Abstract
Purpose
Pediatric sedation is commonly required to obtain high-quality images in magnetic resonance imaging (MRI). We performed a systematic review and meta-analysis to assess the effects of dexmedetomidine sedation for MRI in children.
Methods
A systematic review was conducted to find all randomized controlled trials concerning dexmedetomidine sedation for MRI in children. We searched databases using the Ovid platform in the Cochrane Controlled Trials Register, MEDLINE, and EMBASE. This study was registered in the PROSPERO database: CRD42020198368.
Results
Seven studies and 753 participants were included. Dexmedetomidine sedation showed a significantly delayed onset time [weighted mean differences (WMD) = 8.13 min, 95% confidence interval (CI) 4.64 to 11.63, I2 = 98%] and recovery time (WMD = 5.22 min, 95% CI 0.35 to 10.09, I2 = 92%) compared to propofol, ketamine, and midazolam sedation. There was no difference in quality of sedation [risk ratio (RR) = 1.25, 95% CI 0.92 to 1.69, I2 = 89%], or incidence of sedation failure (RR = 1.39, 95% CI 0.53 to 3.66, I2 = 83%) between groups. Although a significantly decreased heart rate (WMD = − 17.34 beats/minute, 95% CI − 22.42 to − 12.26, I2 = 96%) was observed, bradycardia that required treatment was not increased (RR = 8.00, 95% CI 1.02 to 62.64, I2 = 0%). Dexmedetomidine sedation had a lower incidence of desaturation events (RR = 0.42, 95% CI 0.20 to 0.86, I2 = 4%). However, there was no difference in incidence of postoperative vomiting (RR = 0.42, 95% CI 0.15 to 1.17, I2 = 17%) between groups.
Conclusions
Dexmedetomidine sedation provided a similar sedation quality with a reduced incidence of desaturation events. However, the delayed onset and recovery times were drawbacks. The clinical significance of bradycardia is considered to be low. GRADE assessment revealed the quality of the evidence in this meta-analysis ranged from very low to moderate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) is a non-radiative and painless examination that produces detailed visualization of the organs and structures. Although its role is expanding in diagnosis of pediatric diseases, it is difficult for infants and young children to remain still for the duration of the MRI exam due to loud noises, the narrow space, and immobilization [1]. Therefore, sedation or general anesthesia is requested with increasing frequency to obtain qualified images in children, and a recent survey conducted by the pediatric committee of the Society of Cardiovascular Magnetic Resonance showed that 64% of children younger than 8 years underwent general anesthesia for MRI, while 23% underwent sedation [2].
The aim of MRI sedation is not only to obtain high-quality images, but also to achieve maximum patient safety. However, sedation comes with a risk of airway collapse and hemodynamic instability [3]. Furthermore, despite many studies on MRI sedation in children, the most appropriate sedation method has not been established [4].
Among the sedative drugs, dexmedetomidine is a specific and highly selective alpha-2 adrenoreceptor agonist with both analgesic and sedative effects [5, 6]. Although it has the advantage of less respiratory depression, its sympatholytic effects have the risk of dose-dependent bradycardia [7, 8]. The overall effectiveness of dexmedetomidine sedation compared with other sedation methods for MRI in children remains incompletely evaluated [9, 10]. Therefore, we performed this systematic review and meta-analysis of randomized controlled trials on dexmedetomidine sedation compared to other sedation methods to assess the efficacy and safety of dexmedetomidine sedation for MRI in children.
Materials and methods
We used a systematic approach to locate publications that evaluated the efficacy and safety of dexmedetomidine sedation for MRI in children. This systematic review and meta-analysis is performed according to the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Cochrane Review Methods [11]. This study was registrated in the PROSPERO database: CRD42020198368.
