Abstract
Purpose
To provide optimal conditions for neurophysiological monitoring and rapid awakening, remifentanil is commonly used during pediatric spinal surgery. However, remifentanil may induce hyperalgesia and increase postoperative opioid requirements. We evaluated the potential of methadone or magnesium to prevent remifentanil-induced hyperalgesia.
Methods
Using a prospective, randomized, blinded design, adolescents presenting for posterior spinal fusion to treat idiopathic scoliosis were assigned to receive desflurane with remifentanil alone (REMI), remifentanil + methadone (MET) (0.1 mg/kg IV over 15 min), or remifentanil + magnesium (MAG) (50 mg/kg bolus over 30 min followed by 10 mg/kg/h). Primary outcomes were opioid requirements and postoperative pain scores. Secondary outcomes included intraoperative anesthetic requirements, neurophysiological monitoring conditions, and emergence times.
Results
Data analysis included 60 patients. Total opioid requirement (hydromorphone) in the REMI group (received perioperatively and on the inpatient ward) was 0.34 ± 0.11 mg/kg compared to 0.26 ± 0.10 mg/kg in the MET group (95% confidence interval (CI) of difference: − 0.14, − 0.01; p = 0.035). The difference in opioid requirements between the REMI and MET group was related to intraoperative dosing (0.04 ± 0.02 mg/kg vs. 0.02 ± 0.01 mg/kg; 95% CI of difference: − 0.01, − 0.02; p = 0.003). No difference was noted in pain scores, and no differences were noted when comparing the REMI and MAG groups.
Conclusion
With the dosing regimens in the current study, the only benefit noted with methadone was a decrease in perioperative opioid requirements. However, given the potential for hyperalgesia with the intraoperative use of remifentanil, adjunctive use of methadone appears warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During anesthesia for posterior spinal fusion, the anesthetic regimen is tailored to facilitate neurophysiological monitoring of spinal cord integrity using somatosensory (SSEP) and motor evoked potentials (MEP) [1,2,3,4,5,6]. When changes occur during neurophysiological monitoring, the anesthetic technique must allow for a rapid “wake-up” test to document the validity of these findings. In such protocols, a continuous infusion of remifentanil is a key component as it allows for the rapid control of hemodynamic parameters and the provision of intensive analgesia with a rapid recovery time [4, 6, 7]. Despite the efficacy of remifentanil, given the avidity with which it binds to opioid receptors, acute tolerance may occur which increases postoperative opioid requirements by 20–30% [8,9,10,11]. It has been postulated that the hyperalgesia associated with remifentanil may be modulated through the N-methyl-d-aspartate (NMDA) receptor system [12]. Attempts to modulate the response using pre-emptive opioid administration and other pharmacologic agents such as ketamine have resulted in variable success [13, 14].
Magnesium is an inexpensive agent with a wide therapeutic threshold which has been studied during orthopedic surgery, particularly in the adult population where it has been shown to reduce postoperative opioid requirements and intraoperative anesthetic needs [15,16,17,18,19]. Magnesium produces a voltage-gated non-competitive blockade at the NMDA receptor, preventing the binding of glutamate [15]. Other investigators have suggested the use of methadone as a means of improving postoperative analgesia following major spinal surgery and blunting remifentanil-induced hyperalgesia [20,21,22]. Despite this information, there remain limited data focusing on the adolescent population undergoing posterior spinal fusion for idiopathic scoliosis, with no data providing comparable assessments of the intraoperative use of magnesium or methadone as opposed to remifentanil alone. The current prospective, randomized trial investigates the efficacy of these two agents on the perioperative course of adolescents undergoing posterior spinal fusion.
