Abstract
Background
To evaluate the effectiveness of diphenhydramine, an antihistamine with anti-muscarinic properties, for prevention of postoperative catheter-related bladder discomfort (CRBD).
Methods
Ninety-six ASA physical status I and II adult female patients (20–60 years) scheduled for elective gynecologic laparoscopic surgery were included. Patients were randomized into two groups of 48 patients each. All patients received a detailed preoperative explanation of the possible consequences of CRBD. The control group received normal saline 2 ml, whereas the diphenhydramine group received diphenhydramine 30 mg intravenously after induction of general anesthesia. Then, all patients were catheterized with a 14F Foley catheter and the balloon was inflated with 10 ml of distilled water. All patients who complained of CRBD in the postoperative room were appeased with nursing. Ketorolac 30 mg was used as the rescue drug on patients’ request or when the patient was evaluated as having moderate or severe CRBD. Bladder discomfort and its severity were assessed at 1, 2 and 6 h postoperatively. The severity of CRBD was graded as none, mild, moderate and severe. Adverse effects of diphenhydramine such as sedation, dry mouth or GI upset were recorded.
Results
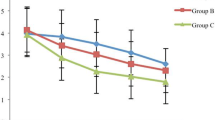
The incidence of CRBD was lower in the diphenhydramine group compared with the control group at 2 h (34.8 vs. 58.7%, p = 0.02) and 6 h (23.9 vs. 56.5%, p < 0.01) postoperatively. Diphenhydramine treatment also reduced the severity of CRBD at 6 h postoperatively (p = 0.01). Moreover, the request for rescue for CRBD was lower in diphenhydramine group at 2 h (8.7 vs. 26.1%, p = 0.03). There were no significant differences in side effects, such as sedation, dry mouth or gastrointestinal upset between the two groups (p > 0.05).
Conclusion
Prophylactic diphenhydramine 30 mg at induction of general anesthesia reduced the incidence and severity of postoperative bladder discomfort without significant side effects in patients receiving gynecologic laparoscopic surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catheter-related bladder discomfort (CRBD) can be troublesome in some patients who are catheterized intraoperatively. These patients often complain of an urge to void or discomfort in the suprapubic region upon awakening from anesthesia and in the postoperative period due to bladder irritation by the urinary catheter [1, 2]. The clinical symptoms of CRBD are similar to those of overactive bladder (OAB), including urinary frequency, urgency, with or without urge incontinence [3, 4]. Although anti-muscarinic drugs are the mainstay of treating OAB in clinical practice, there is still no well-established regimen for the management of CRBD. Muscarinic receptor antagonists such as oxybutynin and tolterodine, routinely used for the treatment of OAB, were also reported to be effective in the prevention of CRBD [5, 6]. Moreover, ketorolac was also demonstrated effective in relieving symptoms of CRBD possibly via a mechanism of anti-inflammation of bladder mucosal injury [7].
Diphenhydramine has been widely employed in the perioperative period for the management of allergic reaction and prevention of postoperative nausea and vomiting [8]. In addition to its well-known H1-antihistamine action, it also possesses anti-muscarinic properties. Thus, diphenhydramine might be able to reduce or even resolve the problem of CRBD by inhibiting smooth muscle spasm of the urinary bladder via its anti-muscarinic action. Moreover, diphenhydramine should be administered via an intravenous route, at the time of induction of general anesthesia as oral medication is not allowed. This study was conducted to evaluate the efficacy of diphenhydramine 30 mg administered intravenously after anesthesia induction in the incidence and severity of CRBD in patients undergoing gynecologic laparoscopic surgery.
Methods
The study protocol was approved by the Institutional Review Board of Human Research at Chi Mei Medical Center (IRB number: 09709-005), and written informed consent was obtained from all patients. A total of 130 female patients between 20 and 60 years of age (ASA Grade I or II) scheduled for gynecologic laparoscopic surgery and requiring intraoperative catheterization were assessed in this double-blind, randomized, placebo-controlled study. The exclusion criteria were bladder outflow obstruction, overactive bladder (urination frequency > 3 times in the night or > 8 times in 24 h), end-stage renal disease (urine output < 500 ml per 24 h), morbid obesity (body mass index over 40), asthma, hyperthyroidism, pregnancy or baby nursing, narrow-angle glaucoma, disturbance of the central nervous system, substance abuse, chronic pain, and cardiovascular, hepatic disease or any psychiatric disease. A total of 34 patients were excluded after assessing for eligibility (Fig. 1).
