Abstract
Purpose
A few randomized controlled trials (RCTs) have compared crystalloid-based goal-directed fluid therapy (GDFT) with starch-based GDFT in patients undergoing major surgical procedures with conflicting results. In this meta-analysis, colloid-based GDFT was compared with crystalloid-based GDFT.
Methods
In this meta-analysis, RCTs comparing colloid- and crystalloid-based GDFT in patients undergoing non-cardiac surgery were included. Binary outcomes were reported as risk ratio (RR) and continuous outcomes were reported as mean difference (MD) with 95% confidence interval (95% CI). PubMed, PubMed central, The Cochrane Library database and EMBASE were searched for potentially eligible trials from inception to 28 February 2020.
Results
Data of 2392 patients from nine RCTs were included in this meta-analysis. Mortality at the longest available follow-up [RR (95% CI) 1.44 (0.88, 2.34); p = 0.15], postoperative kidney dysfunction [RR (95% CI) 1.07 (0.72, 1.60); p = 0.73], postoperative length of hospital stay [MD (95% CI) – 0.29 ( – 1.25, 0.66) d; p = 0.55], cardiovascular complications [RR (95% CI) 1.20 (0.50, 2.88); p = 0.68], wound complications [RR (95% CI) 1.08 (0.76, 1.54); p = 0.66], pulmonary complications [RR (95% CI) 0.90 (0.71, 1.140); p = 0.40] and bleeding [RR (95% CI) 1.24 (0.77, 1.99); p = 0.37] were similar in both the groups. Postoperative major complications were also similar between patients who received colloid and crystalloid [RR (95% CI) 0.79 (0.48, 1.29); p = 0.34].
Conclusion
Colloids in goal-directed fluid therapy protocol does not offer any benefit over crystalloid-based goal-directed fluid therapy protocol in patients undergoing major non-cardiac surgical procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perioperative fluid therapy in patients undergoing major surgical procedure is linked with postoperative clinical outcome [1]. Both the amount and type of fluid administered in the perioperative period contribute to the postoperative outcome. ‘Goal-directed fluid therapy (GDFT)’ is considered to reduce postoperative mortality and morbidity in various clinical scenarios [2]. Most commonly, GDFT is achieved by optimization of stroke volume by intravenous fluid administration. A prior meta-analysis has demonstrated that GDFT reduces postoperative abdominal complications, with no demonstrable effect on mortality or length of hospital stay [3]. The majority of the randomized controlled trials (RCT) used intermittent synthetic colloid boluses such as starch or gelatin, for goal-directed management. Recently colloids, especially synthetic starches, have been challenged in critically ill patients as they offer no benefit over crystalloid, and possibly increase the need for renal replacement therapy and blood transfusion [4, 5]. Several researches were conducted on perioperative use of hydroxyethyl starch, the most commonly used synthetic colloid. A systematic review and meta-analysis of 19 RCTs consisting of more than 1500 patients failed to demonstrate any effect of starch on postoperative kidney dysfunction. It also failed to demonstrate any benefit of colloid over crystalloid [6]. Moreover, use of starch was associated with kidney dysfunction both in critically ill patients and cardiac surgical patients which needs serious attention [7, 8].
Recently, a number of RCTs have compared crystalloid-based GDFT with starch-based GDFT in patients undergoing major surgical procedures, with a few RCTs showing benefit of starch over crystalloid, and the rest showing no benefit [8,9,10,11,12,13,14,15]. However, the majority of the RCTs had small samples. Hence, this systematic review and meta-analysis of RCTs was planned to identify whether colloid-based GDFT was superior to crystalloid-based GDFT.
Method
The PRISMA guidelines were followed for conducting and reporting of this meta-analysis and systematic review [16]. The protocol of this meta-analysis was registered in PROSPERO (CRD42019131745).
