Abstract
Purpose
Transesophageal Doppler (TED)-guided intraoperative fluid therapy has shown to noninvasively optimize intravascular volume and reduce postoperative morbidity. The aim of this study was to compare the effects of Doppler-guided intraoperative fluid administration and central venous pressure (CVP)-guided fluid therapy on renal allograft outcome and postoperative complications.
Methods
A prospective nonrandomized active controlled study was conducted on end-stage renal disease patients scheduled for living donor renal transplant surgery. 110 patients received intraoperative fluid guided by corrected flow time (FTc) and variation in stroke volume values obtained by continuous TED monitoring. Data of 104 patients in whom intraoperative fluid administration was guided by CVP values were retrospectively obtained for a control.
Results
The amount of intraoperative fluid given in the study group (12.20 ± 4.24 ml/kg/h) was significantly lower than in the controls (22.21 ± 4.67 ml/kg/h). The amount of colloid used was also significantly less and fewer recipients were seen to require colloid (69 vs 85 %). The mean arterial pressures were comparable throughout. CVP reached was 7.18 ± 3.17 mmHg in the study group. It was significantly higher in the controls (13.42 ± 3.12 mmHg). The postoperative graft function and rate of dysfunction were comparable. Side-effects like postoperative dyspnoea (4.8 vs 0 %) and tissue edema (9.6 vs 2.7 %) were higher in the controls.
Conclusions
FTc-guided intraoperative fluid therapy achieved the same rate of immediate graft function as CVP–guided fluid therapy but used a significantly less amount of fluid. The incidence of postoperative complications related to fluid overload was also reduced. The use of TED may replace invasive central line insertions in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal transplant surgery is the treatment of choice for patients with end-stage renal disease; however, these patients have a 10–30 times higher incidence of cardiovascular diseases [1]. Accelerated atherosclerosis, ischemic heart disease, dilated cardiomyopathy, concentric left ventricular hypertrophy, pericardial effusion and low ejection fraction may be seen. Perioperative cardiac complications are witnessed in 6 % of recipients [2]. There is a narrow margin of safety with intravenous hydration as the patients oscillate between hypovolemia and hypervolemia [3]. These two factors combined make fluid management in these patients highly critical. Traditionally, central venous pressure (CVP)-guided large volumes of fluids are infused intraoperatively. Excessive fluid load is said to decrease the incidence of postoperative acute tubular necrosis (ATN) [4] and delayed graft function (DGF) [5]. The general recommendation is to keep mean arterial pressure (MAP) >90 mmHg [6] and CVP at 12–14 mmHg [1] at the time of reperfusion of the transplanted kidney. However, this approach subjects vulnerable patients to complications of volume overload, pulmonary edema and prolonged requirement of ventilatory support. Recent studies have utilized transesophageal Doppler (TED) in guiding perioperative volume therapy. It is known to optimize intravascular volume and to reduce perioperative morbidity [7, 8]. To the best of our knowledge, this approach has not been utilized for renal transplant surgery to date. This study was designed to observe the effects of intraoperative Doppler-guided fluid administration on renal allograft outcome and postoperative complications.
Methods
This prospective nonrandomized active controlled study was performed after clearance from the Institutional Ethical Committee, registration in the Clinical Trial Registry of India (CTRI/2013/12/004251) and obtaining written informed consent from the patients. The study included patients scheduled for living donor renal transplantation; however, patients undergoing second transplantation and patients re-explored after surgery were excluded.