In the preoperative holding area, the remaining 96 patients were randomly assigned to two groups of 48 each with a computer-generated random number table. The study medications diluted with 2 ml normal saline (NS) were prepared by a nurse anesthetist. Patients in the control group received normal saline (NS) 2 ml alone, whereas patients in the diphenhydramine group received diphenhydramine 30 mg diluted by NS to a total volume of 2 ml. These medications were given intravenously after the induction of general anesthesia by another nurse anesthetist who was blinded to the study medication.
Induction of anesthesia was standardized in all patients with fentanyl 3 μg/kg, lidocaine 1 mg/kg and propofol 2 mg/kg. Tracheal intubation was facilitated with rocuronium 0.6 mg/kg. Urinary catheterization was done with a 14 Fr Foley catheter and its balloon was inflated with 10 ml distilled water after induction of anesthesia. K–Y jelly (a water base lubricating gel) was used to lubricate the catheter which was later fixed in the suprapubic area with an adhesive tape without any traction and was always left to free drainage. Anesthesia was maintained with sevoflurane (50% air in oxygen) at 0.7–1.3 end-tidal minimum alveolar concentration. Additional fentanyl 50 μg was given if the heart rate or blood pressure increased more than 20% from the preoperative value during surgery. Ventilation was controlled mechanically and adjusted to maintain end-tidal CO2 at 30–35 mmHg throughout the surgery. Additional rocuronium was also given as required. At the end of surgery, neuromuscular blockade was reversed with neostigmine (0.04 mg/kg) and glycopyrrolate (0.01 mg/kg). After the patient had spontaneous respiration and became conscious, the trachea was extubated and the patient was moved to the postanesthesia care unit (PACU).
All patients were closely observed for 6 h after surgery (2 h in PACU and 4 h in general ward). Bladder discomfort was assessed at 1, 2 and 6 h postperatively by a senior anesthesia resident who was blinded to the medication received by the patient. The severity of bladder discomfort was graded as mild (reported by the patient only on questioning), moderate (reported by the patient without questioning; not accompanied by any behavioral response), or severe (reported by the patient and accompanied by behavioral responses). Behavioral responses observed included flailing limbs, strong vocal response and attempts to remove the catheter [6]. If bladder discomfort was not relieved by nursing appease, ketorolac 30 mg IV was administered as the rescue drug on patients’ requests. Additional dose at 15 (if body weight < 60 kg) or 30 mg (if body weight ≥ 60 kg) was given on second request. The maximal allowable ketorolac dose was limited to 60 mg during the 6 h study period.
Postoperative pain was scored at 1, 2, 6 h after surgery using a 10-cm visual analog scale (VAS; from 0 = no pain to 10 = most severe pain). Meperidine 0.5 mg/kg IV was used for acute pain control [9]. An additional dose was given when requested. The total analgesic consumption of meperidine and ketolorac for rescue of CRBD was also recorded. Adverse effects such as postoperative nausea and vomiting, sedation, dry mouth, or GI upset were evaluated. The level of sedation in this study was classified as: 0 = awake; 1 = drowsy, responds to verbal commands; 2 = asleep, responds to touch; 3 = asleep, responds to pain stimuli.
Study outcomes
The primary outcome was the incidence of CRBD at these three time points. Secondary outcomes included the intensity of CRBD and the incidence of ketorolac rescue for CRBD. VAS pain score and side effects, such as dry mouth, sedation and gastrointestinal upset served as tertiary outcomes.
Sample size calculation
The reported incidence of bladder discomfort secondary to intraoperative catheterization ranged from 52 to 60% [1, 5]. Based on the assumption that the incidence of CRBD would be decreased by 30% after diphenhydramine treatment, the sample size was predetermined using a power analysis with a significant level of α = 0.05 (one-sided) and power of 1 − β = 0.8. The results indicated that 41 patients would be needed for each group. Allowing for potential drop-outs, we enrolled 48 patients in each group.
Statistics
The differences in severity of discomfort (mild, moderate, and severe) and the incidence of bladder discomfort between groups were analyzed by Chi square test. Pain at the operative site was measured on a VAS score and analgesic requirements were compared between groups by Student's t test. SPSS 14.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. A value of p < 0.05 was considered statistically significant.
Results
One hundred and thirty patients were assessed from October 2008 to August 2009, and 34 patients were excluded because of ineligibility. Therefore, a total of 96 patients were enrolled in this study. Four patients were excluded from the analysis due to conversion to exploratory laparotomy in four patients and needed patient-controlled analgesia (PCA) for postoperative pain control. The remaining 92 patients were evaluated in this study (Fig. 1). There were no significant differences between diphenhydramine and control groups in patient characteristics, surgery duration, PONV or analgesic requirement (p > 0.05) (Table 1).