Data sources and literature sources
The OVID platform was used for examining the relevant literature. We searched MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE (R) Daily, and Ovid MEDLINE (R) from 1946 to the present (OVID platform); the Cochrane Controlled Trials Register (OVID platform), the Cochrane Database of Systematic Reviews (OVID platform), and EMBASE (from 1974) (OVID platform) from inauguration to July 22, 2020. We additionally searched all the relevant literature in the Web of Science, KoreaMed databases, and Google Scholar. The main keywords were dexmedetomidine, magnetic resonance imaging, MRI, nuclear magnetic resonance, child, and randomized controlled trial. The detailed search strategy for MEDLINE is described in Supplementary Table 11. These search strategies were modified for the EMBASE and the Cochrane Database of Systematic Reviews.
Study selection
Two reviewers (JYK and DWK) independently identified all the studies using predefined selection criteria. A third reviewer (KNK) arbitrated disagreements in primary study selection. Studies were included in this meta-analysis if they fulfilled the following criteria: (1) Literature type: randomized controlled trials in any published international journals in the English language; (2) Subjects: patients aged younger than 20 years undergoing sedation for MRI examination; (3) Interventions: studies evaluating the efficacy and safety of dexmedetomidine sedation; (4) Outcomes: the primary outcomes were sedation onset and recovery times; secondary outcomes were quality of sedation, sedation failure rate, hemodynamic changes during sedation, and adverse effects such as incidence of hypotension, bradycardia, desaturation, nausea, and vomiting. The outcome variables were mean differences or the incidence of events between groups. The exclusion criteria were as follows: (1) patients aged older than 20 years; (2) patients with developmental delay, psychological disorder, cognitive impairment, or severe central nervous system disorder; (3) combination regimen with other sedative agents that might influence the sedation effects.
Data extraction
The data were independently extracted by two reviewers (JYK and DWK) using a specific pre-designed data extraction form. The extracted data were verified by the third reviewer (KNK). The following variables were extracted: (1) number of patients and patient characteristics; (2) details of the sedative method and drug dosage used as the intervention; (3) means and standard deviations of the outcome data or incidence of events; (4) time points at which outcome data were assessed; and (5) incidence of adverse events for each method. If any of these data were not described in studies, we requested the data via email.
Assessment of methodological quality
Assessments of the risk of bias were performed independently by two reviewers (KNK and JYK) using the Cochrane risk-of-bias tool [11]. The methods for generating random sequences, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incompleteness of outcome data, selective reporting of outcomes, and other possible sources of risk of bias were evaluated to assess the quality of randomized controlled studies.
Quality of the evidence
The grade of the outcome evidence was evaluated using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) assessments [11]. Two reviewers (KNK and JYK) independently estimated the quality of each outcome. The five categories of GRADE quality assessment are limitations of design, inconsistency, indirectness, imprecision, and publication bias. A “GRADE summary of findings” table was presented by the GRADE profiler (GRADEpro) and included the following outcomes: (1) sedation onset time; (2) sedation recovery time; (3) quality of sedation; (4) mean arterial pressure; (5) heart rate; (6) incidence of desaturation; and (7) incidence of postoperative vomiting.
Statistical analysis
Continuous data were presented as mean difference and their associated 95% confidence interval (CI). They were analyzed using weighted mean differences (WMD) determined via the generic inverse variance method. Binary outcomes were presented as a risk ratio (RR) with 95% CI. Heterogeneity between studies was evaluated using the χ2 test and the I2 statistic [12]. An I2 statistic > 50% and a χ2 test with a P value < 0.10 were considered to indicate statistical heterogeneity. When significant statistical heterogeneity was detected without small-study effects, we used random-effects models.
We performed a subgroup analysis according to sedation regimens to assess the efficacy and safety of each sedation method. If the number of included studies was greater than 10, we used funnel plots to assess the publication bias of the studies included in this meta-analysis. All statistical analyses were conducted using the Cochrane Collaboration Review Manager software (RevMan version 5.4.).
Results
Identification of studies
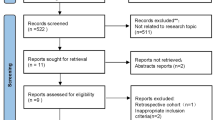
Initial database searches identified 501 publications. After 241 duplicate articles were removed, we additionally excluded 229 publications by screening their titles and abstracts which did not satisfy the selection criteria. For the remaining 31 publications, review of the full manuscripts was performed, and 24 were excluded because of different study designs (13 articles), combination interventions involving other drugs (nine articles), a retracted publication (one article), and inappropriate outcome data (one article). Consequently, seven studies [13,14,15,16,17,18,19] and 753 participants were included in this meta-analysis (Fig. 1).