Materials and methods
This study was approved by the Nationwide Children’s Hospital (NCH) Institutional Review Board (IRB13-00036) and registered at ClinicalTrails.gov (NCT01795495). An investigational new drug (IND) application was approved by The Food and Drug Administration (FDA) prior to beginning the study (IND117889). Written informed consent was obtained from a parent and assent from the patient. The primary objective of the study was to determine the effects of intraoperative methadone and magnesium on postoperative opioid requirements and pain scores. The secondary objectives were to determine the effects of methadone and magnesium on the intraoperative inspired concentration of desflurane to maintain a bispectral index (BIS) at 50–60; the dose of remifentanil required to maintain the mean arterial pressure (MAP) at 55–65 mmHg; the need for supplemental agents for blood pressure control despite a maximum remifentanil infusion of 0.3 µg/kg/min; the total dose of rocuronium administered during dissection through the paravertebral muscles (mg/kg); time from turning supine at the completion of the case until eye opening, time until the patient is able to follow commands, time to tracheal extubation; and hospital length of stay (LOS).
Given the need for neurophysiological monitoring and the potential for a “wake-up test”, the anesthetic regimen for posterior spinal fusion includes a titrated technique to ensure a limited effect on MEP and SSEP monitoring with a rapid awakening throughout the procedure. We have previously reported our experience with the use of a volatile anesthetic agent-based technique using desflurane combined with remifentanil [6]. This desflurane–remifentanil technique remains our standard intraoperative anesthetic for such cases and was the technique chosen for this study. The efficacy of neurophysiologic monitoring including SSEP (amplitude and latency, measured at the posterior tibial nerve tract) and MEP (mA required to elicit the response) were compared between each experimental group and the control group at 4 time points: baseline, 30 min after baseline (at least 60 min after rocuronium), after anchor insertion, and at instrumentation completion. If available, left and right data points were averaged at each time. Subcortical (P31) and cortical SSEP (P37) data were analyzed separately.
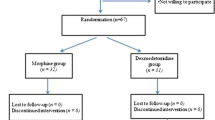
Following the consent procedure, the patients were randomized via lottery to one of 3 groups. The three groups included: remifentanil alone (REMI), remifentanil + methadone (MET) (0.1 mg/kg IV over 15 min, administered just after induction of anesthesia), or remifentanil + magnesium (MAG) (50 mg/kg bolus over 30 min followed by 10 mg/kg/h). The remainder of the intraoperative care followed our standard practice for anesthetic care during posterior spinal fusion [6]. This included oral premedication with midazolam 20 mg followed by the inhalation of 70% nitrous oxide in oxygen to provide analgesia for the placement of a peripheral intravenous cannula. Anesthesia was then induced with propofol 2.5 mg/kg and remifentanil 2.5 µg/kg. Neuromuscular blockade to facilitate endotracheal intubation was accomplished with rocuronium (0.3 mg/kg). Following anesthetic induction and endotracheal intubation, a second peripheral intravenous cannula and a radial arterial cannula were placed. No additional neuromuscular blockade was administered until after the patient was turned prone onto the Jackson table and baseline neurophysiological monitoring was obtained. After this, incremental doses of rocuronium (0.1 mg/kg) were administered during the dissection of paravertebral muscles as requested by the surgeons. Maintenance anesthesia consisted of desflurane titrated to maintain the BIS at 50–60 and a remifentanil infusion starting at 0.05 µg/kg/min and increased up to 0.3 µg/kg/min to maintain the mean arterial pressure (MAP) at 55–65 mmHg. If the MAP was greater than 65 mmHg despite a remifentanil infusion at 0.3 µg/kg/min, labetalol (0.1–0.2 mg/kg) was administered as needed. Following the start of maintenance anesthesia, patients in the remifentanil arm received no additional opioids other than the remifentanil infusion. For patients in the methadone group, methadone (0.1 mg/kg) was administered over 15 min. Due to the concerns of magnesium potentially interfering with MEP monitoring, magnesium was administered after the baseline set of MEPs was obtained. Magnesium was administered as a bolus of 50 mg/kg over 30 min followed by 10 mg/kg/h for the duration of the surgery.
