Abstract
Purpose
There have been no large-scale studies on whether metformin therapy might have a potential benefit for lowering mortality. Thus, this study aimed to investigate the association between prior metformin therapy and the development of sepsis as well as the association between prior metformin therapy and 30-day mortality in sepsis patients.
Methods
We evaluated adult diabetes patients registered in the 2010 sample cohort database of the National Health Insurance Service in South Korea. Diabetes was identified according to the International Classification of Disease-10 diagnostic system (E10–E14). The cohorts were divided into the metformin user group (i.e., those who had been prescribed continuous oral metformin over a period of ≥ 90 days) and the control group (i.e., all other individuals). The primary endpoint was the development of sepsis between 2011 and 2015, and the secondary endpoint was 30-day mortality among diabetes patients diagnosed with sepsis.
Results
In total, 77,337 patients (34,041 in the metformin user group and 43,296 in the control group) were included in the analysis, among whom 2512 patients (3.2%) were diagnosed with sepsis between 2011 and 2015. After propensity score adjustment, metformin use was not significantly associated with both the risk of sepsis (OR: 0.92, 95%CI 0.82–1.03; P = 0.143) and the risk of 30-day mortality after diagnosis of sepsis (OR: 0.94, 95%CI 0.75–1.17; P = 0.571).
Conclusions
Prior metformin therapy was not significantly associated with the risk of sepsis and 30-day mortality after diagnosis of sepsis among diabetes patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a life-threatening illness caused by dysregulated host immune response due to infection [1]. It occurs in 6% of all hospitalized adults in the United States [2], and its incidence is continuously increasing [3]. Further, a 2011 retrospective in-patient study in the United States reported an all-cause mortality rate of sepsis of 14.8% [4]. With respect to economic burden, sepsis has also been considered a significant concern [5], with the median hospital cost of sepsis treatment per patient estimated at approximately $16,000 [4]. Therefore, efforts for preventing sepsis and lowering mortality in sepsis patients have been emphasized.
Metformin is a biguanide drug that is most commonly prescribed for the management of type 2 diabetes mellitus [6]. In addition to its well-known hypoglycemic effect, some previous studies have suggested that metformin might inhibit the expression of pro-inflammatory factors in vitro and ameliorate inflammatory injuries in vivo [7,8,9,10]. Although the mechanism is unclear and controversial, metformin has been reported to inhibit the activity of the mitochondrial respiratory complex, which decreases the generation of ATP and activates adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) [11]. AMPK activation have been confirmed to have a protective effect for sepsis in animal studies [12,13,14], indicating that AMPK plays a significant role in the pathogenesis of sepsis. Moreover, a recent meta-analysis of five cohort studies concluded that preadmission metformin use is associated with lower mortality, as evidenced by an odds ratio (OR) of 0.59 (95% confidence interval (CI) 0.43–0.79) [15]. However, the five cohort studies had a relatively small sample size, of which four were retrospective observational studies from a single institution [16,17,18,19], and only one was a prospective observational study [20]. Additionally, the meta-analysis focused only on mortality in sepsis patients and did not investigate the protective effect of metformin against the development of sepsis.
Therefore, this study aimed to investigate whether metformin helped prevent sepsis, using a sample cohort from South Korea. Additionally, we aimed to investigate the association between prior metformin therapy and 30-day mortality in sepsis patients.
Methods
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (X-1904-532-901) and the Health Insurance Review and Assessment Service (NHIS-2019-2-142).
Data source
The sample cohort database of the National Health Insurance Service (NHIS) was developed to provide data for academic research of the general population in South Korea. The database comprises a stratified random sample of one million people who were registered with the NHIS since 2002. It was designed to be representative of the national population with regard to demographic and socioeconomic information. The cohort was dynamic and followed up until the end of 2015. It was supplemented with additional cohort data, including data on infants, to allow for attrition due to death and loss to follow-up. Each year subjects were added to the cohort to replace people who had died or emigrated in the previous year, using stratified extraction methods to ensure that the cohort was representative of the national population [21].
Population
We evaluated adult diabetes patients (age, ≥ 18 years) registered in the 2010 cohort database of the NHIS. Diabetes was identified according to the International Classification of Disease (ICD)-10 diagnostic system (E10–E14). Individuals who died in 2010 and those who emigrated between 2011 and 2015 were excluded, as they could not be followed up.