Eligibility criteria
Published RCTs comparing colloid-based GDFT with crystalloid-based GDFT regimen in adult patients undergoing non-cardiac surgeries were included in this meta-analysis. RCTs, which used any synthetic colloid such as starch, gelatin or dextran as intravenous fluid boluses for goal-directed therapy, were included in this meta-analysis. Any validated method of intravascular volume optimization such as measurement of cardiac output and/ or cardiac index, stroke volume variation, pulse pressure variation, and corrected flow time were considered as ‘GDFT’. RCTs that reported at least one postoperative complication were included in this meta-analysis.
Exclusion criteria
RCTs in patients undergoing cardiac surgery were not included in this meta-analysis.
Information sources
PubMed, PubMed central, The Cochrane Library database and EMBASE were searched for potentially eligible trials from inception to 28 February 2020. No language restriction was applied in the search strategy. We also manually searched references of the previously published relevant meta-analyses.
Search strategy
The following keywords were used to search the databases: “randomized controlled trial, randomized clinical trial, colloid, crystalloid, goal directed therapy, goal directed fluid therapy”. Details of PubMed/ PubMed Central, The Cochrane Library database and EMBASE search strategy have been provided in appendix 1.
Study selection
Title and abstracts of the possibly eligible trials were independently searched by two authors (AT and SM). Then, full texts of the potentially eligible trials were retrieved and assessed for inclusion in this meta-analysis. Any disagreement between the two review authors were discussed and solved in consultation with the third review author (SB).
Data collection process
Required data from the eligible RCTs were extracted by two independent authors (AT and SM) from the included trials and all data were initially tabulated in a Microsoft Excel™ (Microsoft Corp., Redmond, WA) data sheet. All data were cross-checked by the third review author (SB).
Data items
The following data were retrieved from the full text: first author, year of publication, country where work was done, sample size, inclusion criteria of the patients, hemodynamic optimization target (e.g. stroke volume, pulse pressure variation, cardiac index, corrected flow time etc.), details of colloid or crystalloid administered, blood loss, postoperative outcome (major complications, postoperative organ dysfunction, postoperative intensive care unit (ICU) admission, postoperative hospital and ICU length of stay and mortality at the longest reported follow-up).
Risk of bias in individual studies
The methodological quality of the included RCTs were assessed by two independent authors (SM and SB). The following methodological questions were searched from the studies as per the Cochrane methodology (yes, no or uncertain): method of randomization, allocation concealment, blinding of the participants and personnel, blinding of outcome assessment, incomplete data reporting, selective reporting and any other bias [17].
Summary measures and synthesis of results
Predefined primary outcome of this meta-analysis was ‘number of patients with at least one postoperative complication’. Predefined secondary outcomes were ‘mortality at longest available follow-up’, incidence of acute kidney injury (AKI), length of hospital stay, any reported organ specific complications (cardiovascular complications, wound complications, pulmonary complications and bleeding) and incidence of major postoperative complications (as defined by the trial authors).
For a continuous variable, the mean and standard deviation (SD) values were extracted from both arms of the trial, a mean difference (MD) was computed at the study level, and a weighted mean difference was computed to pool the results across all RCTs. If the values were reported as median and an inter-quartile range or total range of values, the mean value was estimated from a previously described method [18]. The risk ratio (RR) for each trial and pooled RR using the inverse variance method were calculated for binary variables. All statistical variables were calculated with 95% confidence interval (95% CI). The Q-test was used to analyze the heterogeneity of trials. Considering the possible heterogeneity due to study design and patients’ population, we used a random effect model for all pooled analysis. Pooled analysis was conducted in RevMan software (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Publication bias was tested by Egger’s regression test. A meta-regression was also planned to assess the effects of sample size, baseline risk of events in control group patients and year of publication on postoperative outcome in case more than ten trials are included. Missing outcome data were estimated by mice package in R (R Development Core Team, 2010; R Foundation for Statistical Computing, Vienna, Austria) by predictive mean matching. Each outcome was assessed by GRADE (Grading of Recommendations, Assessment, Development and Evaluations) methodology which considered risk of bias, imprecision, inconsistency, indirectness and publication bias to determine the ‘quality of evidences’ [19].