All patients received dexamethasone (4 mg) and ondansetron (4 mg) for the prevention of postoperative nausea and vomiting. Intravenous acetaminophen (15 mg/kg up to 1000 mg) was administered intraoperatively and continued every 6 h for 36 h postoperatively as an adjunct to opioid analgesia. Following completion of the instrumentation and the need for neurophysiological monitoring, the remifentanil infusion was discontinued and hydromorphone (incremental doses of 0.2 mg) administered to achieve a respiratory rate of 8–12 breaths/minute. When the surgical procedure was completed, the patient was turned supine and the trachea extubated once awake. Additional doses of hydromorphone were administered following tracheal extubation and in the post-anesthesia care unit (PACU) as needed to optimize analgesia. Postoperative analgesia as provided by hydromorphone delivered via a patient-controlled analgesia (PCA) device with a bolus dose of 4–10 µg/kg, a lockout period of 10 min, and no basal infusion. If analgesia was inadequate, the bolus dose was increased up to 20 µg/kg. A basal infusion (2–5 µg/kg/hr) was added for patients with severe pain (pain scores ≥ 7) if increasing the bolus did not provide adequate analgesia. On postoperative day one, intravenous ketorolac was started and continued for a total of 20 doses.
Study outcomes were compared separately between REMI and MET groups, and REMI and MAG groups using unpaired t-tests. Opioid requirements were expressed as mg/kg of hydromorphone in the OR, in the PACU, on the inpatient ward for 24 postoperative hours, and for the following combinations: OR + PACU; and OR + PACU + the inpatient ward. The recorded scores for pain using visual analog scale pain scores (VAS, 0–10 scale) were averaged during the patient’s time in the PACU; while on the inpatient ward; and all observations in the PACU and on the ward. Remifentanil dosing (including any bolus doses) was divided by the patient’s weight and the anesthesia duration, and expressed in µg/kg/min. Desflurane concentrations were averaged over all available data points (collected each 30 min) during the case. Total labetalol dosing was expressed in mg/kg, with values of 0 assigned to cases not requiring this medication. A sample size of 20 patients per group was planned to achieve 86% power for detecting a 1 standard deviation (SD) pairwise group difference in mean opioid requirements at a confidence level of 95%. Data analysis was performed using Stata/IC 13.1 (College Station, TX, USA: StataCorp, LP), and two-tailed p < 0.05 was considered statistically significant. Hypothesis tests comparing MET to REMI and MAG to REMI were not adjusted for multiple comparisons, due to the intent to separately analyze potential benefits of methadone and magnesium.
Results
Sixty-three subjects were enrolled in the study, of whom 3 were withdrawn due to deviations from the study protocol. The remaining 60 subjects included 10 boys and 50 girls with an average age of 15.0 ± 1.6 years. They were randomly assigned to the study groups as follows: 19 in REMI, 22 in MET, and 19 in MAG. Descriptive characteristics of each group are summarized in Table 1. Intraoperative adverse events included excessive bleeding in 1 patient (MET). Postoperative adverse events included ileus in 1 patient (REMI) and readmission due to pain and constipation in 1 patient (MAG).
Data on the primary outcomes are compared between MET and REMI and between MAG and REMI in Table 2. Total opioid requirement (hydromorphone) in the REMI group (OR, PACU, and on the inpatient ward) was 0.34 ± 0.11 mg/kg compared to 0.26 ± 0.10 mg/kg in the MET group (95% confidence interval (CI) of difference: − 0.14, − 0.01; p = 0.035). The total opioid requirement in the MAG group was 0.38 ± 0.10 mg/kg (95% CI of difference compared to REMI: − 0.03, 0.12; p = 0.243). Sub-analysis of OR, PACU, and inpatient ward dosing (Table 2) revealed that the MET group had lower intraoperative opioid (hydromorphone) requirements than the REMI group (0.02 ± 0.01 mg/kg vs. 0.04 ± 0.02 mg/kg; 95% CI of difference: − 0.01, − 0.02; p = 0.003), but the opioid requirements did not differ from the REMI group in the PACU (p = 0.465) or on the inpatient ward (p = 0.072). The averages of all available VAS pain scores in the PACU and on the inpatient ward were 5 ± 2 in the REMI group, 5 ± 2 in the MET group (p = 0.386 vs. REMI), and 5 ± 2 in the MAG group (p = 0.391 vs. REMI).
