Abstract
Purpose
Many studies have been published on the beneficial effects of oral carbohydrate solutions (OCS) administered prior to surgery. However, the risk of pulmonary aspiration cannot be excluded in all patients undergoing anesthesia. But, there are few studies on the safety of OCS at lung aspiration.
Methods
Experiments were conducted with mice (Nine- to ten-week-old male BALB/c mice weighted 23–26 g). Lung aspiration was performed by intratracheal administration of OCS and its major constituents, fructose and maltodextrin. Bronchoalveolar lavage fluid (BALF) was collected 3 and 24 h after lung aspiration. The level of Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2) were measured in BALF. The total white blood cell, neutrophil counts, wet to dry ratio and histological examination were performed in BALF and lung tissue, respectively, at 24 h after aspiration.
Results
The OCS increased the level of TNF-α, IL-6 and MIP-2 at 3 h and the neutrophil count at 24 h in BALFs, compared to a phosphate-buffered saline (PBS) group. The increase in IL-6 level induced by OCS was maintained for 24 h. The OCS also increased the number of white blood cells and the percentage of neutrophils in BALFs. Compared to fructose, maltodextrin significantly increased the production of MIP-2 in BALFs. OCS and maltodextrin also increased neutrophil recruitment in lung tissue.
Conclusion
Aspiration of OCS may cause inflammation of the lungs. The preoperative use of OCS may require caution under specific clinical conditions, such as patients at risk of lung aspiration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pre-operative fasting is performed to reduce the incidence of aspiration pneumonia due to reflux of gastric contents during anesthesia [1, 2]. However, this leads to thirst, hunger, and metabolic disturbances such as glucose intolerance, in the fasting patient [3]. Recently, Enhanced Recovery After Surgery (ERAS) programs attempted to reduce insulin resistance through preoperative oral carbohydrate loading [4]. Oral carbohydrate solution (OCS) ingestion prior to surgery can reduce the inconvenience to the patient, enhance postoperative recovery and shorten the hospital stay [5,6,7].
OCS consists of water and nutritional substances, such as carbohydrates and amino acids. The composition and type of nutrients affect the gastric emptying time. For example, the gastric emptying time for OCS containing the only carbohydrate is faster than that of OCS containing amino acids [8, 9]. The concentration and total amount of carbohydrates in OCS also affect gastric emptying [10]. Recent studies showing that complex carbohydrates can improve insulin resistance suggest that OCS containing complex carbohydrates is more suitable for preoperative carbohydrate loading than an OCS containing only simple carbohydrate [11, 12]. Based on these results, the commercialized OCS is a mixture of simple sugar and complex carbohydrate. However, few studies have reported that complex carbohydrates improve postoperative outcomes compared to simple carbohydrates.
Aspiration pneumonia is a serious complication of general anesthesia with a mortality rate of 5% [13]. However, a previous study has reported that OCS administered 4 h before surgery did not develop aspiration pneumonitis [14]. The gastric emptying time is influenced by several factors associated with surgery and anesthesia [15]. Gastric emptying time can be delayed by medical conditions, such as diabetes mellitus and intestinal obstruction, as well as by opioid use. In addition, the stomach may remain full after the recommended fasting time up to 2% in patients despite there was no reason to delay gastric emptying [16]. These results suggest that there is a possibility that the OCS administered before surgery may cause lung aspiration in specific clinical conditions. However, there were few studies on the safety of the OCS aspirated to the lung. Therefore, we investigated the effect of the constituents of OCS on pulmonary inflammation following aspiration into the lungs.
Methods
All animal experiments were approved with the institutional Animal Care and Use Committee (CNU IACUC-H-2017-14) protocols. Nine to ten-week old male BALB/c mice (23–26 g) were purchased from Samtako Science (Daejeon, Korea). The mice were housed in a temperature-controlled facility (22 ± 2 °C) and maintained under a 12/12 h light/dark cycle with ad libitum access to food and water for at least 3 days.
Materials
A commercially available OCS (12.8 g/100 mL maltodextrin, 2 g/100 mL fructose, No-NPO®; Daesang Wellife, Seoul, South Korea) was used. Maltodextrin and fructose were purchased from Sigma Aldrich (St. Louis, MO).