Study characteristics and patient populations
The included articles were published between 2005 and 2018 in five different countries: USA (1), China (1), India (1), Turkey (2), and Egypt (2). Four studies [13, 14, 16, 18] compared the effects of intravenous dexmedetomidine with those of intravenous propofol. One study compared intravenous dexmedetomidine with ketamine [15], and one study compared intramuscular dexmedetomidine with ketamine [17]. One study compared intravenous dexmedetomidine with intravenous midazolam [19]. Table 1 summarizes the characteristics of the included studies.
Quality of included studies
Although all included studies used a random allocation method, two did not describe in detail the specific random method [13, 16]. Allocation concealment was explained in detail in one study [15], and four studies [16,17,18,19] minutely described their blinding methods. The risk of allocation concealment and blinding was unclear in the other studies. The risks of incomplete outcome data, selective reporting, and other bias in the included studies were low. Supplementary Fig. 1a and b presents the risk-of-bias graph and summaries.
Sedation onset and recovery time
The onset time was defined as the time from administration of sedative to achievement of adequate sedation (Ramsay sedation score 4–6). Our meta-analysis revealed a significantly delayed onset time with dexmedetomidine sedation compared to other sedation methods (WMD = 8.13 min, 95% CI 4.64 to 11.63, P < 0.00001, I2 = 98%) (Fig. 2a). A subgroup analysis comparing dexmedetomidine with propofol revealed a significantly delayed onset time in dexmedetomidine sedation compared to propofol (WMD = 6.05 min, 95% CI 3.13 to 8.97, P < 0.0001, I2 = 92%) and a delay compared to ketamine (WMD = 11.05 min, 95% CI 8.90 to 13.21, P < 0.00001, I2 = 87%) (Fig. 2a).
There was significantly delayed recovery time between dexmedetomidine sedation and other sedation methods (WMD = 5.22 min, 95% CI 0.35 to 10.09, P = 0.04, I2 = 92%) (Fig. 2b). A subgroup analysis comparing dexmedetomidine with propofol revealed a significantly delayed recovery time (WMD = 10.10 min, 95% CI 2.93 to 17.27, P = 0.006, I2 = 79%) (Fig. 2b).
Sedation quality and incidence of sedation failure
Sedation quality was assessed based on the number of high-quality images obtained from MRI scanning or the number of instances of no movement during the MRI scan. There was no difference between dexmedetomidine sedation and other sedation methods in quality of sedation (RR = 1.25, 95% CI 0.92 to 1.69, P = 0.15, I2 = 89%) (Fig. 3a). In subgroup analysis, dexmedetomidine sedation showed a higher quality of sedation compared to midazolam sedation (RR = 4.00, 95% CI 2.11 to 7.58, P < 0.0001) (Fig. 3a). There was no difference in incidence of sedation failure between groups (RR = 1.39, 95% CI 0.53 to 3.66, P = 0.50, I2 = 83%) (Fig. 3b).
Hemodynamic variables
This meta-analysis demonstrated that mean arterial pressure did not differ between dexmedetomidine sedation and other sedation methods (WMD = 2.22 mmHg, 95% CI − 8.71 to 13.15, P = 0.69, I2 = 99%) (Fig. 4a). In terms of heart rate, dexmedetomidine sedation significantly decreased heart rate (WMD = − 17.34 beats/minute, 95% CI − 22.42 to − 12.26, P < 0.00001, I2 = 96%) (Fig. 4b). All subgroup analyses of dexmedetomidine sedation compared with propofol, ketamine, and midazolam demonstrated a significantly decreased heart rate (Fig. 4b).