A detailed history was obtained and preoperative investigations (hemoglobin, coagulation studies, serum electrolytes, plasma proteins, liver function test, chest radiography, electrocardiography and echocardiography) were performed. All patients underwent hemodialysis 24 h before surgery. The procedures were performed under balanced general anesthesia with mechanical ventilation. Induction of anesthesia was carried out with 0.03 mg/kg intravenous (iv) midazolam, 2 µg/kg fentanyl, propofol 1 % as required by the patient, and 0.5 mg iv atracurium. The trachea was intubated with an appropriate size PVC cuffed endotracheal tube. A 7.5-F triple lumen central venous catheter was inserted with full aseptic precautions either in the right or left internal jugular vein. Monitoring included electrocardiogram, invasive arterial blood pressure, CVP, pulse oximetry, capnography, core temperature and urine output. A 14-F esophageal Doppler probe (Cardio Q™, Deltex Medical Ltd, Chichester, UK) was introduced orally after intubation and positioned approximately 35–40 cm from the teeth. The patient’s age, height and weight were entered into the monitor and stroke volume (SV), cardiac output (CO) and corrected flow time (FTc) were noted. Maintenance of anesthesia was performed with isoflurane (0.4–0.8 %) + air + oxygen + intermittent atracurium boluses. Analgesia was obtained with hourly 1 µg/kg fentanyl. At the end of surgery, isoflurane was discontinued and muscle relaxation reversed. Patients were extubated when the following criteria were met (institute policy)—eye opening and protrusion of tongue on command, tidal volume ≥5 ml/kg, respiratory rate ≤30/min, maintaining peripheral oxygen saturation (SpO2) >94 % on fractional inspiratory oxygen (FiO2) 0.4, end-tidal carbon-dioxide (etCO2) in the range of 36–40 mmHg, and powerful hand grip present [9]. The patients were transferred to the Kidney Transplant Unit (KTU) for further care. Monitors were attached to the patient and SpO2 was continuously monitored. The patients were kept on room air. Oxygen was delivered with a face mask at FiO2 0.4 if SpO2 went <92 %.
Fluid management
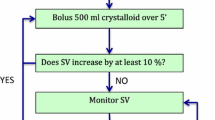
The patients were given normal saline at 10 ml/kg/h until completion of vascular anastomosis, following which urine output replacement was performed until the end of surgery. In addition, the FTc value was observed every 15 min. If the value was <350 ms, 250 ml of hydroxyethyl starch was administered and variation in SV was noted. If the variation in SV was <10 %, normal saline infusion was continued and fresh readings taken every 15 min. Fluid therapy was continued normally if FTc was >350 ms (Fig. 1). Fluid given was calculated as total fluid given, including blood products and colloids, before reperfusion of the graft, divided by the patient’s body weight and duration from initiation of anesthesia until allograft reperfusion (in hours). The trigger for blood transfusion was hemoglobin <7 g/dl. Fresh frozen plasma was given at the discretion of the anesthetist due to increased oozing from the tissues. Platelets were administered to patients with preoperative platelets <80,000 × 109/l.
It is institutional policy to keep MAP >90 mmHg at the time of graft reperfusion and thereafter. IV boluses (6 mg) of mephentermine were given when required to maintain MAP intraoperatively. In the event of a postoperative drop in MAP to <90 mmHg, fluid intake and urine output charts were seen to ensure positive fluid balance and noradrenaline infusion was then initiated at 0.05 µg/kg/min and titrated onwards to maintain MAP.
All the living kidney donors underwent nephrectomy under general anesthesia. They received 1,500 ml normal saline and 1,500 ml Ringer’s solution, supplemented by crystalloid titrated to match the urine output from the start of surgery until the renal vessels were clamped. The harvested kidney was immediately submerged in sterile ice and rinsed with heparin 5,000 IU added to cold University of Wisconsin solution until the venous effluent was clear.
Immunosuppressant medication: all patients received oral tacrolimus 0.12 mg/kg, oral prednisolone 20 mg and mycophenolate mofetil 500 mg tds 2 days prior to the day of surgery. On the morning of surgery, they received basiliximab 20 mg through their iv catheter. 500 mg methylprednisolone iv was given during anastomosis of vessels. Postoperatively, tacrolimus was titrated as per recommended dose levels, prednisolone was tapered to 5 mg od over a period of 3 months and mycophenolate continued. Drug modifications were carried out by the nephrologists in cases of graft dysfunction.