Results
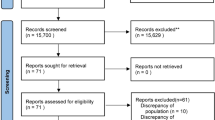
Initial database searching revealed 8564 articles and after duplicate removal and screening n = 319 relevant articles were assessed for inclusion in this meta-analysis. Searching of the other sources revealed 18 other articles. Finally, the data of 2392 patients from nine RCTs were included in this meta-analysis [9,10,11,12,13,14,15, 20, 21]. A PRISMA flow diagram showing the process of study selection has been depicted in Fig. 1. One RCT was not included because it included no outcome that could be pooled from it [22]. Characteristics of the included trials including the amount of study fluid received in each group at individual trial have been reported in Table 1. The review authors’ judgment about each risk of bias in the individual trail has been depicted in Fig. 2.
Our original plan was to analyze the ‘number of patients with at least one postoperative complication’ as primary outcome. However, as no study explicitly reported this outcome, the rest of the prespecified additional outcomes were analyzed. Eight of the included trials reported postoperative mortality, and mortality at longest available follow-up was found to be similar in both the groups [RR (95% CI) 1.44 (0.88, 2.34); p = 0.15, I2 = 0.0%; n = 2322; quality of evidence: low]. Standard deviations of length of hospital stay were not reported for two trials [11, 13] and those were estimated by multiple imputation with predictive mean matching. Postoperative kidney dysfunction was also similar in both the groups [RR (95% CI) 1.07 (0.72, 1.60); p = 0.73; I2 = 29%; quality of evidence: low]. Postoperative hospital length of stay was also similar in the two groups [MD (95% CI) – 0.29 ( – 1.25, 0.66) day; p = 0.55, I2 = 82%; n = 2392, quality of evidence: moderate]. Forest plots showing RR and MD of mortality and length of hospital stay at study level and pooled analysis level have been depicted in Fig. 3. The incidence of postoperative cardiovascular complications was similar between patients who received colloid and crystalloid [RR (95% CI) 1.20 (0.50, 2.88); p = 0.68; I2 = 56%; quality of evidence: very low]. Postoperative wound complication rate [RR (95% CI) 1.08 (0.76, 1.54); p = 0.66; I2 = 1%; quality of evidence: very low], pulmonary complications [RR (95% CI) 0.90 (0.71, 1.14); p = 0.40; I2 = 47%; quality of evidence: low] and bleeding [RR (95% CI) 1.24 (0.77, 1.99); p = 0.37; I2 = 7%; quality of evidence: very low] were also similar between both the groups. Four RCTs reported postoperative major complications and it was found to be similar between patients who received colloid and crystalloid [RR (95% CI) 0.79 (0.48, 1.29); p = 0.34; I2 = 62%; quality of evidence: very low]. The summary of findings as per GRADE methodology has been provided in Fig. 4. Quality of evidences for ‘length of hospital stay’, downgraded because of significant heterogeneity, and ‘postoperative kidney dysfunction’, downgraded because of different definitions of ‘kidney dysfunction’, were used in the different trials (Table 2).
A sensitivity analysis was performed excluding the study of Futier et al. [21], as 0.9% saline was used as opposed to balanced salt solution and fluid therapy protocol was used in the postoperative period. However, mortality at longest follow-up (p = 0.53), length of hospital stay (p = 0.76) and postoperative kidney dysfunction (p = 0.45) remained similar in both the groups. Another sensitivity analysis was performed excluding the studies by Lindroos et al. [20] and Tyagi et al. [11], as these RCTs were conducted in a non-abdominal surgical setting. Mortality at the longest follow-up (p = 0.15), length of hospital stay (p = 0.45) and postoperative kidney dysfunction (p = 0.71) remained similar in both the groups even in this sensitivity analysis. Lastly, we did another sensitivity analysis excluding the study of Yates et al. [13] as it included only severe kidney dysfunction requiring dialysis; we found that postoperative kidney dysfunction was similar (p = 0.70).
