Abstract
Purpose
We conducted a retrospective study to evaluate the efficacy and duration of pain relief for idiopathic trigeminal neuralgia (TN) patients after continuous radiofrequency (CRF) combined with pulsed radiofrequency (PRF) treatment of the Gasserian ganglion (GG).
Methods
Twenty-one patients were treated with pulsed RF for 6 min repeated after rotating the needle tip by 180°, at a pulse width of 10 ms and at 45 °C. This was followed by conventional RF at 60 °C for 60 s, repeated after needle rotation by 180°, then finally at 65 °C for 60 s also repeated after needle rotation by 180°. Patients were assessed for pain intensity and consumption of analgesics at baseline and 7 days, 1 month, 6 months, and 12 months after the procedure. The patients’ global impression of change (PGIC) scale was also assessed 7 days, 1 month, 6 months, and 12 months after the procedure. The incidence of facial dysthesia was evaluated 7 days after the procedure.
Results
Excellent pain relief was achieved for 15 of 21 patients (71.4 %) after 1 week, 1 month, and 6 months. and for 14 of 21 patients (66.7 %) after 12 months. Consumption of analgesics was significantly reduced for more than 6 months, and for fifteen patients the PGIC scale result was very much improved 12 months after the procedure compared with baseline. Eighteen of the 21 patients (85.7 %) experienced facial dysthesia 1 week after the procedure.
Conclusion
Excellent pain relief and reduced consumption of analgesics for more than 6 months were observed in patients who received PRF combined with CRF to the GG for treatment of idiopathic TN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout the world, trigeminal neuralgia (TN) is regarded as the worst pain among many facial pain patients [1]. The pathophysiology is still unproved, but TN occurs mostly because focal demyelination at the root entry zone of blood vessels or tumors results in aberrant depolarization, ectopic changes, and ephaptic transmission [2]. TN is commonly attributed to vascular anomalies, with 80 % of cases involving the superior cerebellar artery (SCA), cerebello-pontine angle tumors, multiple sclerosis (MS) (2–4 % of MS patients suffer from TN), trigeminal schwannoma, medial sphenoid wing meningioma, or secondary to facial trauma or surgery (trigeminal neuropathy) [3]. Treatment of TN is either conservative or interventional. Pharmacotherapy with carbamazepine is tried early in cases of TN. Carbamazepine may reduce symptoms in 70 % of cases but with many side effects [2, 4]. Other medications with similar efficacy include oxycarbazepine, gabapentin, pregabalin, baclofen, valproate, clonazepam, phenytoin, and lamotrigime [5]. Oxycodone has proved to be an efficacious opioid for treatment of neuropathic pain and is used to treat resistant cases of TN [6]. Interventional therapy for TN varies, is of variable efficacy and safety, and results in different periods of time before recurrence of symptoms. The most clinically appropriate maneuvers include: surgical microvascular decompression (MVD), stereotactic radiation therapy: gamma knife (SGK), percutaneous balloon microcompression, percutaneous glycerol rhizolysis, percutaneous radiofrequency (RF) of the gasserian ganglion, and gasserian ganglion stimulation and/or neuromodulation [2, 7]. Of these options only pulsed RF and MVD are regarded as restorative methods that palliate pain while preserving the neural tissues [7]. According to many reports, MVD is most likely to result in a successful cure [8]; it does, however, involve open craniotomy with associated mortality and morbidity and cannot be tolerated by elderly patients or those with co-morbidity. Recently pulsed radiofrequency (PRF) has proved to be of high clinical value for treatment of chronic pain; it is a safe, minimally invasive, and simple technique [9]. Some clinical trials have found PRF to be reasonably efficacious in treatment of TN [10] whereas others have reported it as an ineffective measure compared with conventional RF [11]. The effect on idiopathic TN of recent types of PRF, for example with the newest guidance techniques, 3D computed tomography (CT) [7], or modified of PRF neuromodulation [12], have been investigated.
In this retrospective study, modified PRF combined with conventional thermocoagulative RF, was used in an attempt to combine the safety and efficacy of neuromodulation with the known long term efficacy of neuroablation for patients with idiopathic TN.