The perioperative CVP, systolic, diastolic, and MAP, CO, SV, and FTc were recorded. The donor parameters, human leukocytes antigen (HLA) typing and glomerular filtration rate (GFR) of donor kidneys were also noted. After extubation, all the patients were assessed for tissue edema in the face, eyelid and conjunctiva [10]. The parameters of renal graft function, serum creatinine levels on days 0, 1, 2, 4, 7, 10 and at 1, 3 and 6 months as well as urine output until completion of surgery and on days 0, 1, 2, 7 and 10 were recorded. Patients were divided into four groups post transplant—(i) normal graft function, (ii) slow graft function (SGF), (iii) DGF, and (iv) patients with immediate good graft function but a rise in creatinine was observed at 1-month follow-up after transplant. SGF was defined as plasma creatinine level >3 mg/dl and no dialysis within 1 week of transplant [11]. DGF was defined as requirement of dialysis in the first week post transplant [5]. A renal biopsy was performed in all patients with graft dysfunction. Acute rejection (ARE) was defined according to the 2007 Banff criteria [12]. ATN was defined as urine output <400 ml/day and exclusion of other causes by allograft biopsy [13].
For comparison, the data of living donor renal transplant recipients, operated on from June 2011 to May 2012, were collected in the same format via use of the hospital information system and perioperative records. CVP-guided intraoperative fluid therapy was used in these patients. Normal saline and hydroxyethyl starch was given at the discretion of the anesthetist. The fluid intake maintained CVP >15 mmHg at the time of clamp opening and thereafter. The criteria for blood product administration, extubation and use of oxygen in the postoperative period were similar to that in the study group. Information about the presence of visible tissue edema, and requirement of supplementary oxygen in the postoperative period was obtained from perioperative and KTU records.
Postoperatively, 100 % replacement of hourly urine output was performed in the first 48 h and then 80 % replacement of daily urine output until discharge. Postoperative fluid management was similar in both groups.
The aim was to evaluate the effect of Doppler-guided intraoperative fluid management on renal allograft function and register the incidence of SGF, DGF and postoperative ATN, and other postoperative complications. In an observational study, Feriss et al. [13] showed ATN in 28 of 77 patients (36.4 %). Taking a power of 90 % and α = 0.05, a sample size of 60 was required in each group to reduce the incidence of ATN to 10 %.
Statistical analysis was performed using SPSS software version 16 (Chicago, USA). Continuous variables are presented as mean ± SD and categorical values as percentages. Intergroup differences between demographic profile, perioperative hemodynamic values, volume of fluid used, donor profile, urine output and laboratory values were compared using the unpaired Student t test. For categorical values, chi-squared test was used. Z test was applied to see if the proportion of side-effects and type of graft dysfunction in both groups were statistically significant. Binary logistic regression (multivariate) was used to identify cumulative effects of factors affecting graft function (graft dysfunction was taken as a dependent factor). Statistical significance was defined as p < 0.05.
Results
This study was conducted from June 2012 to May 2013. 118 living donor renal transplants were registered for the trial. Four patients were re-explored within 5 days after surgery. Four died in the follow-up period due to sepsis. The six-month survival rate was 96.6 %. Data of the remaining 110 patients were noted.
112 living donor renal transplants conducted a year earlier were used as controls. One patient was re-explored. Three died of sepsis in the follow-up period. Four were cases of second transplant. The six-month survival rate was 95.5 %. Data of the remaining 104 patients were collected and analysed.
The demographic profile of the patients, donors, donor kidney GFR, and clinical data of surgery (Table 1) were comparable in both groups. HLA mismatches were also comparable. Hypertension was the commonest comorbidity with 97/110 patients (88.18 %) in the study group and 88/104 (85 %) in the controls being affected. Diabetes mellitus was seen in 30 (27.2 %) and 32 (30.76 %) in the study group and controls, respectively. Associated coronary artery disease was seen in 22/110 (20 %) patients in the study group and 20/104 (19 %) in the controls. Preoperative pleural effusion was present in 2/110 (1.8 %) in the study group and 1/104 (0.09 %) in the controls. Dilated cardiomyopathy was seen in 3 patients in each group, i.e., 2.7 % in the study group and 2.8 % in the controls. Echocardiographic findings showed left ventricular hypertrophy in 81/110 (73.6 %) in the study group and in 77/104 (74 %) in the controls. Left ventricular ejection fraction <50 % was seen in 26/110 (23.6 %) in the study group and 25/104 (24 %) in the controls. Pulmonary artery hypertension >35 mmHg was seen in 6/110 (5.4 %) in the study group and 4/104 (3.8 %) in the controls. Mean preoperative hemoglobin was 7.5 g/dl (6.1−10.5) in the study group and 7.2 g/dl (6.3−11.0) in the controls. Mean preoperative creatinine was 4.99 ± 1.32 and 4.83 ± 1.48 mg/dl in the study group and the controls, respectively. There was no significant difference in renal ischemia time (55 ± 18 min in the study group and 52 ± 20 min in the controls).