Experiment protocol
The first experiment was conducted to investigate if OCS aspirated to the lungs causes inflammation. Mice were randomly allocated to one of three groups: sham (tracheal exposure only), phosphate-buffered saline (PBS; PBS aspiration) and OCS (OCS aspiration). The mice are anesthetized by intraperitoneal of ketamine (80 mg/kg) and dexmedetomidine (0.5 mg/kg). The depth of anesthesia was confirmed by pinching the paw using tweezers. The mice were fixed in the supine position on a heating pad maintained at 38 °C. The trachea was exposed by performing a sterile surgical procedure. The exposed trachea was slowly injected with 80 μL of PBS or OCS using a 1 mL subcutaneous syringe with a 31- gauge 5/16-inch needle. Next, the skin was sutured, the mice were returned to their cages until awakening. The second experiment was conducted to determine which components of OCS is the main inflammatory inducer. OCS consists mainly of simple carbohydrates such as fructose and complex carbohydrate such as maltodextrin [17]. Mice were randomly allocated (n = 6 mice per group) to fructose group and maltodextrin group. Fructose and maltodextrin solutions were prepared at the same concentration as per the OCS. In the third experiment, aspirated solutions were calibrated to have a pH 1.8 similar to that of gastric juice, like the clinical situation. The pH of the solutions before titration was OCS = 4.67, PBS = 7.28, maltodextrin 6.68, and fructose = 7.26, respectively. 80 μL of each experimental solution calibrated to a pH of 1.8 ± 0.05 using hydrochloric acid was aspirated into mice (n = 6 per group). The pH was measured using a pH meter (FiveEasy™ F20-Std-Kit, Mettler Toledo).
Harvest of bronchoalveolar lavage
Bronchoalveolar lavage fluids (BALFs) were sampled 3 or 24 h after thracheal injection. Next, 1 mL of PBS (pH 7.2) were instilled and gently aspirated three times. The fluid recovery rate was > 80%. Total cell counts in the BALFs were measured with a hemocytometer after red blood cell lysis. Next, BALFs were stained with Wright–Giemsa to determine the percentage of neutrophil.
Enzyme-linked immunosorbent assay of cytokines
The BALF was centrifuged at 1000 ×g for 10 min (CF-RD, Sakura, Tokyo, Japan) at 4 °C to remove the cells. The cell-free supernatant was stored at − 80 °C for measurement of the tumor necrosis factor-α (DY410), interleukin-6 (DY406), and macrophage inflammatory protein-2 (DY452) levels by enzyme-linked immunosorbent assay (ELISA), using DuoSet kits obtained from R&D Systems (Minneapolis, MN, USA) according to the manufacturer’s instructions. The optical density of each sample were determined using a microplate reader (Tecan™ Infinite M200) set to 450 nm. Each sample were analyzed in duplicate and the average values were used.
Harvest of lung tissue
Mice were sacrificed 24 h after injection and the lungs were fixed with formalin (5%) at a pressure of 20 cm H2O by tracheal administration. The left lung of each mouse was embedded in paraffin, sectioned to a thickness of 5 μm, and stained with hematoxylin and eosin (H&E).
Lung injury histology score
The lung injury scores were calculated by a pathologist blinded to the experimental design. The histologic score of each slide was measured using the lung injury scoring system recommended by the American Thoracic Society [18].
Wet to Dry lung weight ratio
Pulmonary edema due to the aspirated solution was confirmed using the wet and dry ratio of the lung tissue. After collecting the lung tissue on both sides, the blood on the surface was removed and the weight was measured immediately. The lung tissue was dried in a 60 °C oven for 7 days and the dry weight was measured. The Wet to Dry ratio was calculated using the wet and dry weights.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). The Kolmogorov–Smirnov test was used to determine whether the data were normally distributed. Statistical significance was evaluated by Student’s t-test and one-way analysis of variance (ANOVA) with Bonferoni’s post hoc test, for comparison of two and more than two groups. The Kruskal–Wallis H-test and Bonferroni-corrected Mann–Whitney U tests were used. A value of P < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS software (version 20.0.0; IBM Corp, Armonk, NY).
Results
OCS increases the inflammatory response when aspirated into the lungs
We determined whether OCS could affect the expression of an inflammatory mediator such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and macrophage inflammatory protein-2 (MIP-2) following aspiration into the lungs. OCS increased level of TNF-α, IL-6 and MIP-2 in BALFs 3 h after aspiration into the lung when compared to PBS group (Fig. 1a–c). The increase in the IL-6 level was maintained even after 24 h in the OCS group (Fig. 1d–f). Because the accumulation of neutrophils in the alveolar space and lung interstitium is prominent after 24 h administration lipopolysaccharide into the lungs [19, 20], we investigated the effect of OCS on neutrophil recruitment to the alveolar space 24 h after its administration. The administration of OCS into the lungs resulted in significant increases in the total number of cells and the percentage of neutrophils in BALFs, compared to the sham or PBS group (Fig. 2).