Discussion
We found no benefit of colloid-based GDFT over crystalloid-based goal-directed fluid therapy in terms of postoperative mortality, length of hospital stay or any other organ-specific complications. The incidence of postoperative kidney dysfunction was also similar in both the groups.
Goal-directed fluid therapy in major non-cardiac surgery is a long-debated matter in perioperative medicine. A meta-analysis of 41 RCTs reported that colloid-based goal-directed fluid therapy was associated with less postoperative wound infection, abdominal complications and hypotension over conventional fluid therapy [3]. However, no mortality benefit was obtained in that meta-analysis. Another subsequent meta-analysis of 95 RCTs, including both cardiac and non-cardiac surgical patients, reported that GDFT was associated with significant mortality benefit over conventional fluid therapy [22].
Although colloid-based GDFT was compared with conventional fluid therapy in a number of RCTs, only a few authors compared crystalloid boluses for GDFT with colloid-based GDFT, generating conflicting results. Kabon et al. reported that postoperative complications including kidney dysfunction were similar between patients undergoing moderate- to high-risk abdominal surgery, in patients receiving colloid or crystalloid within a goal-directed fluid therapy protocol [9]. In contrast, Joosten et al. reported a reduction in postoperative morbidity and complications with the use of colloid-based GDFT [10]. Our meta-analysis failed to find any benefit of colloid in terms of postoperative complications or any organ-specific morbidity. Our finding probably highlights the fact that the amount of fluid administered during surgery is more important than whether colloid or crystalloid is used.
Nowadays, the use of synthetic colloids, especially starches, is being discouraged in critically ill patients due to increased requirement of renal replacement therapy and possibly increased mortality [4, 23]. In surgical patients, the clinical effects of synthetic colloid are less clear. A retrospective study reported increased incidence of acute AKI in orthoptic liver transplant patients with the use of starch when compared to human albumin [24]. A meta-analysis by Ramussen et al. reported higher postoperative bleeding with the use of starch in comparison to crystalloid in non-cardiac surgery patients [25]. In another well-conducted meta-analysis of 13 RCTs, Gilles et al. reported no differences in the incidence of postoperative AKI [6]. It is worth mentioning that the included trials were small in sample size and the event rate of AKI was also low; hence, these findings require validation in large RCTs. In our meta-analysis, we have found that incidence of postoperative kidney dysfunction was not higher in patients who received colloid as compared to crystalloid. As patients undergoing routine surgery are rarely septic, starches may have minimal detrimental effect on kidney function in this setting.
The length of hospital stay was similar in our analysis irrespective of the type of intravascular fluid used. Som et al. [3] also reported a similar postoperative hospital length of stay with the use of colloid-based GDFT as opposed to standard fluid therapy. However, use of colloid-based fluid therapy reduced the number of patients with at least one postoperative complication. Despite the common concern of coagulopathy [26], in our meta-analysis, we did not find any increased incidence of postoperative bleeding with the use of starch.
Strength and limitation
The most important strength of this meta-analysis is the absence of any statistical heterogeneity in ‘postoperative mortality’, which is an important patient-centric outcome. However, this meta-analysis has several limitations. Other than postoperative mortality, significant statistical heterogeneity was found in most of the other outcomes. Moreover, clinical heterogeneity is also possible because of different inclusion criteria and fluid therapy protocol. Hence, the quality of evidences was downgraded to ‘low’ to ‘very low’. Event rates in the postoperative outcomes were also small, which could again contribute to the downgrading of the quality of the evidences.
Conclusion
The use of colloids in goal-directed fluid therapy protocol does not offer any benefit over crystalloid-based goal-directed fluid therapy protocol in patients undergoing major non-cardiac surgical procedures. However, no increased incidence of kidney dysfunction was found with the use of colloid.