Prior metformin therapy as exposure variable
The diabetes patients were divided into the metformin user group, defined as those who had been prescribed continuous oral metformin over a period of ≥ 90 days, and the control group, defined as all other subjects. The classification of metformin use was based on the metformin prescription data from October 2009 to December 2010. This was done to remove immediate exposure to metformin (< 90 days) before outcome evaluation (i.e., development of sepsis) from January 2011 as lag time approach in this study [22]. In addition, the metformin users were divided into two groups according to daily dosage, namely the high daily dosage group (> 1 g/day) and the low daily dosage group (≤ 1 g/day).
Study endpoints
The primary endpoint of this study was development of sepsis between 2011 and 2015, which was defined as registered sepsis (including septic shock: A40*, A41*, R65.2*, R572, and B377) during this period. The secondary endpoint was 30-day mortality among diabetes patients who developed sepsis between 2011 and 2015. The 30-day mortality was defined as any mortality within 30 days after the diagnosis of sepsis.
Confounders
The following variables were considered as confounders in this study: (1) demographic data (age and sex); (2) socioeconomic data (income level in deciles and place of residence in 2010 [Seoul, metropolitan cities, and other]); (3) the 2010 Charlson comorbidity index, which was calculated using registered ICD-10 diagnostic codes between 2009 and 2010 in the NHIS database (e-Appendix 1); (4) total number of hospital visit days in 2010; and (5) use of other anti-diabetic medications (sulfonylureas, alpha-glucosidase inhibitors, thiazolidinediones, and insulin). In the classification of place of residence, Seoul, the capital city, was assigned a separate category, and the cities of Incheon, Kwangju, Busan, Ulsan, Daegu, and Daejeon were classified as metropolitan cities. The number of hospital visit days included the number of hospital outpatient clinic visits and days spent on admission, but it did not include outpatient visits to primary care physicians. For example, an individual who visited a hospital outpatient clinic five times and was admitted to the hospital for 3 days would be considered to have had 8 hospital visit days. In the analysis, the number of hospital visit days was categorized into five groups (0, 1–7, 8–29, 30–90, and > 90 days).
Statistical analysis
The baseline characteristics were presented as means with standard deviations for continuous variables or frequencies with percentages for categorical variables. We performed propensity score (PS) matching to reduce confounders in observational studies [23] using the nearest neighbor method with a 1:1 ratio, without replacement, and a caliper width of 0.1. Logistic regression analysis was performed to calculate PSs as a logistic model, and all covariates were included in the PS model. The absolute value of standardized mean difference (ASD) was used to determine the balance between the metformin user group and the control group before and after PS matching. ASDs between the two groups were set to below 0.1 to determine whether the two groups were well balanced through PS matching. After confirming good balance between the two groups through PS matching, we performed a univariable logistic regression analysis for the development of sepsis between 2011 and 2015 in the PS-matched cohort. As a first sensitivity analysis, because observational studies often suffer from immortal bias because of the time period from the treatment to event (i.e., development of sepsis in this study) [24], we used the landmark method to determine the development of sepsis in the PS-matched cohort [25]. In the landmark method, we performed four logistic regression analysis for the development of sepsis in four different periods (i.e., 2011–2015, 2012–2015, 2013–2015, and 2014–2015), considering that metformin users or the control patients might die due to another disease (e.g., heart failure or trauma) before the development of sepsis.
Next, we performed multivariable logistic regression analysis for the development of sepsis between 2011 and 2015 in the entire 2010 sample cohort to (1) determine whether the results obtained from the PS-matched cohort were generalizable to the entire sample cohort in 2010 and (2) investigate the risk for development of sepsis among metformin users with other important covariates in context, not isolated. All covariates were included in the multivariable model for adjustment, and there was no multicollinearity in all multivariable models of the entire cohort with a variance inflation factor of < 2.0. Hosmer–Lemeshow statistics was used to confirm goodness of fit of multivariable models as P > 0.05. Additionally, we performed two subgroup analyses in the multivariable logistic regression analysis: (1) subgroup analysis according to the daily dose of metformin was performed to investigate whether the daily dosage of metformin influences the development of sepsis, (2) subgroup analysis according to severity of diabetes mellitus, because it might affect the use of anti-diabetic medication. On the basis of ICD-10 codes in 2010, the total cohort was divided into two groups, namely, diabetes mellitus with chronic complication and diabetes mellitus without chronic complication, as shown in e-Appendix 1.
