Abstract
Background
Rocuronium bromide (Rb) is a rapid onset, intermediate-acting neuromuscular blocking agent that is suitable for continuous administration. The appropriate rate of rocuronium administration is, however, difficult to determine due to large interindividual differences in sensitivity to rocuronium. The aim of this study was to clarify whether the simulated rocuronium concentration at the time of recovery to %T1 > 0 % after the initial administration of rocuronium is a good indicator of optimal effect-site concentrations during continuous rocuronium administration.
Methods
Twenty-one patients were anesthetized with propofol. After induction, Rb 0.6 mg/kg was administered intravenously, and nerve stimulation using the single stimulation mode was conducted every 15 s. When %T1 recovered to >0 % after the initial administration of Rb, the effect-site concentration of rocuronium, calculated by pharmacokinetic simulation with Wierda’s set of parameters, was recorded and defined as the recovery concentration (Rb r.c.). The administration rate of rocuronium was adjusted to maintain the Rb r.c. during surgery. Rb administration was discontinued just before the end of surgery, and the recovery time until %T1 > 25 % was recorded. Plasma Rb concentrations were measured at 1 and 3 h after the initiation of continuous Rb administration.
Result
The mean Rb r.c. was 1.56 ± 0.35 μg/ml, with minimum and maximum values of 1.09 and 2.08 μg/ml, respectively. The %T1 did not increase above 10 % in any of the patients during continuous administration of Rb, and the recovery period to %T1 > 25 % ranged from 9 to 29 min. The effect-site concentrations of Rb calculated with Wierda’s parameters significantly correlated with plasma concentrations (P < 0.01) at both 1 and 3 h after the initial administration of Rb.
Conclusion
The results suggest that our method may be one of the most reliable protocols for the continuous administration of Rb described to date for maintaining suitable muscle relaxation during surgery without excessively prolonged effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rocuronium bromide (Rb) is an aminosteroidal, nondepolarizing, intermediate-acting neuromuscular blocking agent. Its metabolites have only 5 % the potency of rocuronium and are almost undetectable in the human body [1], making it clinically useful as a continuous infusion.
Neuromuscular blocking agents are standardly administered intermittently during general anesthesia. However, the continuous administration of drugs provides a more stable blood concentration and is more useful in maintaining patient stability under conditions with varying requirements, such as during surgery. Continuous infusions of rocuronium within a short period do not result in accumulation of the drug [2], and its administration for longer durations in intensive care patients results in only a moderately prolonged recovery time [3].
There are large interindividual differences in the sensitivity to rocuronium due to the many factors which affect the pharmacokinetics and pharmacodynamics of rocuronium, such as age, gender, body weight, skeletal muscle mass, and sensitivity of the neuromuscular receptor. Appropriate titration of rocuronium to the requirements of an individual patient provides sufficient neuromuscular blocking effects while avoiding excessive drug effects. Consequently, it is essential to be able to determine the appropriate rate of administration of rocuronium. The suitability of rocuronium for continuous infusion is well known, but the optimal rate of administration of a continuous infusion of rocuronium has not yet been clearly defined. Several studies have reported that the dose of rocuronium required to induce muscle relaxation does not decrease over time [2, 4, 5]. The differences among individuals in terms of their sensitivity to neuromuscular blocking agents in general and rocuronium in particular may be affected by several factors. Xue et al. reported a gender-related difference in sensitivity to rocuronium, with women being approximately 30 % more sensitive to rocuronium than men [6]. Bevan et al. found that although rocuronium had a similar potency in elderly and young patients, the onset and recovery of neuromuscular blockade were slower in the elderly [7]. Several studies have reported that, in obese patients, the duration of action of rocuronium was significantly prolonged when the drug was infused according to total body weight (TBW) [8, 9]. Moreover, geographical differences in rocuronium potency and duration of action have also been found [10].
The purpose our study was to clarify whether the simulated rocuronium concentration, based on TBW, at the time of recovery to %T1 (first twitch) > 0 % after the initial administration of rocuronium is a good indicator of optimal effect-site concentrations during continuous rocuronium administration. To this end, we investigated continuous administration doses of rocuronium resulting from individual variability under propofol–remifentanil anesthesia, by calculating the relationship between muscle relaxation effects and effect-site concentrations of rocuronium obtained using simulation software.