Effect of oral carbohydrate solution (OCS) on the bronchoalveolar lavage fluid (BALF) levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and macrophage inflammatory protein-2 (MIP-2) at 3 h (a, b, c) and 24 h (d, e, f) after administration into the lung. PBS: phosphate-buffered saline. Data are mean ± SEM (n = 6). *P < 0.05
Maltodextrin is responsible for neutrophil recruitment to the lung
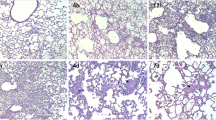
Because OCS led to significant neutrophil infiltration in the alveolar space (Fig. 2), we determined which components of OCS induced significant infiltration of the alveolar wall and lung parenchyma. There was no significant difference between the fructose and maltodextrin in the level of TNF- α and IL-6 in BALFs 3 h after administration to lung (Fig. 3a-b). However, maltodextrin significantly increased the level of MIP-2 in BALFs 3 h after administration to the lung, compared to fructose (Fig. 3c). But, there was no statistical difference in the level of TNF-α, IL-6 and MIP-2 in BALFs between the two groups 24 h after lung aspiration (Fig. 3d, e). Light microscopy showed greater infiltration of the neutrophils in the alveolar wall and pulmonary interstitium in the OCS and maltodextrin group compared to PBS group. The lung injury score was also higher in the OCS and maltodextrin group compared to PBS group (Fig. 4).
Microscopic findings and Lung injury scores. Histologic examination (H-E staining, original magnification × 200) of the lung was performed 24 h after administration of PBS, OCS, fructose, and maltodextrin into the lung. a PBS, b OCS, c fructose, d maltodextrin (Scale bar = 50 μM.). Data are mean ± SEM (n = 6). *P < 0.05
OCS also plays a major role in increasing the inflammatory responses when aspirated with hydrochloric acid
PBS or OCS combined with hydrochloric acid to a pH 1.8 similar to the upper range of typical gastric contents were injected into the lungs in mice. Even when the pH of PBS or OCS were calibrated similarly to that of the gastric content, OCS increased level of TNF-α, IL-6 and MIP-2 in BALFs 3 h after aspiration into the lung when compared to PBS group (Fig. 5a–c). In addition, wet to dry lung weight ratio also increased in the OCS group compared to the PBS group (Fig. 5d). Next, maltodextrin, whose pH was calibrated to be similar to gastric acid, significantly increased the level of MIP-2 in BALFs 3 h after administration to the lung, compared to the pH-corrected fructose (Fig. 5g). But, there was no statistical difference in the level of TNF-α and IL-6 in BALFs (Fig. 5e, f), consistent with the above findings (Fig. 3a–c).
Effect of OCS with hydrochloric acid (a, b, c, d), fructose (FRU) and maltodextrin (MTD) with hydrochloric acid (e, f, g) on the levels of TNF-α, IL-6, and MIP-2 in bronchoalveolar lavage fluid (BALF) at 3 h after and wet to dry lung weight ratio (Wet/Dry Ratio) at 24 h after administration into the lung. Data are mean ± SEM (n = 6). *P < 0.05
Discussion
In the present study, we found that OCS increased the level of inflammatory mediators, such as TNF-α, IL-6 and MIP-2 in BALF and the severity of lung injury following aspiration to the lungs. In addition, OCS further increased the production of inflammatory mediators in BALF even when administered with acid to the lungs, and exacerbated acid aspiration-induced lung injury, as evidenced by the increase in the wet/dry weight lung ratio. Although there are no reports of aspiration pneumonia due to preoperative OCS administration, the result of the present study shows that OCS may cause inflammation when aspirated to the lungs [1, 21]. Aspiration pneumonia may be thought to occur only when the stomach is full, but it also occurs when there are small contents in the stomach. Stimulation like airway manipulation during light anesthesia can induce active vomiting or gastro-esophageal reflux episodes independent of the volume of gastric contents [22, 23]. Micro-aspiration may occur even in patients with endotracheal intubation [24, 25]. Therefore, in patients undergoing general anesthesia, the possibility of occurrence of aspiration into the lungs should not be excluded.