For the secondary endpoint (30-day mortality) among patients who were diagnosed with sepsis during 2011–2015, we performed both PS matching and multivariable logistic regression analysis again using the same method. The results of the logistic regression models were presented as odds ratio (OR) with 95% confidence interval (CI). All statistical analyses were performed using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria), and P < 0.05 was considered statistically significant.
Results
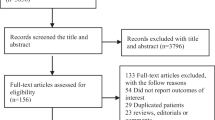
In total, 78,876 adult diabetes patients were initially screened. Of these, 52 patients who emigrated between 2011 and 2015 and 1487 patients who died in 2010 were excluded from analysis. Thus, 77,337 patients (34,041 patients in the metformin user group and 43,296 patients in the control group) were included in the analysis. After the first PS matching, 41,608 patients (20,804 patients in each group) were included in the final analysis. Of the 77,337 diabetes patients, 2512 patients (3.2%; 1,045 patients in the metformin user group and 1,467 patients in the control group) were diagnosed with sepsis between 2011 and 2015. After the second PS matching, 1344 patients (672 patients in each group) were included in the secondary analysis. The patient selection flow chart is presented in Fig. 1. The results of the comparison of characteristics between the metformin user group and the control group in the entire cohort and in patients who developed sepsis are presented in Tables 1 and 2, respectively. All ASDs were below 0.1, indicating that all covariates between the two groups were well balanced through PS matching.
Development of sepsis according to prior metformin therapy
Table 3 shows the results of analysis of development of sepsis between 2011 and 2015 in diabetes patients before and after PS matching. After PS matching, the incidence rate of sepsis in the metformin user group and the control group was 2.9% (598/20,804) and 3.1% (649/20,804), respectively. In the logistic regression analysis, metformin use was not significantly associated with the risk of developing sepsis (OR: 0.92, 95% CI 0.82–1.03; P = 0.143). In sensitivity analysis according to daily metformin dosage, both low daily dosage (P = 0.775) and high daily dosage (P = 0.437) were not significantly associated with the risk of sepsis. The sensitivity analyses in both the multivariable logistic regression model in the entire cohort showed a similar tendency (Table 5) and in the PS-matched cohort according to survival time showed a similar tendency (e-Appendix 2).
Thirty-day mortality after diagnosis of sepsis in diabetes patients
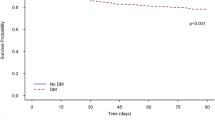
Table 4 shows the results of analysis with respect to the 30-day mortality after diagnosis of sepsis before and after PS matching. After PS matching, the 30-day mortality rate in the metformin user group was 35.9% (241/672), while it was 37.4% (251/672) in the control group. In the logistic regression analysis, metformin use was not significantly associated with the risk of 30-day mortality (OR: 0.94, 95% CI 0.75–1.17; P = 0.571). The sensitivity analysis of the multivariable logistic regression model in the entire cohort showed a similar tendency (Table 5).
Subgroup analysis
Table 5 also shows the results of subgroup analyses according to daily dosage of metformin, and severity of diabetes mellitus. Both low and high daily dosage of metformin user group were not associated with development of sepsis, compared to control group (P = 0.084 and P = 0.428, respectively). Metformin user group was not associated with development of sepsis compared to control group in both groups: diabetes without and diabetes with chronic complication (P = 0.966 and P = 0.099, respectively). A similar trend was observed on subgroup analyses with regard to the secondary endpoint (30-day mortality) among sepsis patients.
Discussion
This population-based cohort study showed that prior metformin therapy in diabetes patients was not significantly associated with the development of sepsis. Additionally, prior metformin therapy was not associated with 30-day mortality in diabetes patients diagnosed with sepsis. This association was coincident regardless of the daily dosage of metformin and severity of diabetes mellitus.