Data on secondary outcomes are summarized in Tables 3 and 4. The intraoperative remifentanil dose was lower in the MET group than in the REMI group (0.16 ± 0.04 µg/kg/min vs. 0.19 ± 0.03 µg/kg/min; p = 0.016) and was similar between the REMI group and MAG group (0.20 ± 0.03 µg/kg/min). Total labetalol dose was lower in the MET group than in the REMI group (0.06 ± 0.10 mg/kg vs. 0.17 ± 0.19 mg/kg; p = 0.018), but was similar between the REMI group and the MAG group (0.22 ± 0.29 mg/kg; p = 0.550). Total rocuronium dose, MEP threshold, SSEP amplitude, SSEP latency, and hospital LOS did not differ between the REMI group and either the MAG or MET groups. The MET group did not differ from the REMI group in times to eye opening, following commands, or tracheal extubation; while in the MAG group, times to eye opening and following commands were approximately 1 min shorter than in the REMI group (Table 3).
Discussion
In the current study, we evaluated the intraoperative and postoperative effects of the administration of either methadone or magnesium on the basic intraoperative regimen of desflurane–remifentanil. In that context, the only differences we noted in the current study were between the REMI and MET groups with no difference in the MAG group. With the administration of methadone after anesthetic induction, the total perioperative hydromorphone requirements were lower and, intraoperatively, we noted a decrease in both the remifentanil requirements, as well as labetalol requirements to maintain the desired MAP to provide controlled hypotension. The controlled hypotension is utilized as part of our standard intraoperative technique to limit allogeneic transfusion requirements. When considering the effects of methadone on perioperative hydromorphone requirements, the clinical impact was primarily noted intraoperatively, as hydromorphone was administered in the operating room based on respiratory rate prior to tracheal extubation. The hydromorphone requirements were approximately half that of patients who did not receive methadone. We would postulate that the effect of methadone may have been related primarily to its analgesic effects, thereby limiting intraoperative and postoperative opioid needs directly, or a secondary effect related to a decrease in intraoperative remifentanil requirements, and hence blunting of remifentanil-induced hyperalgesia.
One interesting yet difficult to explain finding was that time to eye opening as well as time to tracheal extubation were more rapid in patients who received magnesium when compared to the remifentanil group. No difference was noted when compared to the methadone group. While both of these endpoints met statistical significance, the findings were of limited clinical significance as the average time difference was only 1.1 min and 1.8 min, respectively. As there was no difference in intraoperative anesthetic requirements for remifentanil and desflurane between the two groups, we are unable to postulate a physiologic mechanism for this effect and it may be a chance finding related to the sample size of the study.
Postoperatively, we noted no difference in the hydromorphone requirements during the PACU stay or the initial 24 postoperative hours among the study groups. Although there was no statistically significant difference in PACU dosing, the overall perioperative dosing (OR + PACU) was less with methadone. A larger study might have provided stronger support for a difference in opioid consumption in PACU between the MET and REMI groups. However, it is important to note that no difference with the addition of methadone or magnesium was found, based on pain scores. The intraoperative hemodynamic control was more stable in patients receiving methadone, as we noted decreased intraoperative remifentanil requirements as well as a decrease in the need for supplemental agents to maintain MAP at the desired range of 55–65 mmHg. The decrease in the intraoperative remifentanil requirements may be clinically efficacious as higher intraoperative infusion rates of remifentanil may accelerate acute tolerance and further increase postoperative analgesic requirements [23].