Intraoperative fluid given in both the groups before renal allograft reperfusion was 12.20 ± 4.24 ml/kg/h in the study group and 22.21 ± 4.67 ml/kg/h in the controls (p = 0.001). Only normal saline was used as crystalloid in both groups. The colloid used was hydroxyethyl starch. The use of colloid was observed in 76 patients (69 %) in the study group but was significantly higher in the controls (89; 85.5 %) (Z = −2.86, p = 0.004). The total amount of colloid used was also significantly less in the TED-guided group 6.85 ± 4.0 ml/kg than in the controls (8.45 ± 6.57 ml/kg) (t = 2.16, p = 0.03). Intraoperative blood loss was similar in the study group (535 ± 422 ml) and the controls (500 ± 450 ml). The use of blood products was also comparable.
Figure 2 shows intraoperative changes in systolic, diastolic, MAP and CVP. The MAP at reperfusion in the study group was 108.79 ± 9.31 mmHg while that in controls was 110.62 ± 10.5 mmHg. There was significant intergroup difference (p = 0.001) in CVP values in the study group and the controls at clamp application (8.82 ± 2.84 vs 14.0 ± 3.1 mmHg), reperfusion (7.18 ± 3.17 vs 13.42 ± 3.12 mmHg) and at the end of surgery (6.0 ± 3.27 vs 12.5 ± 3.5 mmHg).
The readings of TED, namely FTc, SV and CO in the study group are shown at four different time points (Table 2). FTc was constantly maintained >330 ms and a rising trend was seen in mean SV and CO.
The renal functions as assessed by urine output and creatinine levels were comparable in both the groups (Fig. 3). Renal allograft dysfunction was seen in 32.7 % in the study group and 31.7 % in the controls. The proportion of type of graft dysfunction was similar in both the groups (Table 3). Renal functions eventually recovered spontaneously or with treatment in all patients except 2 (1.7 %) in the study group and 3 in the controls (2.6 %) who were discharged as dialysis-dependent. The incidence of permanent graft failure was also comparable (p = 0.89).
The incidence of complications and the requirement of vasopressors in the immediate post-reperfusion period as well as later are shown in Table 4. Continued postoperative ventilation was required in 1.8 % of the study group. It was higher although not significantly in the controls (4.8 %, p = 0.22). In all patients requiring postoperative ventilation, the patients were unable to maintain SpO2 >94 % at FiO2 0.4 despite following commands. The ratio between partial pressure of oxygen and inspiratory oxygen was <200. Both the patients in the study group and 3 out of 5 patients in the controls requiring post operative ventilation were patients with preoperative left ventricular ejection fraction <50 %. Post-extubation desaturation in the KTU requiring supplementary oxygen with a face mask at FiO2 0.4 was seen in 5 of 104 patients (4.8 %) in the controls while no such incidence was observed in the study group (p = 0.019). Post-extubation visual tissue edema, assessed by the anesthetist extubating the patients, was also significantly higher in the controls (9.6 %) compared to the study group (2.7 %).
Multivariate logistic regression was applied considering graft dysfunction as a dependent variable. Independent variables were recipient’s age, sex, months of dialysis, duration of surgery, amount of fluid given in ml/kg/h, donor age, donor HLA, donor GFR, MAP and CVP at clamp opening, and requirement of vasopressor and use of blood products. MAP at clamp opening [HR 1.12 (95 % CI 1.00–1.26), p = 0.04] and donor age [HR 1.29 (95 % CI 1.06–1.57), p = 0.01] were found to be independent factors affecting graft function. The effect of HLA matching was determined by assigning discrete number values for different degrees of HLA mismatch. Chi-squared for association of HLA mismatch with graft dysfunction was 24 (p = 0.004).
