimazolam affects IONM during anesthetic management in neurosurgery. Therefore, we reported three patients in which propofol was changed to remimazolam during general anesthesia. TES-MEP measurements were recorded during the process and reproducible TES-MEP responses were obtained intraoperatively.
Case presentations
Written informed consent was obtained from all three patients.
General anesthesia
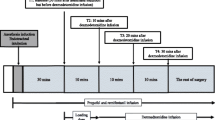
General anesthesia was induced with propofol at a target concentration of 4.0 − 5.0 µg/mL, followed by 0.1 − 0.35 µg/kg/min remifentanil and 0.6 − 0.8 mg/kg rocuronium. After tracheal intubation, anesthesia was maintained with 2.7 − 3.5 µg/mL propofol and 0.1 − 0.35 µg/kg/min remifentanil to maintain the bispectral index (BIS) between 40 and 60. We did not use rocuronium except immediately before intubation. Anesthesia was changed from propofol to remimazolam after each event in each case. As bradycardia and hypotension persisted, remimazolam was changed for prevention. Initially, for the reason described below, only in Case 3, when changing from propofol to remimazolam, 12 mg/kg/h of remimazolam was administered for the first few seconds. After the change, remimazolam (1 mg/kg/h) was administered in all cases. The total dose and duration of anesthesia are shown in Table 1. Circulatory dynamics and BIS before and after the change are shown in Fig. 1. Rocuronium was not used during the operation. Sugammadex was administered to reverse neuromuscular blockade as necessary.
Box plots of (a) heart rate (HR), b mean blood pressure (MBP), and c bispectral index (BIS) before and after the change in three cases in 2 h. Each datum was recorded every 5 min, reported as medians (Interquartile range: IQR, 25th to 75th percentile). The median HR, MBP, and BIS of propofol and remimazolam were 64 bpm (IQR: 57–67.5 bpm) and 68 bpm (IQR: 60.75–69 bpm), 76 mmHg (IQR: 72.5–81 mmHg) and 92 mmHg (IQR: 85.75–95.25 mmHg), and 41 (IQR: 36.25–48.25) and 38.5 (IQR: 30.75–45), respectively
Assessment and measurement of TES-MEP
The central sulcus was identified with a navigation system. Then, corkscrew electrodes were implanted in the skull at C3 and C4 according to the International 10–20 system. TES-MEP was recorded from the abductor pollicis brevis (APB) and abductor hallucis (AH) muscles, using a pair of surface electrodes placed 3 cm apart in each belly and tendon. TES-MEP was derived as a compound muscle action potential (CMAP) after monophasic electrical stimulation with the anode of C3 or C4 on the operation side and the cathode C4 or C3 on the pair side electrodes. The stimulation and recording of TES-MEPs were performed using a Digitimer Multipulse Stimulator (Neuromaster MEE-2000, Nihon Kohden, Tokyo, Japan). The stimulation waveform was a constant current with a rectangular pulse of electrical stimulus intensity (0–200 mA). Other conditions for TES-MEP electrical stimulation were as follows: stimulus duration of 0.3 ms, inter-stimulus interval of 2.0 ms, five train stimulations, band-pass filter of 10–3000 Hz, and a time base of 100 ms [7]. All the above stimulation conditions were fixed before and after the change in anesthetic agents. The stimulus intensity was increased stepwise in 5–10 mA increments, from 50 to 70 mA, to a maximum of 200 mA until the TES-MEP threshold was confirmed. We defined TES-MEPs as 20–30 µV or more based on previous studies [8]. We assumed a stimulus intensity that ensured 50% TES-MEP threshold probability. Amplitudes of TES-MEP were measured during suprathreshold stimulation at ≥ 20% threshold, from peak to peak between consecutive largest peaks at the positive and negative polarities. After recording and measurement, TES-MEP stimulations were left at the interval for at least 60 s, and continuous monitoring was maintained. Data are expressed as mean ± SD.
Case 1
A 66-year-old man complained of dizziness and nausea. He was diagnosed with hemorrhage from a right brainstem cavernous angioma. The patient was followed up. Subsequently, bleeding recurred. Paralysis of the left side of the patient’s body was also developed. Intracranial tumor resection was performed because of the rapid growth of the tumor (Fig. 2a).