Despite its efficacy in providing intense analgesia and yet allowing for rapid awakening, concerns have been expressed over the possible hyperalgesia which may result from the intraoperative use of remifentanil. While it has been postulated that this may be mediated through the NMDA system, limited impact has been demonstrated in various clinical settings with the intraoperative administration of ketamine [12, 13, 24]. Therefore, in the current study, we chose to evaluate the potential impact of two agents which may have effects as NMDA antagonists including magnesium and methadone. One critique of the study design that might impact our finding is the dose of methadone that was selected. The chosen dose was on the lower end of the dosing scale as we did not want to impact postoperative awakening and the goal in our study was to use methadone as an adjunctive agent, focusing on its effects primarily at the NMDA receptor. Higher doses (0.2–0.25 mg/kg) may impact the findings and improve the postoperative impact of this agent. Furthermore, lower doses (0.025–0.05 mg/kg) may impact the NMDA system while limiting the impact on postoperative opioid requirements related to opioid receptor effects. A future trial may be indicated focusing specifically on methadone dosing in this patient population. The dose of magnesium chosen, both for the bolus and infusion therapy, was the same dose used in previous adult studies for patients undergoing major orthopedic surgeries involving the lumbar spine [17]. As with methadone, larger doses may impact the analgesic effects noted with magnesium, but given the effects of magnesium on the neuromuscular junction, these larger doses may also impact MEP monitoring. We have anecdotally noted the brief loss of MEP signals following the administration of a bolus dose of magnesium in a similar clinical scenario. Consistent with the aforementioned adult study, we did not measure magnesium levels. Furthermore, missing data may have affected the precision of some of our estimates; for example, 5 patients (8%) were missing data on the primary outcome of opioid consumption at 1 or more of the study time points.
In summary, with the dosing regimens described in the current study, no clinically significant benefit was noted with the intraoperative administration of magnesium during a desflurane–remifentanil anesthetic for posterior spinal fusion in adolescents. Remifentanil is frequently chosen during neurophysiological monitoring given its lack of context-sensitive half-life and the resultant rapid dissipation of its effects when the infusion is discontinued. These properties, when combined with desflurane, allow for the performance of a wake-up test should irreversible changes be noted on neurophysiological monitoring. However, the hyperalgesia that occurs may be problematic during the immediate postoperative period as the patient emerges from anesthesia where increased pain and opioid requirements may be seen. The current study demonstrates that the administration of methadone may mitigate these effects. The intraoperative administration of methadone in a dose of 0.1 mg/kg resulted in decreased intraoperative requirements for hydromorphone during emergence from anesthesia and improved intraoperative hemodynamic control while decreasing intraoperative remifentanil and labetalol requirements. However, outside of the immediate postoperative period, there was no clinically or statistically significant difference in the clinical course during the postoperative period. We would suggest that future investigations may be warranted to evaluate dose-escalations of methadone in the same or similar clinical settings. Given the potential for hyperalgesia with the intraoperative use of remifentanil, the adjunctive use of methadone may be warranted in this patient population.
References
Padberg AM, Bridewell KH. Spinal cord monitoring: current state of the art. Orthop Clin North Am. 1999;30:407–33.
Intraoperative Neurophysiology Committee. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1990;40:1644–6.
Clapcih AJ, Emerson RG, Roye DP Jr, Xie H, Gallo EJ, Dowling KC, Ramnath B, Heyer EJ. The effects of propofol, small-dose isoflurane, and nitrous oxide on cortical somatosensory evoked potential and bispectral index monitoring in adolescents undergoing spinal fusion. Anesth Analg. 2004;99:1334–40.
Imani F, Jafarian A, Hassani V, Khan ZH. Propofol–alfentanil vs. propofol–remifentanil for posterior spinal fusion including wake-up test. Br J Anaesth. 2006;96:583–6.
Kim WH, Lee JJ, Lee SM, Park MN, Park SK, Seo DW, Chung IS. Comparison of motor-evoked potentials monitoring in response to transcranial electrical stimulation in subjects undergoing neurosurgery with partial vs no neuromuscular block. Br J Anaesth. 2013;110:567–76.
Martin DP, Bhalla T, Thung A, Rice J, Beebe A, Samora W, Klamar J, Tobias JD. Volatile agents or total intravenous anesthesia for neurophysiological monitoring during posterior spinal fusion in adolescents with idiopathic scoliosis. Spine. 2014;39:E1318–24.
Sammartino M, Garra R, Sbaraglia F, De Riso M, Continolo N. Remifentanil in children. Paediatr Anaesth. 2010;20:246–55.