References
Bellamy MC. Wet, dry or something else? Br J Anaesth. 2008;97:755–7.
Chong MA, Wang Y, Berbenetz NM, McConachie I. Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur J Anaesthesiol. 2018;35:469–83.
Som A, Maitra S, Bhattacharjee S, Baidya DK. Goal-directed fluid therapy decreases postoperative morbidity but not mortality in major non-cardiac surgery: a meta-analysis and trial sequential analysis of randomized controlled trials. J Anesth. 2017;31:66–81.
Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8:CD000567.
Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Åneman A, Madsen KR, Møller MH, Elkjær JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Søe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjældgaard AL, Fabritius ML, Mondrup F, Pott FC, Møller TP, Winkel P, Wetterslev J, 6S Trial Group. Scandinavian Critical Care Trials Group. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2016;367:124–34.
Gillies MA, Habicher M, Jhanji S, Sander M, Mythen M, Hamilton M, Pearse RM. Incidence of postoperative death and acute kidney injury associated with i.v. 6% hydroxyethyl starch use: systematic review and meta-analysis. Br J Anaesth. 2014;112:25–34.
Müller RB, Haase N, Lange T, Wetterslev J, Perner A. Acute kidney injury with hydroxyethyl starch 130/0.42 in severe sepsis. Acta Anaesthesiol Scand. 2015;59(3):329–36.
Matsunaga W, Sanui M, Sasabuchi Y, et al. Large volume infusions of hydroxyethyl starch during cardiothoracic surgery may be associated with postoperative kidney injury: propensity-matched analysis. Perioper Med (Lond). 2019;8:13.
Kabon B, Sessler DI, Kurz A. Crystalloid-colloid study team. effect of intraoperative goal-directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology. 2019;130:728–44.
Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, Van Obbergh L, Loi P, Rinehart J, Cannesson M, Van der Linden P. (2018) Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: a randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology. 2018;128:55–66.
Tyagi A, Verma G, Luthra A, Lahan S, Das S, Rai G, Sethi AK. Risk of early postoperative acute kidney injury with stroke volume variation-guided tetrastarch versus Ringer's lactate. Saudi J Anaesth. 2019;13:9–15.
Feldheiser A, Pavlova V, Bonomo T, Jones A, Fotopoulou C, Sehouli J, Wernecke KD, Spies C. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Br J Anaesth. 2013;110:231–40.
Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–9.
Zhang J, Qiao H, He Z, Wang Y, Che X, Liang W. Intraoperative fluid management in open gastrointestinal surgery: goal-directed versus restrictive. Clinics (Sao Paulo). 2012;67:1149–55.
Senagore AJ, Emery T, Luchtefeld M, Kim D, Dujovny N, Hoedema R. Fluid management for laparoscopic colectomy: a prospective, randomized assessment of goal-directed administration of balanced salt solution or hetastarch coupled with an enhanced recovery program. Dis Colon Rectum. 2009;52:1935–40.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.handbook.cochrane.org.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Guyatt GH, Oxman AD, Kunz R, et al. Incorporating considerations of resources use into grading recommendations. BMJ. 2008;336:1170–3.
Lindroos AC, Niiya T, Randell T, Niemi TT. Stroke volume-directed administration of hydroxyethyl starch (HES 130/0.4) and Ringer’s acetate in prone position during neurosurgery: a randomized controlled trial. J Anesth. 2014;28:189–97.
Futier E, Garot M, Godet T, et al. Effect of hydroxyethyl starch vs saline for volume replacement therapy on death or postoperative complications among high-risk patients undergoing major abdominal surgery: The FLASH randomized clinical trial. JAMA. 2020;323:225–36.
Lindroos AC, Niiya T, Silvasti-Lundell M, Randell T, Hernesniemi J, Niemi TT. Stroke volume-directed administration of hydroxyethyl starch or Ringer's acetate in sitting position during craniotomy. Acta Anaesthesiol Scand. 2013;57:729–36.