Importantly, while most studies focus on mortality after sepsis diagnosis [16,17,18,19,20], this study focused on the incidence of sepsis among diabetes patients who use metformin in a nationwide sample cohort. Metformin is known to be associated with AMPK activation [11], which is known to have a protective effect against sepsis-induced organ injury [12]. Moreover, a recent study reported that disruption of the AMPK-dependent immunometabolism pathway might contribute to the development of sepsis [26]. As such, metformin therapy might have a potential benefit for preventing sepsis, but this has not been investigated previously. To our knowledge, our study is the first to show that metformin might have no significant preventive effect against sepsis in diabetes patients. In addition, we also found that the daily dose of metformin was not associated with the risk of sepsis.
Because of the observational study design, an important issue in this study was controlling for bias. First, immortal time bias, which is a well-known bias in observational studies [24], might be present in our study. For example, the risk of sepsis in the control group might be higher if patients in the metformin user group died in 2011 due to any other disease (e.g., cardiovascular disease) before the development of sepsis and the control group survived beyond 2011. To minimize this bias, we performed landmark analysis for the development of sepsis in the PS-matched cohort [25] and also included many confounders that might be closely related to death due to other diseases before sepsis development, including comorbidities and socioeconomic factors. Second, there might be a time-lag bias, and this might also affect the results of this observational study [27]. For instance, the results might be biased because of inadequate duration of exposure for the event to occur if a patient received a prescription for metformin from December 2010 within 30 days. To control for this bias, we defined metformin users using a lag-time approach as those who received prescriptions for metformin over 90 days until December 2010 [22]. Through this approach we excluded immediate exposure to metformin among diabetes patients before evaluating the development of sepsis from January 2011. In addition, we performed both PS adjustment and multivariable adjustment in the entire cohort to enhance the robustness of our findings.
Furthermore, aside from the main findings, we also found no significant association between 30-day mortality and prior metformin therapy in diabetes patients who developed sepsis. The association between prior metformin therapy and 30-day mortality is controversial. While some studies showed no significant association between prior metformin therapy and mortality in sepsis patients [18,19,20], others reported that prior metformin therapy was significantly associated with lower mortality in sepsis patients [16, 17]. Although a recent meta-analysis reported that prior metformin therapy was associated with lower mortality in sepsis patients [15], it involved a relatively small sample size, thus possibly limiting the robustness of its findings.
Our study has several limitations. First, some important variables, including body mass index, were not included in both PS adjustment and multivariable adjustment because they were not included in the NHIS data set. Second, we defined the comorbidities using ICD-10 codes to calculate Charlson comorbidity index, but the diseases specified by the ICD-10 codes might differ from the actual underlying diseases in the diabetes patients. Third, PS adjustment and multivariable adjustment could control only for known confounders; thus, there might be residual confounders that could have influenced the study findings. Fourth, our analysis was based on metformin prescription data in the NHIS database; it did not assess compliance among those classified as metformin users. Fifth, it is possible that patients in the control group may have been prescribed metformin, while patients in the metformin group might have stopped the treatment since 2011. Lastly, regarding analysis for 30-day mortality in sepsis patients, we did not adjust for the severity of sepsis (such as acute physiology and chronic health evaluation II score or simplified acute physiology score II) in this study. Considering these limitations, the results of this study should be interpreted cautiously, and further prospective, large population-based cohort studies are needed to confirm these findings.
In conclusion, prior metformin therapy had no significant association with sepsis risk and 30-day mortality after sepsis diagnosis. This association was coincident regardless of daily dosage of metformin, and severity of diabetes mellitus. Our study suggests that the association of prior metformin therapy with both risk and outcome of sepsis remains controversial, and future studies on this topic are needed.
References
Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M, Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):775–87.
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE, Jernigan JA, Martin GS, Septimus E, Warren DK, Karcz A, Chan C, Menchaca JT, Wang R, Gruber S, Klompas M, Program CDCPE. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318(13):1241–9.
Hajj J, Blaine N, Salavaci J, Jacoby D. The “Centrality of Sepsis”: a review on incidence, mortality, and cost of care. Healthcare (Basel). 2018;6(3).
Nguyen AT, Tsai CL, Hwang LY, Lai D, Markham C, Patel B. Obesity and mortality, length of stay and hospital cost among patients with sepsis: a nationwide inpatient retrospective cohort study. PLoS ONE. 2016;11(4):e0154599.
Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Crit Care Med. 2015;43(9):1945–51.
Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–76.
Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425(4):866–72.
Kuo CL, Ho FM, Chang MY, Prakash E, Lin WW. Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J Cell Biochem. 2008;103(3):931–40.
Liu G, Wu K, Zhang L, Dai J, Huang W, Lin L, Ge P, Luo F, Lei H. Metformin attenuated endotoxin-induced acute myocarditis via activating AMPK. Int Immunopharmacol. 2017;47:166–72.
Yuan H, Li L, Zheng W, Wan J, Ge P, Li H, Zhang L. Antidiabetic drug metformin alleviates endotoxin-induced fulminant liver injury in mice. Int Immunopharmacol. 2012;12(4):682–8.
An H, He L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J Endocrinol. 2016;228(3):R97–106.
Escobar DA, Botero-Quintero AM, Kautza BC, Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS, Gomez H. Adenosine monophosphate-activated protein kinase activation protects against sepsis-induced organ injury and inflammation. J Surg Res. 2015;194(1):262–72.
Liu Z, Bone N, Jiang S, Park DW, Tadie JM, Deshane J, Rodriguez CA, Pittet JF, Abraham E, Zmijewski JW. AMP-activated protein kinase and glycogen synthase kinase 3beta modulate the severity of sepsis-induced lung injury. Mol Med. 2016;21(1):937–50.
Mulchandani N, Yang WL, Khan MM, Zhang F, Marambaud P, Nicastro J, Coppa GF, Wang P. Stimulation of brain AMP-activated protein kinase attenuates inflammation and acute lung injury in sepsis. Mol Med. 2015;21:637–44.
Liang H, Ding X, Li L, Wang T, Kan Q, Wang L, Sun T. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care. 2019;23(1):50.
Doenyas-Barak K, Beberashvili I, Marcus R, Efrati S. Lactic acidosis and severe septic shock in metformin users: a cohort study. Crit Care. 2016;20:10.
Green JP, Berger T, Garg N, Suarez A, Hagar Y, Radeos MS, Panacek EA. Impact of metformin use on the prognostic value of lactate in sepsis. Am J Emerg Med. 2012;30(9):1667–733.
Jochmans S, Alphonsine JE, Chelly J, Vong LVP, Sy O, Rolin N, Ellrodt O, Monchi M, Vinsonneau C. Does metformin exposure before ICU stay have any impact on patients' outcome? A retrospective cohort study of diabetic patients. Ann Intensive Care. 2017;7(1):116.
Park J, Hwang SY, Jo IJ, Jeon K, Suh GY, Lee TR, Yoon H, Cha WC, Sim MS, Carriere KC, Yeon S, Shin TG. Impact of metformin use on lactate kinetics in patients with severe sepsis and septic shock. Shock. 2017;47(5):582–7.
van Vught LA, Scicluna BP, Hoogendijk AJ, Wiewel MA, Klein Klouwenberg PM, Cremer OL, Horn J, Nurnberg P, Bonten MM, Schultz MJ, van der Poll T. Association of diabetes and diabetes treatment with the host response in critically ill sepsis patients. Crit Care. 2016;20(1):252.
Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the national health insurance service-national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15.
Arfe A, Corrao G. The lag-time approach improved drug-outcome association estimates in presence of protopathic bias. J Clin Epidemiol. 2016;78:101–7.
Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–24.
Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087.
Mi X, Hammill BG, Curtis LH, Lai EC, Setoguchi S. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Stat Med. 2016;35(26):4824–36.
Huang J, Liu K, Zhu S, Xie M, Kang R, Cao L, Tang D. AMPK regulates immunometabolism in sepsis. Brain Behav Immun. 2018;72:89–100.
Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012;35(12):2665–733.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
540_2020_2753_MOESM2_ESM.docx
e-Appendix 2. Logistic regression analysis for development of sepsis in PS-matched cohort according to survival time PS, propensity score (DOCX 13 kb)
About this article
Cite this article
Oh, T.K., Song, IA. Association between prior metformin therapy and sepsis in diabetes patients: a nationwide sample cohort study. J Anesth 34, 358–366 (2020). https://doi.org/10.1007/s00540-020-02753-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02753-3