Patients and methods
This study was conducted in Shaalan surgery center, Mohandsen, Cairo, from January 2011 to March 2014. Approval was obtained from the local ethics committee before retrospective collection of patient’s data. Twenty-one subjects with TN, of either sex, at least 18 years of age were patients:
-
1
with paroxysmal pain ranging from a fraction of second to minutes affecting one or more of the three divisions of the fifth cranial nerve, diagnosed in accordance with the International Headache Society [13] and with a visual analogue score (VAS) for pain of at least 7 or more for a minimum of 3 months;
-
2
with a stable analgesic regimen for 2 weeks (consisting of at least two analgesics, including anticonvulsants); and
-
3
examined by use of MRI of the brain to exclude secondary neuralgia.
Patients with local infection at the needle puncture, coagulopathy, severe mental or psychiatric disorders or a history of drug abuse, high intracranial tension and history of MVD, SGK, balloon compression, RF treatment, or glycerol injection were excluded from the study. The possibility of vascular loop compression and other causes of TN was excluded, both clinically and by MRIA, by the neurosurgical consultant.
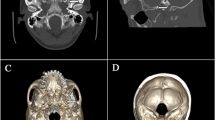
In the operating theater, standard monitors (ECG, noninvasive blood pressure monitoring, and pulse oximetry) were connected to the patient, and O2 was administered via a nasal prong. The patient was placed in the supine position with slight hyperextension of the neck to facilitate the submental view by fluoroscopy. Conscious sedation was complemented by use of fentanyl, 1 μg/kg, and midazolam, 0.05 mg/kg, after 5 min and 2 min; respectively, before local anesthesia infiltration at the site of puncture, and by propofol, 0.75 mg/kg, shots during the needle journey through foramen ovale and during TRF periods. After proper sterilization of the skin and draping, the classic Hartle technique was used to reach the Gasserian ganglion. Fluoroscopy was adjusted in the submental view (caudocranial by 30°–50°) with slight obliqueness (10°–30°) to visualize the foramen oval at the inner side of the mandibular ramus of the affected side. The site of needle entry was 2–3 cm lateral to the angle of the mouth. The RF needle (Neurotherm 22–100–5 mm active tip, curved) was inserted after injection of lidocaine 1 % infiltration. The tunnel view technique for the needle path was tried, aiming at the pupil in anterior view and the mid-zigoma in lateral view. The RF needle passed end-on (Fig. 1) until a depth of 5–7 cm, then a dead lateral view of the skull base was used to verify the position of the needle tip at the basal part of the mid cranial fossa. The proper needle tip position was just past the angle made by the clivius with the petrosal ridge of the temporal bone.
Sensory stimulation with the RF equipment was conducted and parathesia of the affected branch was achieved at 0.1–0.3 V (50 Hz), keeping in mind that the mandibular part of the Gasserian ganglion is ventrolateral and the ophthalmic rootlets are postrolateral. Motor stimulation (2 Hz) was achieved with masseter contraction at 0.1–0.3 V. After sensory and motor stimulation, RF therapy was started by use of the RF generator (Neurotherm 1100), in the sequence:
-
first, pulsed RF for 6 min repeated after rotating the needle tip by 180°, at a pulse width of 10 ms and at 45 °C; then
-
conventional RF at 60 °C for 60 s (twice after needle rotation by 180°); and, finally
-
conventional RF at 65 °C for 60 s (also twice).
All patients were transferred to the recovery room, vital signs were monitored, and ice packs were applied to the patients’ faces to reduce facial ecchymosis. Age, sex, branch affected, VAS, PGIC scale (patients’ global impression of change) [14], and consumption of analgesics (pre-procedure and post-procedure) after 1 week, 1 month, 6 months, and 12 months were recorded. Less than 50 % improvement in VAS was regarded as unsatisfactory block; 50–80 % improvement of VAS was regarded as satisfactory block; more than 80 % improvement in VAS was regarded as excellent pain relief. Adverse effects, for example facial hematoma, pain at puncture site, dysthesia, hypothesia/anesthesia, anesthesia dolorosa, masseter weakness, corneal anesthesia and lost corneal reflex, keratitis, CSF rhinorrhea, caverno-carotid fistula, palsies of cranial nerves III, IV, and VI, and meningitis were also recorded.
Statistical analysis
Statistical analysis was performed by use of the software package StatView for Windows, version 4.57 (Abacus Concepts, Berkeley, CA, USA). Results are reported as mean ± SD, median (range), and number. The Wilcoxon signed-rank test was used for analysis of scores, and the paired t test was used for analysis of parametric data. A P value less than 0.05 was regarded as significant.
Results
The patients’ characteristics are listed in Table 1.
There was significant decrease in VAS score 1 day, 1 week, and 1 and 6 months after the procedure compared with pre-procedure (Table 2).