Materials and methods
This study was approved by the Ethics Committee of Kagoshima University Hospital and registered with the UMIN Clinical Trials Registry (UMIN 000012313) on November 18, 2013. It was conducted in accordance with the principles of the Declaration of Helsinki, and prior written informed consent was obtained from each patient. Twenty-one patients aged 28–77 years with ASA physical status 1–2 who were undergoing elective surgery were enrolled. Patients with renal, hepatic, or neuromuscular diseases were excluded. Patients receiving medications known to interfere with neuromuscular blocking agents, such as antibiotics and anticonvulsants, as well as obese patients [body mass index (BMI) > 30 kg/m2], were also excluded.
All patients fasted overnight and were not premedicated. Electrocardiography, non-invasive blood pressure, pulse oximetry, and bispectral index (BIS; Aspect Medical Systems, Norwood, MA) were monitored upon the arrival of the patient in the operating room. An intravenous catheter was inserted into a forearm vein, and a cannula was inserted into the radial artery under local anesthesia. On the other arm, neuromuscular monitoring was performed using a train-of-four watch (TOF Watch SX; Organon, Osaka, Japan). The monitoring device was stabilized and calibrated according to good clinical research practices described in pharmacodynamic studies of neuromuscular blocking agents [11]. The acceleration transducer was placed in the hand adaptor, and the patient’s fingers were fixed to an arm board.
Anesthesia was induced and maintained with propofol and remifentanil. The rate of propofol infusion was adjusted using a target-controlled infusion pump (TE-371TCI; Terumo Corp., Tokyo, Japan) with a target concentration of 2.5–4.0 μg/ml based on TBW to maintain the BIS value within the range of 40–60. The remifentanil infusion was adjusted in the range of 0.1–0.5 μg/kg/min based on ideal body weight (IBW), depending on surgical invasiveness. After the induction of anesthesia and stabilization of the muscle response to ulnar nerve stimulation, the TOF monitor was calibrated to obtain maximal nerve stimulation. Rb, 0.6 mg/kg of TBW, was administered intravenously, and nerve stimulation using the single stimulation mode at 1 Hz was conducted every 15 s. Tracheal intubation was performed when the first twitch (T1) decreased to 0 %, indicating full neuromuscular block; patients were mechanically ventilated during surgery. The doses of propofol and rocuronium were adjusted according to TBW. During all studies, core temperature of the patient was maintained above 36.0 °C and palm temperature above 32.0 °C using a Bair Hugger forced-air warming device (Arizant Healthcare, Inc., Eden Prairie, MN).
When %T1 increased to more than 0 % in successive measurements after the initial administration of rocuronium, we defined the effect-site concentration of rocuronium by the Wierda pharmacokinetic–pharmacodynamic model [1] as the Rb recovery concentration (Rb r.c.). At this point, continuous administration of rocuronium was started at 7 μg/kgTBW/min, and the administration rate of rocuronium was adjusted to maintain Rb r.c. during surgery. This was achieved by changing the infusion rate of rocuronium in increments or decrements of 1 μg/kg/min every 10 min. The anesthesiologist did not refer to the state of neuromuscular relaxation, as indicated on the neuromuscular monitor, when adjusting the rocuronium infusion rate, and it had been decided a priori to discontinue the experiments when the %T1 increased to >15 % during continuous rocuronium administration. Rocuronium administration was discontinued just before the end of surgery, and the time until recovery of %T1 to >25 % was measured. The plasma concentrations of rocuronium in ten of the 21 patients at 1 and 3 h after the start of continuous rocuronium administration were also measured. Briefly, blood samples were collected from the radial artery and centrifuged for 10 min at 3000 rpm. The separated plasma (samples 2 ml) was added to 1 ml of 0.1 M dibasic sodium phosphate hydrate and stored at −20 °C or lower until the measurement of plasma rocuronium concentrations by liquid chromatography–tandem mass spectrometry according to the method of Valadares de Moraes et al. [12]. We adopted the Wierda pharmacokinetic–pharmacodynamic model to simulate drug pharmacokinetics and compared the correlation between plasma and simulated effect-site concentrations of rocuronium to investigate the efficacy of this simulation method.