Discussion
Proper perioperative fluid management is one of the most important aspects governing hemodynamic functions in any surgical patient. A transplanted kidney is denervated and lacks autoregulation, thus making the role of proper hydration and perfusion even more important.
Isotonic crystalloids are preferred as the intraoperative fluid of choice [14]. Different crystalloid solutions have been used for volume restoration and plasma expansion during kidney transplantation [15–17]; however, normal saline is still most commonly used [18]. Our intraoperative fluid of choice was normal saline. Fluid administration of kidney donors should also be generous (10–20 ml/kg/h) [14]. The same hydration policy was used in this study. Mannitol 20 % was given intravenously to the recipients immediately before allograft reperfusion as it is known to confer protection against cortical ischemia and decrease the incidence of post-transplant dialysis; however, it has no effect on long-term graft function [19].
In the present study, TED-guided SV and FTc values were utilized for intraoperative fluid management. Doppler ultrasound technology analyzes blood flow in the descending aorta. Integrating the velocity–time curve gives the distance travelled by the blood following cardiac systole and multiplying this by the cross-sectional area (estimated by a nomogram) derives SV and CO [20]. The systolic flow time is corrected for heart rate using a modification of Bazett’s equation [21]. The FTc so obtained indicates the preload. An FTc in the descending aorta of 330–360 ms is generally considered normal [22]. A shortened FTc indicates either hypovolemia or peripheral vasoconstriction. If a bolus of fluid fails to increase SV by at least 10 %, hypovolemia is less likely despite lowered FTc. In such cases, causes of increased afterload must be looked for [8]. The use of TED to optimize intravascular volume resulted in the administration of significantly less fluid than when CVP alone was used to guide fluid therapy. The amount of colloid used as well as the number of people requiring colloid was also significantly reduced. Despite providing less fluid and reaching lower CVP there was no increase in the rate of ATN, and slow, delayed or permanent graft function.
Campos et al. [23], in a retrospective study of 1,966 renal transplants, found that the mean CVP at clamp opening and its variations showed no impact on graft function. This may explain adequate graft function despite low CVP in our study group. Administration of whole blood and FFP transfusions are considered to be associated with higher ARE and chronic graft dysfunction [24]. However, in our study no such association was observed. Multivariate logistic regression analysis showed donor age and intraoperative MAP to influence graft dysfunction and HLA mismatching was shown to have some association. De Gasperi et al. [24] also found significant correlation between donor age and graft failure and Campos et al. [23] showed that MAP ≥93 mmHg was associated with greater graft survival.
The incidence of complications like requirement of postoperative ventilation due to the inability to maintain adequate oxygenation was higher though not significant in controls. This complication was observed more in patients with impaired cardiac functions. The rate of visual tissue edema and post-extubation desaturation was significantly higher. Doppler-guided fluid therapy optimized the amount of intraoperative fluids given and hence reduced the rate of negative effects of fluid overload on cardiorespiratory physiology of these previously compromised patients.
The strengths of this study are the large sample size and comparison with data obtained from patients operated upon by the same surgical team. The medical, surgical, anesthesiological and postoperative management was same in both groups, differing only in intraoperative fluid intake. The limitations of the study are the fact that there was no blinding and the control data were obtained retrospectively. Nevertheless, this study provides rationale to further investigate the possibility of replacing invasive central lines with noninvasive TED to guide fluid administration in renal transplant recipients.
To conclude, FTc and SVvariation-guided fluid therapy protects these high risk patients from unnecessary fluid overload as seen in CVP-guided fluid administration without adversely affecting 6-month post-transplant outcome.
References
Martinez BS, Gasanova I, Adesanya AO. Anesthesia for kidney transplantation: a review. J Anesth Clin Res. 2013;4:270–6.
Humar A, Kerr SR, Ramcharan T, Gillingham KJ, Matas AJ. Peri-operative cardiac morbidity in kidney transplant recipients: incidence and risk factors. Clin Transplant. 2001;15(3):154–8.
Yee J, Parasuraman R, Narins RG. Selective review of key preoperative renal electrolyte disturbances in chronic renal failure patients. Chest. 1999;115(5):149s–57s.