Diffusion tensor imaging (DTI) and T2-weighted coronal and axial magnetic resonance imaging (MRI) show a right brainstem cavernous angioma (a). Upper left: expressed DTI and observed tractography of the corticospinal tract (white arrow) located near the cavernous angioma. Upper middle and right: expressed preoperative and postoperative MRI, respectively. Corticospinal tract (white arrow). T2-weighted sagittal MRI shows the preoperative (lower left) and postoperative (lower right) intradural extramedullary tumor at the level of the 7th to 9th thoracic vertebrae (b)
Baseline TES-MEP measurements were recorded. TES-MEP was measured during intracranial tumor removal under a microscope. Since delayed awakening due to prolonged propofol use was expected, the anesthetic agent was changed from propofol to remimazolam. Upon propofol and remimazolam treatment, heart rate (HR) was observed to be 65 and 65 bpm, mean blood pressure (MBP) was 80 and 95 mmHg, and BIS was 45 and 35, respectively. Vasopressors were not required before or after administration of anesthetics, and BIS recovered to 45 in approximately 1 h. Even after prolonged propofol anesthesia, the amplitude of TES-MEP during remimazolam anesthesia was larger than that during propofol anesthesia (Fig. 3a). The TES-MEP amplitudes of propofol and remimazolam were 461.5 ± 150 µV and 590.5 ± 100.9 µV, respectively. This was maintained until the end of surgery. TES-MEP during remimazolam was comprehensively documented. Postoperatively, the patient had mild right peripheral facial nerve palsy and dysarthria. However, his left motor function improved.
Transcranial electrical stimulation-motor-evoked potential (TES-MEP) during anesthesia with propofol, followed by remimazolam. The continuous intravenous infusion of propofol at a target concentration of 2.7 − 3.5 µg/mL was switched to remimazolam in all cases. a Case 1: MEP amplitude was decreased during infusion of propofol, which increased after starting remimazolam at 1 mg/kg/h. b Case 2: MEP amplitude was not changed after changing to remimazolam. c Case 3: MEP amplitude was decreased after infusion of a loading dose of remimazolam 12 mg/kg/h for a few seconds, which increased during continuous infusion of 1 mg/kg/h remimazolam. APB abductor pollicis brevis, Amp amplitude (mean ± SD), Lat latency (mean ± SD), MEP motor-evoked potential, TES transcranial electrical stimulation
Case 2
A 60-year-old man with a history of atypical meningioma, including two recurrences, underwent surgery. Subsequent radiotherapy was performed. Following 60 Gy irradiation, recurrent foci were identified. Since recurrence occurred approximately 2 months after the previous reoperation, further management of the disease with radiotherapy alone was not possible. Therefore, a third intracranial tumor resection was performed. A preoperative examination revealed no motor dysfunction. Initially, the induction and maintenance doses of propofol were the same as those used in Case 1. Ephedrine was used several times to maintain HR and MBP, which were 60 bpm and 75 mmHg, respectively. The lowest HR during propofol infusion was 40 bpm. Due to concerns regarding severe bradycardia during surgery, the anesthesia was changed from propofol to remimazolam during the operative procedures. BIS was 45 but temporarily fell to 30 after the change to remimazolam. HR and MBP after the change were maintained at 60 bpm and 85 mmHg, respectively, with remimazolam when ephedrine was not used. The infusion rate of remifentanil was maintained at 0.14 µg/kg/min with no change. The TES-MEP was measured well, while anesthesia was maintained with remimazolam, and could be used for monitoring motor function during intracranial surgery. The TES-MEP measurements fluctuated within the safe range during tumor removal. The TES-MEP amplitudes of propofol and remimazolam were 1542 ± 127 µV and 1698 ± 211 µV, respectively. No significant changes were observed (Fig. 3b). Immediately after surgery, the patient had mild fine motor deficits in the right upper extremity and aphasia. Complete recovery was achieved over time.