Statistical analysis
The results are shown as the mean ± standard deviation (SD). The factors affecting the values of Rb r.c. were examined, and the relationships between Rb r.c. and BMI as well as age were analyzed using both linear and multiple regression analysis. Linear analyses were also performed to assess the correlations between plasma and effect-site concentrations of rocuronium; the relationship between both concentrations was also analyzed by Bland–Altman analysis. P values of <0.05 were considered to be statistically significant.
Results
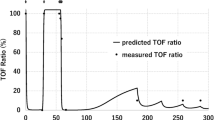
The characteristics of the 21 patients (reported as mean ± SD) are shown in Table 1. Rocuronium was administered continuously at a rate of 6.9 ± 2.8 (range 4–11) μg/kg/min; at this infusion rate, supplemental rocuronium infusion was not needed by any of the patients because there were no episodes of bucking and movement by the patients or requests for intervention from the surgeon due to insufficient neuromuscular blockade. The average rates of continuous administration were derived by calculating the total doses of rocuronium administered and total duration of administration. The distribution of Rb r.c. when the first twitch (T1) was >0 % in all patients is shown in Fig. 1. The Rb r.c. was 1.56 ± 0.35 μg/ml, with minimum and maximum values of 1.09 and 2.08 μg/ml respectively. Individual variations in Rb r.c. were very large, although the distribution was normal. For each patient, the percentage of time during continuous rocuronium administration when the T1 corresponded to values of T1 = 0 % and 0 < T1 < 10 % was 47 ± 40 % and 53 ± 39 %, respectively. T1 did not increase to >10 % during continuous rocuronium administration in any of the patients. Figure 2 shows the recovery period from the time of discontinuation of rocuronium administration until the point when T1 increased to >25 %. Recovery periods ranged from 9 to 29 min, and there was no significant correlation with the duration of rocuronium infusion. There was a significant correlation between Rb r.c. and the BMI of the patients (Fig. 3a). However, patient age did not affect the values of Rb r.c. (Fig. 3b), and there were no significant gender-related differences in the values of Rb r.c. (data not shown). Multiple regression analysis performed to assess the independent determinants of Rb r.c. showed that BMI, but not age, was a variable that significantly correlated with Rb r.c. values (Table 2). The plasma rocuronium concentrations measured in ten of the 21 patients at two time-points during surgery, namely, 1 and 3 h after starting continuous rocuronium administration, were used in a Bland-Altman analysis to investigate the relationship between plasma and effect-site concentrations. Although there was a considerable bias between plasma and effect-site concentrations of rocuronium (Fig. 4a), the simulated effect-site concentrations by the Wierda pharmacokinetic–pharmacodynamic model significantly correlated with plasma concentrations at both 1 and 3 h after the initiation of continuous rocuronium administration (Fig. 4b).
Effect-site concentrations of rocuronium at the time of recovery of %T1 (first twitch) to >0 % (n = 21). Upper part Box-plot of the effect-site concentration of rocuronium where the vertical line in the box represents the mean, and error bars represent the minimum and maximum values. Rb Rocuronium bromide
Time to recovery of the twitch response to %T1 > 25 % after the administration of rocuronium had been discontinued (n = 21). The plot on the left indicates the individual recovery periods in each patient (filled circle for each patient). In the box-plot on the right, the horizontal line in the box represents the mean, and error bars represent the minimum and maximum values
a The bias between simulated effect-site concentrations (A) and plasma concentrations (B) of rocuronium at 1 and 3 h after starting continuous rocuronium administration (n = 10), as determined by Bland–Altman analysis. X-axis Average of A and B, Y-axis differences between A and B. b Relationships between effect-site concentrations and plasma concentrations of rocuronium at 1 and 3 h after initiation of continuous rocuronium infusion (n = 10)
Discussion
The results of this study show that the continuous infusion of rocuronium at a dose equivalent to the simulated effect-site concentration at the time when %T1 recovers to >0 % can provide similar optimal muscle relaxant effects as that achieved by the bolus administration of rocuronium. In this study, %T1 values could be maintained at <10 % during continuous rocuronium administration and were 0 % in nearly half the procedures, indicating that sufficient muscle relaxation during surgery was achieved using our procedure. Moreover, time to recovery of %T1 to >25 % was less than 30 min in all cases. Kotake et al. reported times to recovery of %T1 to >25 % of 22 ± 10 to 24 ± 7 min after bolus administration of rocuronium 0.15 mg/kg [13]. The muscle relaxant effects of