Excellent pain relief was reported for fifteen patients after 1 week, and 1 and 6 months; however, satisfactory pain relief was reported for one of these 12 months after the procedure.
Satisfactory pain relief was reported for four patients after 1 week, and 1, 6, and 12 months after the procedure.
Unsatisfactory pain relief was reported in two patients after 1 week, and 1, 6, and 12 months after the procedure.
Oxycodone consumption was significantly reduced after 1 week, and 1 and 6 months after the procedure compared with pre-procedure. There was significant decrease in carbamazepine consumption after 1 week, and 1, 6, and 12 months after the procedure compared with pre-procedure (Table 2).
According to the PGIC, very much improvement was observed for 15 patients, much improvement for three patients, minimum improvement for one patient, and no change for two patients 12 months after the procedure.
Two cases of hematoma and two cases of bradycardia, which were treated with atropine sulfate 0.01 mg/kg, were recorded. The incidence of facial dysthesia was 85.7 % (18 patients) in the first week. Masseter weakness occurred for one patient and was obvious after 1 month. No cases of anesthesia dolorosa, infection or cranial nerve palsies were reported.
Discussion
Trigeminal neuralgia is relatively a common cause of orofacial pain [15]. There is no accurate information about its prevalence in Egypt, China, and many other countries.
The only strong opioids with possible efficacy against neuropathic pain such as TN are oxycodone and methadone [6]. Oxycodone, only, is available in our country and was used for selected patients in our study if the pain was severe (VAS ≥ 7).
RF therapy is either PRF or RFTC. RFTC interrupts the nociceptive impulses by thermocoagulating the neural tissues; possible patient complications are discomfort, motor weakness, and deafferentation pain. RFTC has been used over 30 years and the technique is well defined and adjusted [7]. Results supporting use of RFTC are abundant, and include the Erdine study (prospective, double blind, comparing PRF with RFTC, 20 patients in each group). The study reported that RFTC is an effective technique for TN, in contrast with PRF which is almost ineffective [11]. Van Boxem et al. [16] also concluded that RFTC was superior to PRF for management of idiopathic TN.
Kanpolat et al. [17] reported results from a large retrospective study of 1600 patients who underwent RFTC. Early pain relief was observed for 97.6 % of patients, 92 % after 6 months, 57.7 % after 5 years and 41 % after 10 years of follow-up. No mortality was reported. Complications were: pain recurrence for 7.7 % in the early period (less than 6 months) and for 17.4 % in late follow up. Diminished corneal sensation was experienced by 5.7 % of cases, masseter weakness by 4.1 %, dysthesia by 1 %, anesthesia dolorosa by 0.8 %, keratitis by 0.6 %, transient cranial nerves III, IV palsy by 0.8 %, CSF leakage by 2 patients, cortico-cavernous fistula by one patient, and aseptic meningitis by one patient [17]. Raj et al. stated that early success after conventional RF is 97.4–100 %. Complications may be 80 % for facial numbness, 0.3–4 % for anesthesia dolorosa, 7 % for corneal anesthesia, and 24 % for masseter weakness. They limit PRF for TN to post-herpetic neuralgia of cranial V and for treating anesthesia dolorosa after RFTC [18].
Pulsed RF has been described by Sluijter et al. [19]. It is a technique which uses RF oscillating at 2 Hz with voltage output of 45 V, frequency 500 kHz, 20 ms duration, and 480 ms interval. These settings enable the heat “generated during treatment period of 20 ms” to dissipate during the resting interval of 480 ms; thus the needle temperature is kept below 42 °C; there is, therefore, no damage of the neural tissues (neuromodulative not neuroabalative) [19]. The exact central or peripheral neuromodulative effects of PRF are controversial and many theories have been proposed, for example altered C-fos gene expression [20] and long-term suppression of nociceptive A delta and C fibers and sensory ganglia by conditioning stimulation (PRF can stimulate the nerve fibers at the normal rhythm of 1–2 Hz) [21]. Moreover, PRF may disturb microtubules, mitochondria of the afferent axons of C-fibers [22].
Several workers have reported the beneficial effect of PRF for treatment of TN. Van Zundert et al. reported that PRF improved VAS for 3 of 5 patients with idiopathic TN, i.e. 60 % [8]. Despite the small scale of Van Zundert’s work, his results were better than those of the Erdine study, which reported improvement for only 2 of 20 patients with TN, i.e. 10 %, and for 3 months only [11].