Case 3
A 73-year-old man presented to our hospital complaining of sensory disturbance in the lower extremities and gait disturbance. The MRI scan revealed an intradural extramedullary tumor at the level of the 7th to 9th thoracic vertebrae (Fig. 2b). The patient was followed up. Subsequently, surgery was performed because of bladder and rectal disorders. After confirming the TES-MEP derivation, the lowest HR and MBP during propofol infusion were 46 bpm and 40 mmHg, respectively. Several doses of ephedrine and phenylephrine were administered while maintaining an HR of 60 bpm and MBP of 70 mmHg; however, they were not permitted after the start of the surgery. Hence, the anesthetic agent was changed from propofol to remimazolam. Initially, remimazolam was loaded at 12 mg/kg/h for approximately 10 s before the dose was reduced for maintenance. BIS was 45, but it fell to 20 right after the loading. HR and MBP were maintained at 60 bpm and 80 mmHg without a vasopressor. TES-MEP was recorded at the same maintenance dose as in the other two cases. However, the amplitude decreased from that of the baseline measurement of propofol for approximately 30 min. Thereafter, the amplitude of TES-MEP recovered over time. BIS was 35 at that point. Tumor removal was performed under a microscope. Intraoperatively, the amplitude of the AH decreased to approximately 20% of the baseline. This was determined to be a true positive, because there was no decrease in amplitude from the APB and contralateral AH. Therefore, surgery was terminated after partial resection of the tumor in the area near the pyramidal tract. The TES-MEP APB amplitudes of propofol and remimazolam were 581.5 ± 91.3 µV and 634 ± 82.7 µV, respectively (Fig. 3c). Postoperatively, the patient presented with temporary moderate motor deficits in the lower extremities, while other symptoms remained unchanged.
Discussion
For intraoperative MEPs, propofol causes a lesser decrease in the excitability of the lower motor neurons than volatile anesthetics, but a comparable effect on the GABAA receptors in synaptic models, with suppression of that MEP amplitude at higher doses [9, 10]. Although these shortcomings of propofol are well known, the new intravenous anesthetic agent, remimazolam, has only been approved for clinical use in Japan since 2020. Remimazolam, an ester-based benzodiazepine, is characterized by (1) a high affinity for all subtypes of benzodiazepine GABAA receptors, (2) short-acting sedative, and (3) reversible using flumazenil. Thus, remimazolam is a sedative with high clearance, rapid induction, and prompt awakening from anesthesia [11, 12]. As remimazolam is a novel drug, its influence on IONM is not well understood. Furthermore, the influence of remimazolam on intraoperative MEP has not yet been studied.
In all three cases, TES-MEP was recorded during general anesthesia using remimazolam. This is the first report to measure and compare TES-MEP between propofol and remimazolam in neurological patients. Importantly, we were able to observe changes due to the different anesthetic agents within the same patient; thus, eliminating the risk of error due to TES-MEP variability between patients.
In all three patients, TES-MEP during remimazolam anesthesia was measured and recorded at the time of tumor resection and functioned as well as IONM. In Case 1, the amplitude of TES-MEP during remimazolam was higher than that of propofol, but, which is superior, could not be determined. In addition, more cases are needed to determine how much time affects the suppression of MEPs. In Case 3, a decrease in amplitude was observed, and the postoperative loss of motor function was expected. It has been reported that midazolam, also a benzodiazepine, decreases the amplitude of TES-MEP with increasing injection volume [13]. In our cases, maintenance using remimazolam at 1 mg/kg/h had little effect on TES-MEP. The prolonged use of the drug was tolerated. In Case 3, TES-MEP decreased temporarily after a loading dose of remimazolam (12 mg/kg/h) was administered. This may be because remimazolam regulates TES-MEP in a dose-dependent manner, similar to other intravenous anesthetics [14]. As such, remimazolam may be considered for anesthetic management during TES-MEP measurements, as long as care is taken to avoid overdosing during anesthetics induction and maintenance.