Carbohydrate loading aims to reduce insulin resistance induced by metabolic stress arising from fasting and surgery. In the early days of introducing the concept of carbohydrate loading to reduce insulin resistance, glucose and insulin were injected intravenously. Recently, however, this has been superseded by oral carbohydrate administration, which is a more physiological method [26]. Early OCS used only glucose as a carbohydrate. But, recently introduced OCSs comprise not only simple sugars, such as fructose, but also complex carbohydrates, such as maltodextrin [27, 28]. Maltodextrin can be enzymatically derived from any source of starch, including potato and corn [29]. The advantage of maltodextrin is that it is more soluble than starch, has a lower osmotic pressure, and is rapidly hydrolyzed in the intestines [17]. Glucose polymers such as maltodextrin stimulate water absorption in patients with short bowel syndrome, and in patients suffering from diarrhea [30]. The ratio of fructose to maltodextrin affects stomach fullness, abdominal cramping, and nausea [31].
In the present study, OCS increased the levels of inflammatory mediators, such as TNF-α, IL-6 and MIP-2, and the numbers of white blood cells and neutrophils in BALF. These results suggest that OCS, when aspirated into the lungs, exacerbates the inflammatory response in patients at high risk of lung aspiration. Interestingly, maltodextrin, a component of OCS, significantly increased the level of MIP-2 in BALFs and neutrophils infiltration in the alveolar wall and pulmonary interstitium compared to fructose. However, there was no significant difference in the level of TNF-α or IL-6 between the two groups. Neutrophils are recruited to sites of inflammation and play an important role in lung injury [32]. CXC chemokines, such as IL-8 and MIP-2 (murine IL-8 homologues), play an important role in neutrophil recruitment to sites of inflammation [33]. TNF-α also promotes neutrophil recruitment to sites of inflammation through interaction with MIP-2 [34]. These results showing that maltodextrin increases TNF-α and MIP-2 simultaneously suggest that maltodextrin is responsible for the recruitment of neutrophils into the alveoli by OCS, as well as subsequent histological changes.
Preoperative fasting is essential to prevent aspiration of gastric contents during induction and recovery of general anesthesia. However, prolonged fasting is uncomfortable for patients [35], and together with surgical stress, can lead to protein catabolism and insulin resistance [3, 26]. The metabolic changes induced by preoperative fasting worsen the prognosis in terms of the length of hospital stay and postoperative infection [36]. Recent NPO guidelines have changed such that preoperative clear fluid intake even 2 h prior to surgery is considered safe with no pulmonary aspiration [1, 21]. However, finding that maltodextrin may cause lung injury suggest that attention should be paid to the use of OCS in patients whose gastric emptying time may be prolonged, such as diabetes or morbid obesity, and that the use of OCS containing maltodextrin may be avoided, especially in patients at risk of lung aspiration.
This study has the following limitations. First, we found that oral carbohydrate solution causes inflammation when aspirated into the lungs, but we could not disclose the detail mechanism. Second, since this study was conducted on mice, it is limited to apply to humans. In addition, it is different from clinical situations by inducing aspiration by injecting it directly into the lung. Third, it is known that the presence or absence of particles as well as the pH and volume of the gastric contents are critical factors affecting the severity of aspiration pneumonia. However, the effect of particles in this experiment has not been studied.
In conclusion, oral carbohydrate loading solutions cause an inflammatory response when aspirated into the lungs. Therefore, oral carbohydrate loading solutions, especially those containing maltodextrin, should be used with caution for patients at potential risk of lung aspiration.
References
Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Soreide E, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28(8):556–69.
Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114(3):495–511.
Schricker T, Lattermann R. Perioperative catabolism. Can J Anaesth. 2015;62:182–93.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–8.
Hausel J, Nygren J, Thorell A, Lagerkranser M, Ljungqvist O. Randomized clinical trial of the effects of oral preoperative carbohydrates on postoperative nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2005;92:415–21.
Pogatschnik C, Steiger E. Review of preoperative carbohydrate loading. Nutr Clin Pract. 2015;30:660–4.
Hausel J, Nygren J, Lagerkranser M, Hellstrom PM, Hammarqvist F, Almstrom C, et al. A carbohydrate-rich drink reduces preoperative discomfort in elective surgery patients. Anesth Analg. 2001;93(5):1344–50.
Lobo DN, Hendry PO, Rodrigues G, Marciani L, Totman JJ, Wright JW, et al. Gastric emptying of three liquid oral preoperative metabolic preconditioning regimens measured by magnetic resonance imaging in healthy adult volunteers: a randomised double-blind, crossover study. Clin Nutr. 2009;28(6):636–41.