Chua et al. reported beneficial results from PRF for 36 patients with TN. The percentage with excellent pain relief (>80 % pain relief) was 73.5 % at 2 months, 61.8 % at 6 months, and 55.9 % at 12 months. The percentage with satisfactory pain relief (50–80 % pain relief) was 14.7 % at 2 months and 17.6 % at 6 and 12 months. No hospitalization was needed for any patient and no complications were reported [12]. A recent study of CT-guided PRF for 20 patients with idiopathic TN reported favorable outcome for 7 of 20 patients (35 %) for a reasonable period of time (1 year) [7].
Recent reports have explained why the efficacy of PRF for treatment of chronic pain, including TN, is underestimated. One theory is that in previous work PRF had been applied for 120 ms only, which was insufficient for good neuromodulatory effects on the Gasserian ganglion, as explained by Tanaka et al. [23] after a study on rats.
Similarly, more beneficial effects of PRF in treatment of TN have been obtained by applying PRF for 6 min at a frequency of 4 Hz and with a pulse width of 10 ms (to propagate the electromagnetic field of the RF current) and performing another PRF session after changing the direction of the RF needle (post-stimulation effect) if more than one branch of the 5th nerve was affected [12]. Another explanation is the need for more precise technique by using a 3D CT guided block after mean motor and sensory stimulation of 0.11 V (i.e. very close to the semilunar Gasserian ganglion) [7].
In our study, we used a new modified and adjusted RF protocol of combined RF (PRF and RFTC). We needed PRF for 6 min at a pulse width of 10 ms and applied twice (i.e. total time of PRF = 12 min) to augment the neuromodulatory changes, especially if more than one branch is affected. This was combined with conventional RFTC to obtain the benefits of the solid, well recognized efficacy of RFTC for treatment of TN [11]. However, we tried to keep the temperature below normal (60–65 °C) to lessen the neuroablative effect of RFTC and hence the possibility of complications. Usually, a higher temperature of RF results in a larger lesion, which results in a lower likelihood of recurrence but a greater risk of dysthesia and anesthesia dolorosa [24]. There is, moreover, a recommendation for RFTC lesion of the ophthalmic division to keep the temperature at 60 °C to preserve the corneal reflex [18].
In our study, excellent pain relief was achieved for 15 of 21 patients (71.4 %) after 1 week, 1 month, and 6 months and for 14 of 21 patients (66.7 %) after 12 months. Satisfactory pain relief was achieved for 4 of 21 patients (19.04 %) after 1 week, 1 month, and 6 months and for 5 of 21 patients (23.8 %) after 12 months. Pain relief was unsatisfactory for 2 patients. Facial dysthesia was experienced by 18 of 21 patients (85.7 %) in the 1st week. Masseter weakness occurred for one patient and was obvious after 1 month. No cases of anesthesia dolorosa, infection, or cranial nerves palsies were observed. Our results are comparable with those of Li et al., who designed a prospective comparative study to show the efficacy and safety of RFTC at 75 °C for 2–3 min and for long periods of 4–5 min and for a 3rd group of PRF at 42 °C for 10 min followed by RFTC at 75 °C for 2–3 min. Pain relief was comparable with that for the 3rd group, with over 70 % of patients in the three groups experiencing significant pain relief and improved quality of life at 12 months post-block. The incidence of facial dysthesia was least in the PRF combined group, indicative of the less neurodestructive effects of RF [25].
In our study, no patients became completely pain-free, which may be because of the low temperature used. For most of the patients, however, pain relief was excellent and hence doses of analgesics were reduced compared with pre-procedure.
Conclusion
Pulsed RF for 6 min with a pulse width of 10 ms at 45 °C repeated after rotating the needle tip by 180° followed by conventional RF at 60 °C for 60 s twice and 65 °C for 60 s twice (which could result in less destruction of the target tissue, which is feared to cause anesthesia dolorosa) results in excellent pain relief for more than 70 % of patients and reduces, for 12 months, consumption of analgesics by patients with idiopathic TN.
Recommendation
Larger scale, prospective work encompassing combined PRF and RFTC, separate PRF and separate RFTC groups may yield more solid and clear data.
References
Weigel G, Kenneth F, Casey M. Striking Back Gainsville: Trigeminal neuralgia association. 2002.