Serpa Neto A, Veelo DP, Peireira VG, de Assunção MS, Manetta JA, Espósito DC, Schultz MJ. Fluid resuscitation with hydroxyethyl starches in patients with sepsis is associated with an increased incidence of acute kidney injury and use of renal replacement therapy: a systematic review and meta-analysis of the literature. J Crit Care. 2014;29(185):e1–7.
Hand WR, Whiteley JR, Epperson TI, Tam L, Crego H, Wolf B, Chavin KD, Taber DJ. Hydroxyethyl starch and acute kidney injury in orthotopic liver transplantation: a single-center retrospective review. Anesth Analg. 2015;120:619–26.
Rasmussen KC, Secher NH, Pedersen T. Effect of perioperative crystalloid or colloid fluid therapy on hemorrhage, coagulation competence, and outcome: A systematic review and stratified meta-analysis. Medicine (Baltimore). 2016;95:e4498.
Hahn RG. Adverse effects of crystalloid and colloid fluids. Anaesthes Intens Ther. 2017;49(4):303–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1: Details of search strategy
Appendix 1: Details of search strategy
1. PubMed/PubMed Central
("colloids" [pharmacological action] OR "colloids" [MeSH terms] OR "colloids" [all fields] OR "colloid" [all fields]) AND ("crystalloid solutions" [MeSH terms] OR ("crystalloid" [all fields] AND "solutions" [all fields]) OR "crystalloid solutions" [all fields] OR "crystalloid" [all fields]).
("goals" [MeSH terms] OR "goals" [all fields] OR "goal" [all fields]) AND directed [all fields] AND ("therapy" [subheading] OR "therapy" [all fields] OR "therapeutics" [MeSH terms] OR "therapeutics" [all fields]).
("goals" [MeSH terms] OR "goals" [all fields] OR "goal" [all fields]) AND directed [all fields] AND ("fluid therapy" [MeSH terms] OR ("fluid" [all fields] AND "therapy" [all fields]) OR "fluid therapy" [all fields]).
("goals" [MeSH terms] OR "goals" [all fields] OR "goal" [all fields]) AND directed [all fields] AND ("fluid therapy" [MeSH terms] OR ("fluid" [all fields] AND "therapy" [all Fields]) OR "fluid therapy" [all fields]).
("goals" [MeSH terms] OR "goals" [all fields] OR "goal" [all fields]) AND directed [all fields] AND ("therapy" [Subheading] OR "therapy" [all fields] OR "therapeutics" [MeSH terms] OR "therapeutics" [all fields]) AND major [all fields] AND ("surgery" [subheading] OR "surgery" [all fields] OR "surgical procedures, operative" [MeSH terms] OR ("surgical" [all fields] AND "procedures" [all fields] AND "operative" [all fields]) OR "operative surgical procedures" [all fields] OR "surgery" [all fields] OR "general surgery"[ MeSH terms] OR ("general" [all fields] AND "surgery" [all fields]) OR "general surgery" [all fields]).
2. EMBASE
“colloid:ti,ab,kw” Or 'goal directed fluid therapy' Or 'crystalloid' Or 'goal directed therapy' And 'randomized controlled trial' Or “randomized AND trial”.
3. CENTRAL database
(colloid):ti,ab,kw Or (crystalloid):ti,ab,kw Or (goal directed fluid therapy):ti,ab,kw Or (goal directed therapy):ti,ab,kw And ("randomized clinical trial"):ti,ab,kw Or ("randomized controlled trial"):ti,ab,kw.
About this article
Cite this article
Tyagi, A., Maitra, S. & Bhattacharjee, S. Comparison of colloid and crystalloid using goal-directed fluid therapy protocol in non-cardiac surgery: a meta-analysis of randomized controlled trials. J Anesth 34, 865–875 (2020). https://doi.org/10.1007/s00540-020-02832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02832-5