Incidence of adverse events
The incidence of adverse events of hypotension, bradycardia, desaturation, nausea, and vomiting was evaluated in this meta-analysis. Hypotension and bradycardia were defined as reduction greater than 20% from the baseline level. There was no significant difference in hypotension (RR = 0.83, 95% CI 0.01 to 88.62, P = 0.94, I2 = 81%) or bradycardia (RR = 8.00, 95% CI 1.02 to 62.64, P = 0.05, I2 = 0%) (Supplementary Fig. 2). All included studies reported no instances of clinically significant hemodynamic instability that required intervention. The incidence of desaturation was defined as SpO2 < 90–93%. Dexmedetomidine sedation showed a reduction in desaturation events compared to other sedation methods (RR = 0.42, 95% CI 0.20 to 0.86, P = 0.02, I2 = 4%) (Supplementary Fig. 3A). A subgroup analysis of the comparisons with propofol also showed fewer desaturation events (RR = 0.16, 95% CI 0.03 to 0.88, P = 0.03, I2 = 0%). There was no significant difference in incidence of postoperative vomiting between groups (RR = 0.42, 95% CI 0.15 to 1.17, P = 0.10, I2 = 17%) (Supplementary Fig. 3b). Although patients who received dexmedetomidine sedation experienced a significantly lower incidence of postoperative vomiting compared to ketamine (RR = 0.16, 95% CI 0.03 to 0.86, P = 0.03, I2 = 0%), there was no difference in a subgroup analysis comparing dexmedetomidine with propofol (RR = 1.40, 95% CI 0.28 to 6.99, P = 0.68, I2 = 0%).
Quality of evidence
The grade of outcome evidence was evaluated using the GRADE assessment, and the GRADE summary of findings is presented in Table 2. The overall quality of the evidence in this meta-analysis ranged from very low to moderate. Most studies had problems with inconsistency and imprecision. Because the number of included studies was fewer than 10, publication bias was not evaluated.
Discussion
This meta-analysis demonstrated that dexmedetomidine sedation for MRI in pediatric patients resulted in delayed sedation onset and recovery times and decreased heart rate. There was no difference in sedation quality, incidence of sedation failure, or mean arterial pressure. Dexmedetomidine sedation was also associated with a lower incidence of desaturation events.
There were two previous meta-analyses that compared dexmedetomidine with propofol [9, 10]. These studies included all relevant publications regardless of study design and included one retracted article and retrospective studies. In addition, studies that used inhalation anesthesia with sevoflurane were included. Use of inhalation anesthetic can affect sedation onset and recovery times, hemodynamic changes, and airway obstruction. The use of invasive airway devices such as laryngeal mask airway is considered as general anesthesia [20], and studies that used such devices should be excluded when assessing the effects of dexmedetomidine sedation. All studies in which sedative drugs other than dexmedetomidine were used were excluded to increase the reliability of the current meta-analysis.
The ideal drug characteristics for sedation are rapid onset and recovery. Our meta-analysis revealed a 8.13-min delayed onset with dexmedetomidine sedation. Compared to the rapid onset times of ketamine (1–3 min) [21] and propofol (10–50 s) [22], dexmedetomidine has a relatively slow average onset time (8.6 min) due to the required slow drug injection over 10 min [23]. Considering that rapid onset of sedation is necessary for efficient MRI, delayed onset is a drawback of dexmedetomidine sedation.
Dexmedetomidine, a potential alpha-2 agonist, dose-dependently decreases blood pressure and heart rate 29. In this meta-analysis, children who underwent dexmedetomidine sedation showed a 17.34 beats/minute decrease in heart rate. However, clinically significant bradycardia that required treatment was not observed. Mason et al. reported that dexmedetomidine sedation in pediatric MRI procedures for 747 children lead to 97.6% success in imaging, with bradycardia, which was seen in 16% of the cases, never exceeding a 20% deviation from standard values [23]. Although the risk of bradycardia should always be considered as a predictable physiological response of dexmedetomidine sedation, the decreased heart rate was less clinically relevant and did not usually require treatment [24, 25].