Nakamura M, Uchida K, Akahane M, Watanabe Y, Ohtomo K, Yamada Y. The effects on gastric emptying and carbohydrate loading of an oral nutritional supplement and an oral rehydration solution: a crossover study with magnetic resonance imaging. Anesth Analg. 2014;118(6):1268–73.
Vist GE, Maughan RJ. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J Physiol. 1995;486:523–31.
Crapo PA, Reaven G, Olefsky J. Plasma glucose and insulin responses to orally administered simple and complex carbohydrates. Diabetes. 1976;25:741–7.
Kielhorn BA, Senagore AJ, Asgeirsson T. The benefits of a low dose complex carbohydrate/citrulline electrolyte solution for preoperative carbohydrate loading: focus on glycemic variability. Am J Surg. 2018;215:373–6.
Shime N, Ono A, Chihara E, Tanaka Y. [Current status of pulmonary aspiration associated with general anesthesia: a nationwide survey in Japan] (in Japanese with English abstract). Masui (Jpn J Anesthesiol). 2005;54:1177–85.
Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. 2014(8):Cd009161.
Asai T. Editorial II: who is at increased risk of pulmonary aspiration? Br J Anaesth. 2004;93:497–500.
Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patientsdagger. Br J Anaesth. 2017;118:363–71.
Zadák Z, Kent-Smith L. Basics in clinical nutrition: commercially prepared formulas. E Spen Eur E J Clin Nutr Metab. 2009;4:e212–5.
Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–38.
Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli GF. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther. 2000;13:61–9.
Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res. 2007;33:375–84.
Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration: Application to Healthy Patients Undergoing Elective Procedures: An Updated Report by the American Society of Anesthesiologists Task Force on Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Anesthesiology. 2017;126(3):376–93.
Bannister WK, Sattilaro AJ. Vomiting and aspiration during anesthesia. Anesthesiology. 1962;23:251–64.
Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62.
Blitt CD, Gutman HL, Cohen DD, Weisman H, Dillon JB. “Silent” regurgitation and aspiration during general anesthesia. Anesth Analg. 1970;49:707–13.
Muscedere J, Dodek P, Keenan S, Fowler R, Cook D, Heyland D. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23:126–37.
Nygren J. The metabolic effects of fasting and surgery. Best Pract Res Clin Anaesthesiol. 2006;20:429–38.
Ljungqvist O, Nygren J, Hausel J, Thorell A. Preoperative nutrition therapy—novel developments. Näringsforskning. 2000;44:3–7.
Thiele RH, Raghunathan K, Brudney CS, Lobo DN, Martin D, Senagore A, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond). 2016;5:24.
Hofman DL, van Buul VJ, Brouns FJ. Nutrition, health, and regulatory aspects of digestible maltodextrins. Crit Rev Food Sci Nutr. 2016;56:2091–100.
Sandhu BK, Jones BJ, Brook CG, Silk DB. Oral rehydration in acute infantile diarrhoea with a glucose-polymer electrolyte solution. Arch Dis Child. 1982;57:152–4.
O’Brien WJ, Rowlands DS. Fructose-maltodextrin ratio in a carbohydrate-electrolyte solution differentially affects exogenous carbohydrate oxidation rate, gut comfort, and performance. Am J Physiol Gastrointest Liver Physiol. 2011;300:G181–9.
Salgado RM, Cruz-Castañeda O, Elizondo-Vázquez F, Pat L, De la Garza A, Cano-Colín S, et al. Maltodextrin/ascorbic acid stimulates wound closure by increasing collagen turnover and TGF-β1 expression in vitro and changing the stage of inflammation from chronic to acute in vivo. J TissueViabil. 2017;26(2):131–7.
Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–16.
McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–35.
Tosun B, Yava A, Açıkel C. Evaluating the effects of preoperative fasting and fluid limitation. Int J Nurs Pract. 2015;21:156–65.
Bilku DK, Dennison AR, Hall TC, Metcalfe MS, Garcea G. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15–22.
Acknowledgments
This study was supported by a grant (Number: CRI 16012-1) from Chonnam National University Hospital Research Institute of Clinical Medicine.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kim, J., Kim, HS., Kim, M. et al. Oral carbohydrate solution cause an inflammatory response when aspirated into the lungs in mice. J Anesth 35, 86–92 (2021). https://doi.org/10.1007/s00540-020-02873-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02873-w