rocuronium in our study were not prolonged as those reported previously even through the doses of rocuronium administered were equivalent to those in previous studies. Surprisingly, we found no correlation between Rb r.c. and the effect-site concentration at the point of recovery of %T1 to >25 %. We also found no significant relationship between Rb r.c. and length of the recovery period. These results may indicate that effective rocuronium concentrations close to the end of surgery are affected by factors such as sympathetic nerve activity, blood loss, circulating blood volume, and speed of rocuronium metabolism. Unnecessary overdoses of neuromuscular blocking agents can lead to residual muscle paralysis after surgery. The possibility of residual paralysis is relatively higher in patients receiving pancuronium, which is a long-acting neuromuscular blocking agent [14], but there are several reports of intermediate-acting agents, such as rocuronium, also causing residual postoperative neuromuscular blockade [15–18], despite their effects being antagonized by anticholinesterase drugs. Augammadex, a selective relaxant binding agent, has recently been developed as an antagonist of the effects of neuromuscular agents such as rocuronium and vecuronium. Optimal administration of sugammadex can completely antagonize rocuronium-induced relaxant effects. However, the administration of an insufficient dose of sugammadex is not only unable to reverse muscle relaxation, but it also eliminates the possibility for muscle relaxation rebound [19]. In the present study, Rb r.c. values were highly variable, with minimum and maximal values of 1.04 and 2.19 μg/ml, respectively. This variability and wide range indicate the large differences between the patients enrolled in our study in terms of their sensitivity to neuromuscular blocking agents in general. However, since Rb r.c. can be adjusted depending on personal sensitivity, it is possible to administer a continuous rocuronium infusion at the optimal rate for each patient. Due to such differences in sensitivity to neuromuscular blocking agents, it is impossible to achieve suitable muscle relaxation in all patients by merely using a predetermined dose range during the continuous infusion of rocuronium. The continuous infusion dose of rocuronium for suitable muscle relaxation needs to be individually tailored for each patient based on individual response to the drug in order to avoid inadequate muscle relaxation. We believe that our method, in which the optimal dose of rocuronium is determined for each individual, will be highly efficient in clinical settings. In our study, rocuronium was continuously infused at the rate of 6.9 ± 2.8 μg/kg/min. An earlier study in Japanese patients reported that the mean continuous infusion rate of rocuronium required to maintain %T1 in the range of 3 to 10 % under propofol anesthesia was 7.4 μg/kg/min [20], which is very similar to the rate of rocuronium infusion in our study.
It is difficult to achieve stable muscle relaxation by the intermittent rocuronium administration method. Insufficient effects could cause harmful events, such as a less than optimal surgical field and moving or bucking of the patient. Maintenance of the %T1 at <10 % during all surgical procedures by our protocol can prevent the occurrence of these harmful events and sustain suitable muscle relaxation. In particular, because the diaphragm is more resistant to neuromuscular blocking agents than the adductor pollicis muscle, the present protocol, which continuously maintains %T1 at <10 %, is highly efficient compared to the twitch-based infusion scheme [20].
We found that BMI inversely correlated with Rb r.c. values, although age and gender did not affect these values. The authors of a number of studies have suggested that the dose of rocuronium infusion should be determined based on actual body weight. However, Leykin et al. reported that muscle relaxant effects are prolonged when rocuronium is dosed according to actual body weight in obese patients [8], likely due to the increased amount of fat as a percentage of body weight, since rocuronium is only minimally distributed in fatty tissue. Meyhoff et al. [9] recommended that the dose of rocuronium should be calculated according to IBW in obese patients. Obese patients with a BMI of >30 kg/m2 were excluded from our study. However, in another study in non-obese patients, the duration of action of rocuronium increased with increasing BMI when rocuronium was administered on the basis of actual body weight [21]. Hence, further research is needed to determine whether rocuronium should be administered according to the IBW in non-obese patients. While both age and gender affect an individual’s sensitivity to muscle relaxants, these factors did not contribute to the Rb r.c. values in our study. This result is rather controversial and may be due to the small sample size which did not allow multiple group comparisons to be performed; this is a limitation of this study.