Desaturation event caused by respiratory depression is a common adverse effect during sedation. Interrupting the MRI exam to secure the airway is sometimes required to correct the desaturation, and this inefficiency leads to an increase in cost. Propofol frequently causes dose-dependent respiratory depression, including apnea, hypoventilation, and airway obstruction [26,27,28]. As described earlier, dexmedetomidine sedation, which induces hyperpolarization of norepinephrine receptors in the locus coeruleus, is similar to natural sleep [6, 29]. The locus coeruleus plays a pivotal role in modulation of respiratory controls and sleep [30]. Consequently, dexmedetomidine sedation maintains airway patency and respiratory drive; several studies have demonstrated these advantages [23, 31, 32]. Our meta-analysis also revealed that the incidence of desaturation events was significantly lower with dexmedetomidine sedation.
The incidence of postoperative vomiting was significantly decreased with dexmedetomidine sedation compared with ketamine sedation. However, there were no differences between dexmedetomidine and propofol, which also has antiemetic properties (RR = 1.40, 95% CI 0.28 to 6.99, P = 0.68, I2 = 0%). Binding to the alpha-2 presynaptic inhibitory receptors in the locus coeruleus in the brain decreases noradrenergic activity, and the alpha-2 adrenoreceptor agonist effect of dexmedetomidine results in its antiemetic properties [33, 34]. In addition, since high catecholamine concentrations may induce nausea and vomiting, a decrease of sympathetic tone could explain the antiemetic effect of dexmedetomidine [35].
This meta-analysis has some limitations. First, there was significant heterogeneity among studies. Clinical heterogeneity, such as in sedation drug, and sedative route was identified. Therefore, we performed a subgroup analysis according to sedation regimen. Second, only a relatively small number of patients were included in this meta-analysis. The effects of the intervention can be overestimated in small clinical trials if allocation sequence generation, allocation concealment, and double blinding are not ensured [36]. Third, according to the result of GRADE assessments, the overall quality of evidence in this meta-analysis ranged from very low to moderate owing to problems with inconsistency or imprecision. Lastly, since randomized controlled trials establish their own inclusion and exclusion criteria and have a predefined intervention protocol, the incidence of unusual clinical events such as adverse events can be underestimated. Therefore, although we only included randomized controlled trials in this meta-analysis, caution must be taken when interpreting our results, and well-controlled randomized studies are needed to evaluate the effect of dexmedetomidine sedation for MRI. In addition, additional research is needed to confirm the incidence of complications from dexmedetomidine sedation.
Conclusions
In conclusion, this meta-analysis provided evidence that significantly delayed onset and recovery times were drawbacks of dexmedetomidine sedation. Dexmedetomidine sedation did not differ from other methods in sedation quality, or incidence of sedation failure for MRI exams. Dexmedetomidine sedation was also associated with a lower incidence of desaturation events. Since bradycardia that required treatment was not observed, the clinical significance of lower heart rates following dexmedetomidine sedation is considered to be low.
References
Schulte-Uentrop L, Goepfert MS. Anaesthesia or sedation for MRI in children. Curr Opin Anaesthesiol. 2010;23(4):513–7.
Ahmad R, Hu HH, Krishnamurthy R, Krishnamurthy R. Reducing sedation for pediatric body MRI using accelerated and abbreviated imaging protocols. Pediatr Radiol. 2018;48(1):37–49.
Hallowell LM, Stewart SE, de Amorim ESCT, Ditchfield MR. Reviewing the process of preparing children for MRI. Pediatr Radiol. 2008;38(3):271–9.
Arthurs OJ, Sury M. Anaesthesia or sedation for paediatric MRI: advantages and disadvantages. Curr Opin Anaesthesiol. 2013;26(4):489–94.
Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90(3):699–705.
Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4(5):619–27.
Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesthesia and Analgesia. 2002;95(2):461–6, table of contents.
Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans II hemodynamic changes. Anesthesiology. 1992;77(6):1134–42.
Zhou Q, Shen L, Zhang X, Li J, Tang Y. Dexmedetomidine versus propofol on the sedation of pediatric patients during magnetic resonance imaging (MRI) scanning: a meta-analysis of current studies. Oncotarget. 2017;8(60):102468–73.
Fang H, Yang L, Wang X, Zhu H. Clinical efficacy of dexmedetomidine versus propofol in children undergoing magnetic resonance imaging: a meta-analysis. Int J Clin Exp Med. 2015;8(8):11881–9.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. http://handbook.cochrane.org.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60.