The incidence of CRBD at 1 h postoperatively was not significantly different between diphenhydramine and control groups (41.3 vs. 51.2%, p = 0.30). Diphenhydramine treatment reduced the incidence of CRBD at 2 h (34.8 vs. 58.7%, p = 0.02) and 6 h (23.9 vs. 56.5%, p < 0.01) (Table 2). Absolute risk reduction with diphenhydramine treatment was 24%. Diphenhydramine treatment also decreased the severity of CRBD at 6 h postoperatively (p < 0.05) (Table 2). However, the frequency of request for ketorolac rescue was only significantly lower at 2 h (8.7 vs. 26.1%, p = 0.03, for diphenhydramine and control group, respectively). The total consumption of ketorolac was not significantly different. Postoperative pain scale assessed by VAS was similar in both groups at all time points (p > 0.05) (Table 2). There were no significant differences in postoperative nausea and vomiting, sedation, dry mouth or GI upset between the two groups (p > 0.05) (Tables 1, 3).
Discussion
This is the first clinical study to evaluate the efficacy of diphenhydramine treatment for the prevention of CRBD in patients undergoing gynecologic laparoscopic surgery. Our results showed that preemptive administration of 30 mg diphenhydramine intravenously in the anesthetic induction effectively decreased the incidence and severity of postoperative bladder discomfort. In addition, postoperative adverse effects including PONV, sedation, dry mouth and gastrointestinal upset were not increased in patients who received diphenhydramine treatment compared to control group.
Diphenhydramine, a first-generation antihistamine has been used for treatment of allergic reaction and as an antiemetic in anesthesia. It has an onset of action within 2–3 min after intravenous administration and duration of effect of 4–8 h. In addition to its antihistamine effect, diphenhydramine also possesses significant anti-muscarinic activity and was shown to inhibit the transmission of parasympathetic nerve impulses and thereby reduce spasms of bladder smooth muscle in an in vitro study [8, 10]. Our results demonstrated that preemptive diphenhydramine significantly decreased the incidence of CRBD in the late postoperative period. The lack of significant difference in CRBD between the two groups during the 1-h postoperative period might be attributed to patients not fully awake from anesthesia and thus unable to accurately describe bladder discomfort. Another concern is the dose of diphenhydramine we administered in this study was too low, therefore we only demonstrated a borderline significant result. Moreover, the timing of drug given in the anesthetic induction phase may not be quite justifiable. Drug administered at the end of surgery or another boost dose might improve the effect, however, this needs further study to verify this speculation.
Diphenhydramine is commonly used for antiemesis in the perioperative period [11]. Other reports demonstrated that diphenhydramine provided effective antiemetic efficacy for intravenous patient-controlled analgesia (PCA) [12, 13]. However, in our present study, the incidence of PONV was not decreased in the diphenhydramine group compared to the control group. This was attributed to treatment with a single dose of diphenhydramine which was expected to provide an effect for only about 4–8 h.
In comparison with the classical anti-muscarinic agents (oxybutynin and tolterodine) for overactive bladder, diphenhydramine offers the advantage that it can be administered intravenously in the perioperative period as nil per os (nothing by mouth) is required [6, 14, 15]. In addition, ketamine and tramadol have also been found to attenuate this disturbing problem [5, 16]. The effects are thought to be mediated by their action on muscarinic receptors of the smooth muscles to reduce involuntary contractions of the bladder [17]. However, till now, there is not golden standard for prevention or treatment of postoperative CRBD.
One potential disadvantage of diphenhydramine is the marked drowsiness which may result in delayed arousal after general anesthesia [18]. However, we did not find any significant sedative response in the diphenhydramine group during the postoperative period. No additional adverse effects of treatment, such as dry mouth and GI upset, were noted.
An interesting finding of present study is that although the severity of CRBD was lower in diphenhydramine prophylactic group, the request for rescue treatment was only lower at 2 h evaluation point than the control patients. Not only those had who scored CRBD as moderate or severe, but also some of them who scored the discomfort as mild also requested rescue treatment. Therefore, the total consumption of ketorolac in 0–6 h period was not different between the two groups. This indicated that CRBD is really a distressing experience.
There were several limitations in this study. We only evaluated the effect of diphenhydramine for CRBD in female patients undergoing gynecologic laparoscopic surgery. The role of diphenhydramine in male and other surgical interventions, especially genitourinary surgery and in patients who are catheterized for other medical procedures remains to be determined. Moreover, we only evaluated the effect of a single dose of diphenhydramine on CRBD. Neither dose response titration of diphenhydramine nor the effect of continuing therapy in the postoperative period was evaluated. Further studies in these areas are needed.