To investigate the reliability of the Wierda pharmacokinetic model, we measured plasma rocuronium concentrations in ten patients at 1 and 3 h after initiation of continuous administration of rocuronium. Although there was considerable bias between plasma and effect-site concentrations of rocuronium, the effect-site concentrations of rocuronium simulated using the Wierda pharmacokinetic–pharmacodynamic model were significantly correlated with the plasma concentrations at both time-points. Moreover, comparisons of the two rocuronium concentrations at these two time-points in each patient showed that the differences between the plasma and effect-site concentrations of rocuronium were almost equal. The Wierda simulation model shows not only effect-site concentrations of rocuronium but also estimates plasma drug concentrations. When rocuronium is administered or continuous infusion rates are changed, there is first a rapid change in the estimated plasma concentrations, followed by changes in the effect-site concentrations. However, these concentrations become almost the same within a few minutes. In our study, we adjusted the continuous infusion rate of rocuronium every 10 min, but we did not take blood samples for the measurement of plasma rocuronium concentration when the interval between changes in infusion rates was <10 min. Therefore, we believe that the differences between the estimated plasma concentration and the effect-site concentration were not large. Moreover, we believe that the changes in the relative effect-site concentrations of rocuronium in conjunction with plasma rocuronium concentrations in each patient are important and accurate indicators of the continuous administration dose of rocuronium. Therefore, we also believe that our rocuronium administration method, in which continuous infusion doses are based on simulation using the Wierda pharmacokinetic model, is appropriate. However, we could not measure plasma concentrations of rocuronium in all patients and at the points of %T1 >0 % after bolus administration. This is a limitation of our study, although we believe that there was a correlation between plasma and effect-site concentrations of rocuronium at these time-points.
Since rocuronium does not have cumulative effects, it is not difficult to simulate its effect-site concentration, which may also be one of the reasons that rocuronium is suitable for continuous infusion. We have investigated the optimal dose of continuous rocuronium administration under propofol–remifentanil anesthesia. Intravenous anesthetic agents, such as propofol and fentanyl, do not affect the muscle relaxant effects of rocuronium [22]. Therefore, the anesthetic agents administered in our study did not influence the effects of rocuronium. In contrast, inhaled anesthetics, such as sevoflurane, isoflurane, and desflurane, can potentiate the neuromuscular blocking effects of rocuronium, which would decrease the continuous rocuronium infusion dose requirements [23, 24]. Further research is needed to investigate whether the our method, as described herein, is effective under conditions of inhaled anesthesia.
In conclusion, the determination of the simulated effect-site concentration of rocuronium at the point where T1 recovers to >0 % could define the optimal rate of continuous rocuronium administration. The results of the present study suggest that the method described herein may be one of the more reliable continuous rocuronium administration methods reported to date. We have shown that with our protocol, optimal administration doses can be adjusted in advance without a time lag, enabling successful maintenance of sufficient and stable muscle relaxation without excessively prolonging effects at the end of surgery.
References
Wierda JM, Kleef UW, Lambalk LM, Kloppenburg WD, Agoston S. The pharmacodynamics and pharmacokinetics of Org 9426, a new non-depolarizing neuromuscular blocking agent, in patients anaesthetized with nitrous oxide, halothane and fentanyl. Can J Anaesth. 1991;38:430–5.
Miller DR, Wherrett C, Hull K, Watson J, Legault S. Cumulation characteristics of cisatracurium and rocuronium during continuous infusion. Can J Anaesth. 2000;47:943–9.
Sparr HJ, Wierda JM, Proost JH, Keller C, Khuenl-Brady KS. Pharmacodynamics and pharmacokinetics of rocuronium in intensive care patients. Br J Anaesth. 1997;78:267–73.
Shanks CA, Fragen RJ, Ling D. Continuous intravenous infusion of rocuronium (ORG 9426) in patients receiving balanced, enflurane, or isoflurane anesthesia. Anesthesiology. 1993;78:649–51.
McCoy EP, Mirakhur RK, Maddineni VR, Wierda JM, Proost JH. Pharmacokinetics of rocuronium after bolus and continuous infusion during halothane anaesthesia. Br J Anaesth. 1996;76:29–33.