Yang Z, Song G, Hu W, Dang X, Haijuan G, Yu J. Pharmacological analysis of dexmedetomidine hydrochloride in pediatric anesthesia during magnetic resonance imaging. Pakistan J Pharmac Sci. 2018;31(5(Special)):2209–14.
Kamal K, Asthana U, Bansal T, Dureja J, Ahlawat G, Kapoor S. Evaluation of efficacy of dexmedetomidine versus propofol for sedation in children undergoing magnetic resonance imaging. Saudi J Anaesth. 2017;11(2):163–8.
Eldeek AM, Elfawal SM, Allam MG. Sedation in children undergoing magnetic resonance imaging comparative study between dexmedetomidine and ketamine. Egyptian Journal of Anaesthesia. 2016;32(3):263–8.
Wu J, Mahmoud M, Schmitt M, Hossain M, Kurth D. Comparison of propofol and dexmedetomedine techniques in children undergoing magnetic resonance imaging. Paediatr Anaesth. 2014;24(8):813–8.
Tammam TF. Comparison of the efficacy of dexmedetomidine, ketamine, and a mixture of both for pediatric MRI sedation. Egyptian Journal of Anaesthesia. 2013;29(3):241–6.
Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesthesia & Analgesia. 2006;103(1):63–7, table of contents.
Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94(6):821–4.
Campbell K, Torres L, Stayer S. Anesthesia and sedation outside the operating room. Anesthesiol Clin. 2014;32(1):25–43.
Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet (London, England). 2006;367(9512):766–80.
Roback MG, Carlson DW, Babl FE, Kennedy RM. Update on pharmacological management of procedural sedation for children. Curr Opin Anaesthesiol. 2016;29(Suppl 1):S21-35.
Mason KP, Zurakowski D, Zgleszewski SE, Robson CD, Carrier M, Hickey PR, Dinardo JA. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18(5):403–11.
Mason KP, Lönnqvist PA. Bradycardia in perspective-not all reductions in heart rate need immediate intervention. Paediatr Anaesth. 2015;25(1):44–51.
Mason KP, Zgleszewski SE, Prescilla R, Fontaine PJ, Zurakowski D. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr Anaesth. 2008;18(5):393–402.
Srinivasan M, Turmelle M, Depalma LM, Mao J, Carlson DW. Procedural sedation for diagnostic imaging in children by pediatric hospitalists using propofol: analysis of the nature, frequency, and predictors of adverse events and interventions. J Pediatr. 2012;160(5):801-6.e1.
Mallory MD, Baxter AL, Yanosky DJ, Cravero JP. Emergency physician-administered propofol sedation: a report on 25,433 sedations from the pediatric sedation research consortium. Ann Emerg Med. 2011;57(5):462-8.e1.
Cravero JP, Beach ML, Blike GT, Gallagher SM, Hertzog JH. The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg. 2009;108(3):795–804.
Aghajanian GK, VanderMaelen CP. Alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science (New York, NY). 1982;215(4538):1394–6.
Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, Young CC, Wright DR, Macleod DB, Somma J. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101(5):1066–76.
Sriganesh K, Saini J, Theerth K, Venkataramaiah S. Airway dimensions in children with neurological disabilities during dexmedetomidine and propofol sedation for magnetic resonance imaging study. Turkish J Anaesthesiol Reanimat. 2018;46(3):214–21.
Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115(2):171–82.
Whittington RA, Virág L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg. 2006;102(2):448–55.
Jin S, Liang DD, Chen C, Zhang M, Wang J. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: A PRISMA-compliant meta analysis of randomized controlled trials. Medicine. 2017;96(1):e5770.
Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30(12):1537–41.
Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135(11):982–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was not supported by external funding and the authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kim, J.Y., Kim, K.N., Kim, D.W. et al. Effects of dexmedetomidine sedation for magnetic resonance imaging in children: a systematic review and meta-analysis. J Anesth 35, 525–535 (2021). https://doi.org/10.1007/s00540-021-02946-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-02946-4