Crawford MW, Hickey C, Zaarour C, Howard A, Naser B. Development of acute opioid tolerance during infusion of remifentanil for pediatric scoliosis surgery. Anesth Analg. 2006;102:1662–7.
Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance. Anesthesiology. 2000;93:409–17.
Komatsu R, Turan AM, Orhan-Sungur M, McGuire J, Radke OC, Apfel CC. Remifentanil for general anaesthesia: a systematic review. Anaesthesia. 2007;62:1266–80.
Sharma S, Balireddy RK, Vorenkamp KE, Durieux ME. Beyond opioid patient controlled analgesia: a systemic review of analgesia after major spine surgery. Reg Anesth Pain Med. 2012;37:79–98.
Zhao M, Joo DT. Enhancement of spinal n-methyl-d-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 2008;109:308–17.
Englehardt T, Zaarour C, Naser B, Pehora C, de Ruiter J, Howard A, Crawford MW. Intraoperative low dose ketamine does not prevent a remifentanil-induced increase in morphine requirement after pediatric scoliosis surgery. Anesth Analg. 2008;107:1170–5.
McDonnell C, Zaarour C, Hull R, Thalayasingam P, Pehora C, Ahier J, Crawford MW. Pre-treatment with morphine does not prevent the development of remifentanil-induced hyperalgesia. Can J Anesth. 2008;55:813–18.
Elsharnouby NM, Elsharnouby MM. Magnesium sulphate as a technique of hypotensive anaesthesia. Br J Anaesth. 2006;96:727–31.
Altan A, Turgut N, Yildiz F, Türkmen A, Ustün H. Effects of magnesium sulphate and clonidine on propofol consumption, haemodynamics and postoperative recovery. Br J Anaesth. 2005;94:438–41.
Levaux Ch, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intraoperative magnesium sulphate on pain relief and patient comfort after major orthopaedic surgery. Anaesthesia. 2003;58:131–5.
Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulfate for postoperative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand. 2009;53:1088–91.
Na HS, Lee JH, Hwang JY, Ryu JH, Han SH, Jeon YT, Do SH. Effects of magnesium sulphate on intraoperative neuromuscular blocking agent requirements and postoperative analgesia in children with cerebral palsy. Br J Anaesth. 2010;104:344–50.
Stemland CJ, Witte J, Colquhoun DA, Durieux ME, Langman LJ, Balireddy R, Thammishetti S, Abel MF, Anderson BJ. The pharmacokinetics of methadone in adolescents undergoing posterior spinal fusion. Paediatr Anaesth. 2013;23:51–7.
Sharma A, Tallchief D, Blood J, Kim T, London A, Kharasch ED. Perioperative pharmacokinetics of methadone in adolescents. Anesthesiology. 2011;115:1153–61.
Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. 2011;112:218–23.
Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Remifentanil-acute opioid tolerance and opioid-induced hyperalgesia: a systematic review. Am J Ther. 2015;22:e62–74.
Perelló M, Artés D, Pascuets C, Esteban E, Ey Batlle AM. Prolonged perioperative low-dose ketamine does not improve short and long-term outcomes after pediatric idiopathic scoliosis surgery. Spine. 2017;42:E304–12.
Funding
This project was supported by The Clinical and Translational Intramural Funding Program through the Research Institute at Nationwide Children’s Hospital (Columbus, Ohio).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no other funding or conflict of interest to report.
Ethics
The study was approved by the Institutional Review Board at Nationwide Children’s Hospital (IRB13-00036) and registered at ClinicalTrails.gov (NCT01795495). An investigational new drug (IND) application for this study was approved by The Food and Drug Administration (FDA) prior to study initiation (IND117889).
Additional information
The content is solely the responsibility of the authors and does not necessarily represent the official views of Nationwide Children’s Hospital.
About this article
Cite this article
Martin, D.P., Samora, W.P., Beebe, A.C. et al. Analgesic effects of methadone and magnesium following posterior spinal fusion for idiopathic scoliosis in adolescents: a randomized controlled trial. J Anesth 32, 702–708 (2018). https://doi.org/10.1007/s00540-018-2541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-018-2541-5