In conclusion, intravenous administration of diphenhydramine 30 mg after anesthesia induction in gynecologic laparoscopic surgery patients reduced both the incidence and severity of postoperative CRBD without significant side effects. These findings suggest that prophylactic treatment with diphenhydramine for CRBD is effective in patients undergoing gynecologic laparoscopic surgery, and could be routinely used as a prophylactic drug for CRBD in women receiving gynecological laparoscopic surgery.
References
Bala I, Bharti N, Chaubey VK, Mandal AK. Efficacy of gabapentin for prevention of postoperative catheter-related bladder discomfort in patients undergoing transurethral resection of bladder tumor. Urology. 2012;79(4):853–7.
Wilson M. Causes and management of indwelling urinary catheter-related pain. Br J Nurs. 2008;17(4):232–9.
Leron E, Weintraub AY, Mastrolia SA, Schwarzman P. Overactive bladder syndrome: evaluation and management. Curr Urol. 2018;11(3):117–25.
Tauzin-Fin P, Stecken L, Sztark F. Catheter-related bladder discomfort in post-anaesthesia care unit. Ann Fr Anesth Reanim. 2012;31(7–8):605–8.
Antoniou GA, Giannoukas AD, Georgiadis GS, Antoniou SA, Simopoulos C, Prassopoulos P, Lazarides MK. Increased prevalence of abdominal aortic aneurysm in patients undergoing inguinal hernia repair compared with patients without hernia receiving aneurysm screening. J Vasc Surg. 2011;53(5):1184–8.
Agarwal A, Dhiraaj S, Singhal V, Kapoor R, Tandon M. Comparison of efficacy of oxybutynin and tolterodine for prevention of catheter related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Br J Anaesth. 2006;96(3):377–80.
Park JM, Houck CS, Sethna NF, Sullivan LJ, Atala A, Borer JG, Cilento BG, Diamond DA, Peters CA, Retik AB, Bauer SB. Ketorolac suppresses postoperative bladder spasms after pediatric ureteral reimplantation. Anesth Analg. 2000;91(1):11–5.
Church DS, Church MK. Pharmacology of antihistamines. World Allergy Organ J. 2011;4(3 Suppl):S22–27.
Kulacoglu H, Dener C, Kama NA. Urinary retention after elective cholecystectomy. Am J Surg. 2001;182(3):226–9.
Church MK, Church DS. Pharmacology of antihistamines. Indian J Dermatol. 2013;58(3):219–24.
Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59(2):213–43.
Lu CW, Jean WH, Wu CC, Shieh JS, Lin TY. Antiemetic efficacy of metoclopramide and diphenhydramine added to patient-controlled morphine analgesia: a randomised controlled trial. Eur J Anaesthesiol. 2010;27(12):1052–7.
Lin TF, Yeh YC, Yen YH, Wang YP, Lin CJ, Sun WZ. Antiemetic and analgesic-sparing effects of diphenhydramine added to morphine intravenous patient-controlled analgesia. Br J Anaesth. 2005;94(6):835–9.
Agarwal A, Raza M, Singhal V, Dhiraaj S, Kapoor R, Srivastava A, Gupta D, Singh PK, Pandey CK, Singh U. The efficacy of tolterodine for prevention of catheter-related bladder discomfort: a prospective, randomized, placebo-controlled, double-blind study. Anesth Analg. 2005;101(4):1065–7 (table of contents).
Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration. Application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126(3):376–93.
Gupta D, Agarwal A, Dhiraaj S. Ketamine for treatment of catheter-related bladder discomfort. Br J Anaesth. 2005;95(5):720.
Bai Y, Wang X, Li X, Pu C, Yuan H, Tang Y, Li J, Wei Q, Han P. Management of catheter-related bladder discomfort in patients who underwent elective surgery. J Endourol. 2015;29(6):640–9.
Simons FE, Simons KJ. Clinical pharmacology of H1-antihistamines. Clin Allergy Immunol. 2002;17:141–78.
Acknowledgements
We appreciate Miss Yu-Li Cheng and Shu-Zhen Huang for their excellent help in the evaluation of patients and data collection.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Li, YY., Zeng, YS., Chen, JY. et al. Prophylactic diphenhydramine attenuates postoperative catheter-related bladder discomfort in patients undergoing gynecologic laparoscopic surgery: a randomized double-blind clinical study. J Anesth 34, 232–237 (2020). https://doi.org/10.1007/s00540-019-02724-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-019-02724-3