Van Kleef M, Wilco E Van Genderen, Samer Narouz, Turo J Nurmikko, Jan Van Zuder T, Jose W Geurts, Nagy Mekhail. Trigeminal neuralgia: In evedence- based interventional pain medicine: According to clinical diagnosis.In: Jan Van Zundert, Jacob Patijn, Craig T Hartrick, Arno Lataster, Frank JPM Huygen, Nagy Mekhail, Maarten Van Kleef, editors. 1st edn. Wiely; 2012, chap 1, pp 1–7.
Neurmikko TJ, Eldridge PR. Trigeminal neuralgia pathophysiology, diagnosis and current treatment. Br J anesth. 2001;87(1):117–32.
Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev. 2000;(3):CD001133.
Rozen TD. Trigeminal neuralgia and glossopharyngeal neuralgia. Neurol Clin. 2004;22:185–206.
Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405–16.
Fang Luo, Ying Shen, Tao Wang, Meng Lan Yu, Xiaotong Ji Nan. 3D CT guided pulsed radiofreqency treatment for trigeminal neuralgia. Pain Pract. 2014;14(1):16–21.
Koopman JS, de Vries LM, Dielemen JP, Huygen FJ, Stricker BH, Sturkenboom MC. A nation wide study of three invasive treatment of trigeminal neuralgia. Pain. 2011;152:507–13.
Cha NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications—a review. Acta Neurochir (Wien). 2011;153:763–71.
Van Zundert J, Brabant S, Van de Kelft E, Vercruyssen A, Van Buyten JP. Pulsed radiofrequency treatment of the Grasserian ganglion in patients with idiopathic trigeminal neuralgia. Pain. 2003;104:449–52.
Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of the pulsed with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. Eur J Pain. 2007;11:309–13.
Chua NHL, Halim W, Beeme T, Vissers KCP. Pulsed radiofrequency treatment for trigeminal neuralgia. Anesth Pain. 2012;1(4):257–61.
Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders. Cephalagia 2004;24(suppl 1):9–160 (2nd edition).
Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21.
Eyigor C, Eyigor S, Korkmoz OK, Uyar M. Intraarticular corticosteroid injection versus radiofrequency in painful shoulder: a prospective, randomized, single blinded study. Clin J Pain. 2010;26:386–92.
Van Boxen K, Van Eerd M, Brinkhuizen T, Patijin J, Van Kleef M, Van Zundert J. Radiofrequency and pulsed radiofrequency treatment of chronic pain syndrome: the available evidence. Pain Pract. 2008;8(5):358–93.
Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25 years experience with 1600 patients. Neurosrgery. 2001;48(3):524–34.
Erdine S, Racz G and Noe CE. Somatic blocks of the head and neck. In: Raj PP, Lou L, Edrine S, Staats PS, Waldman SD, Racz G, Hammer M, NIv D, Lopez RR, HeavnerJE, editors. Interventional pain management. Image guided procedures. London: Saunders Elservier; 2008, chap 6, pp. 77–107.
Sluijter ME, Cosman E, Rittman W, VanKleef M. The effect of pulsed radiofrequency field applied to the dorsal root ganglion. A preliminary report. Pain Clin. 1998;11:109–17.
Sluijter ME, Racz G. Technical aspects of radiofrequency. Pain Pract. 2002;2:195–200.
Cosman ER, Cosman ER. Electric thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6(6):405–24.
Erdine S, Bilir A, Cosman ER, Cosman ER. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9(6):407–17.
Tanaka N, Yamaga M, Tateyama S, Umo T, Tsumeyoshi I, Takasaki M. The effect of pulsed radiofrequency current on mechanical allodynia induced with resiniferatoxin in rats. anesth Analg. 2010;111(3):784–90.
Kate G, Andrew S, Paul E. Neuralgia: trigeminal and glossopharyngeal. In: key topics in pain medicine, 3rd ed. Informa healthcare 2007;134–140.
Li Xuanying, Ni Jiaxiang, Yang Liqiang, Baishan Wu, He Mingwei, Zhang Xiushuang, Ma Ling, Sum Haiyam. A prospective study of Grasserian ganglion pulsed radiofrequency combined with continuous radiofrequency for the treatment of trigeminal neuralgia. J Clin Neurosci. 2012;19(6):824–8.
Conflict of interest
No funding was received for this work from any of organizations or companies.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ali Eissa, A.A., Reyad, R.M., Saleh, E.G. et al. The efficacy and safety of combined pulsed and conventional radiofrequency treatment of refractory cases of idiopathic trigeminal neuralgia: a retrospective study. J Anesth 29, 728–733 (2015). https://doi.org/10.1007/s00540-015-2029-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-015-2029-5