Carlier M, Squifflet JP, Pirson Y, Gribomont B, Alexandre GP. Maximal hydration during anesthesia increases pulmonary arterial pressures and improves early function of human renal transplants. Transplantation. 1982;34(4):201–4.
Bacchi G, Buscaroli A, Fusari M, Neri L, Cappuccilli ML, Carretta E, Stefoni S. The influence of intraoperative central venous pressure on delayed graft function in renal transplantation: a single-center experience. Transplant Proc. 2010;42:3387–91.
Toth M, Reti V, Gondos T. Effect of recipients’ peri-operative parameters on the outcome of kidney transplantation. Clin Transplant. 1998;12:511–7.
Abass SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia. 2008;63:44–51.
Schober P, Loer SA, Schwarte LA. Perioperative hemodynamic monitoring with transesophageal Doppler technology. Anesth Analg. 2009;109:340–53.
Roth R, Chowdhury F, Frost E. Extubation: making the unpredictable safer. Anaesthesiology News Guide Airway management. 2012. pp. 69–74. http://anaesthesiologynews.com/download/Extubation_ANGAM12_WM.pdf. Accessed 12 July 2015.
Othman MM, Ismael AZ, Hammouda GE. the impact of timing of maximal crystalloid hydration on early graft function during kidney transplantation. Anesth Analg. 2010;110(5):1440–6.
Tan JC, Busque S, Scandling J. Clinical management. In: Maarteen WT, Chertow GM, editors. Brenner and rector’s the kidney. 9th ed. Elsevier: Saunders; 2012. p. 2523.
Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–60.
Ferris RL, Kittur DS, Wilasrusmee C, Shah G, Krause E, Ratner L. Early haemodynamic changes after renal transplantation: determinants of low central venous pressure in the recipients and correlation with acute renal dysfunction. Med Sci Monit. 2003;9(2):61–6.
Othman MM. Perioperative hydration policy, understanding the complexities of kidney transplantation. In: Jorge O editors. 2011. ISBN: 978-953-307-819-9.
O’Malley CMN, Frumento RJ, Bennett-Guerrero E. Intravenous fluid therapy in renal transplant recipients: results of a US survey. Transplant Proc. 2002;34:3142–5.
O’Malley CMN, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, Bennett-Guerrero E. A randomized, double-blind comparison of lactated Ringer’s solution and 0.9 % NaCl during renal transplantation. Anesth Analg. 2005;100:1518–24.
Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effects of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg. 2008;107:264–9.
Khajavi MR, Etezadi F, Moharari RS, Imani F, Meysami AP, Khashayar P, Najafi A. Effects of normal saline vs. Lactated Ringer’s during renal transplantation. Ren Fail. 2008;30:535–9.
Schnuelle P, van der Woude FJ. Perioperative fluid management in renal transplantation: a narrative review of the literature. Transplant Int. 2006;19:947–59.
Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, Fleming SC. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634–42.
Singer M, Bennett ED. Non-invasive optimisation of left ventricular filling by esophageal Doppler. Crit Care Med. 1991;9:1132–7.
Laupland KB, Bands CJ. Utility of esophageal Doppler as a minimally invasive hemodynamic monitor: a review. Can J Anaesth. 2002;49:393–401.
Campos L, Parada B, Furriel F, Castelo D, Moreira P, Mota A. Do intraoperative hemodynamic factors of the recipient influence renal graft function? Transplant Proc. 2012;44(6):1800–3.
De Gasperi A, Narcisi S, Mazza E, Bettinelli L, Pavani M, Perrone L, Grugni C, Corti A. Perioperative fluid management in kidney transplantation: is volume overload still mandatory for graft function? Transplant Proc. 2006;38(3):807–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
About this article
Cite this article
Srivastava, D., Sahu, S., Chandra, A. et al. Effect of intraoperative transesophageal Doppler-guided fluid therapy versus central venous pressure-guided fluid therapy on renal allograft outcome in patients undergoing living donor renal transplant surgery: a comparative study . J Anesth 29, 842–849 (2015). https://doi.org/10.1007/s00540-015-2046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-015-2046-4