This study has several limitations. First, there were no cases in which the anesthetic agent was changed from remimazolam to propofol. Before clinical application, a crossover study to verify the superiority or inferiority of remimazolam is warranted. Second, we need to clarify the index for the appropriate dose of remimazolam. Propofol can be assessed using BIS; however, it is difficult to adjust the maintenance dose of remimazolam in the same way. Therefore, anesthesiologists might not be able to preset the conditions for an equal depth of anesthesia for each patient.
Conclusion
We reported three patients in which the anesthetic agent was changed from propofol to remimazolam during neurosurgery. We were able to confirm the effects of both anesthetic agents on TES-MEP in the same patient. The results showed that we could stably measure TES-MEP under general anesthesia using remimazolam at 1 mg/kg/h for maintenance. Our findings suggest that TES-MEP can be measured when remimazolam is used and that motor functions can be monitored during surgery.
Change history
30 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00540-022-03146-4
References
Taniguchi M, Cedzich C, Schramm J. Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery. 1993;32:219–26.
Kombos T, Suess O, Ciklatekerlio Ö, Brock M. Monitoring of intraoperative motor evoked potentials to increase the safety of surgery in and around the motor cortex. J Neurosurg. 2001;95:608–14.
Talacchi A, Turazzi S, Locatelli F, Sala F, Beltramello A, Alessandrini F, Manganotti P, Lanteri P, Gambin R, Ganau M, Tramontano V, Santini B, Gerosa M. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. J Neurooncol. 2010;100:417–26.
Rampil IJ, King BS. Volatile anesthetics depress spinal motor neurons. Anesthesiology. 1996;85:129–34.
Kerz T, Hennes HJ, Fève A, Decq P, Filipetti P, Duvaldestin P. Effects of propofol on H-reflex in humans. Anesthesiology. 2001;94:32–7.
Pelosi L, Stevenson M, Hobbs GJ, Jardine A, Webb JK. Intraoperative motor evoked potentials to transcranial electrical stimulation during two anaesthetic regimens. Clin Neurophysiol. 2001;112:1076–87.
MacDonald DB, Skinner S, Shils J, Yingling C. American Society of Neurophysiological Monitoring. Intraoperative motor evoked potential monitoring–A position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013;124:2291–316.
Szelényi A, Senft C, Jardan M, Forster MT, Franz K, Seifert V, Vatter H. Intra-operative subcortical electrical stimulation: a comparison of two methods. Clin Neurophysiol. 2011;122:1470–5.
Kalkman CJ, Drummond JC, Ribberink AA, Patel PM, Sano T, Bickford RG. Effects of propofol, etomidate, midazolam, and fentanyl on motor evoked responses to transcranial electrical or magnetic stimulation in humans. Anesthesiology. 1992;76:502–9.
Walker CT, Kim HJ, Park P, Lenke LG, Weller MA, Smith JS, Nemergut EC, Sciubba DM, Wang MY, Shaffrey C, Deviren V, Mummaneni PV, Chang JM, Mummaneni VP, Than KD, Berjano P, Eastlack RK, Mundis GM Jr, Kanter AS, Okonkwo DO, Shin JH, Lewis JM, Koski T, Hoh DJ, Glassman SD, Vinci SB, Daniels AH, Clavijo CF, Turner JD, McLawhorn M, Uribe JS. Neuroanesthesia guidelines for optimizing transcranial motor evoked potential neuromonitoring during deformity and complex spinal surgery. Spine. 2020;45:911–20.
Wesoowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36:1021–7.
Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60:505–14.
Schönle PW, Isenberg C, Crozier TA, Dressler D, Machetanz J, Conrad B. Changes of transcranially evoked motor responses in man by midazolam, a short acting benzodiazepine. Neurosci Lett. 1989;101:321–4.
Penney R. Use of dexmedetomidine and ketamine infusions during scoliosis repair surgery with somatosensory and motor-evoked potential monitoring: a case report. AANA J. 2010;78:446–50.
Funding
This study did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yamada, S., Akiyama, Y., Tachibana, S. et al. The intraoperative motor-evoked potential when propofol was changed to remimazolam during general anesthesia: a case series. J Anesth 37, 154–159 (2023). https://doi.org/10.1007/s00540-022-03112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-022-03112-0