Xue FS, Tong SY, Liao X, Liu JH, An G, Luo LK. Dose-response and time course of effect of rocuronium in male and female. Anesth Analg. 1997;85:667–71.
Bevan DR, Fiset P, Balendran P, Law-Min JC, Ratcliffe A, Donati F. Pharmacodynamic behavior of rocuronium in the elderly. Can J Anaesth. 1993;40:127–32.
Leykin Y, Pellis T, Lucca M, Lomangino G, Marzano B, Gullo A. The pharmacodynamics effects of rocuronium when dosed according to real body weight or ideal body weight in mobidly obese patients. Anesth Analg. 2004;99:1086–9.
Meyhoff CS, Lund J, Jenstrup MT, Claudius C, Sorensen AM, Viby-Mogensen J, Rasmussen LS. Should dosing of rocuronium in obese patients be based on ideal or corrected body weight? Anesth Analg. 2009;109:787–92.
Dahaba AA, Perelman SI, Moskowitz DM, Bennett HL, Shander A, Xiao Z, Huang L, An G, Bornemann H, Wilfinger G, Hager B, Rehak PH, List WF, Metzler H. Geographic regional differences in rocuronium bromide dose-response relation and time course of action: an overlooked factor in determining recommended dosage. Anesthesiology. 2006;104:950–3.
Fuchs-Buder T, Claudis C, Skovgaard LT, Eriksson LI, Mirakhur RK, Viby-Monngensen J. 8th International Neuromuscular Meeting. Good clinical research practice in pharmacodynamics studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51:789–808.
Valadares de Moraes N, Rocha Lauretti G, Campos de Filgueira G, Carvalho Portes Lopes B, Lanchote VL. Analysis of rocuronium in human plasma by liquid chromatography-tandem mass spectrometry with application in clinical pharmacokinetics. J Pharm Biomed Anal. 2014; 90:180–5.
Kotake Y, Takeda J, Ozaki M, Saeki S, Otagiri T, Kiyama S, Uchimoto R, Iwao Y. Randamized, multicenter study of interaction between Org 9426 (rocuronium bromide) and anesthestic agents in Japanese population (in Japanese with English abstract). Masui (Jpn J Anesthesiol). 2006;55:873–9.
Bissinger U, Schimek F, Lenz G. Postoperative residual paralysis and respiratory status: a comparative study of pancuronium and vecuronium. Physiol Res. 2000;49:455–62.
Hayes AH, Mirakhur RK, Breslin DS, Reid JE, McCourt KC. Postoperative residual block after intermediate-acting neuromuscular blocking drugs. Anaesthesia. 2001;56:312–8.
Kim KS, Lew SH, Cho HY, Cheong MA. Residual paralysis induced by either vecuronium or rocuronium after reversal with pyridostigmine. Anesth Analg. 2002;95:1656–60.
Debaene B, Plaud B, Dilly MP, Donati F. Residual paralysis in the PACU after a single intubating dose of nondepolarizing muscle relaxant with an intermediate duration of action. Anesthesiology. 2003;98:1042–8.
Cammu G, De Witte J, De Veylder J, Bytebier G, Vandeput D, Foubert L, Vandenbroucke G, Deloof T. Postoperative residual paralysis in outpatients versus inpatients. Anesth Analg. 2006;102:426–9.
Eleveld DJ, Kuizenga K, Proost JH, Wierda JM. A temporary decrease in twich response during reversal of rocuronium-induced muscle relaxation with a small dose of sugammadex. Anesth Analg. 2007;104:582–4.
Takagi S, Ozaki M, Iwasaki H, Hatano Y, Takeda J. Effects of sevoflurane and propofol on neuromuscular blocking action of Org 9426 (rocuronium bromide) infused continuously in Japanese patients (in Japanese with English abstract). Masui (Jpn J Anesthesiol). 2006;55:963–7.
Fujimoto M, Tanahira C, Nishi M, Yamamoto T. In non-obese patients, duration of action of rocuronium is directly correlated with body mass index. Can J Anaesth. 2013;60:552–6.
Olkkola KT, Tammisto T. Quantifying the interaction of rocuronium (Org 9426) with etomidate, fentanyl, midazolam, propofol, thiopental, and isoflurane using closed-loop feedback control of rocuronium infusion. Anesth Analg. 1994;78:691–6.
Wulf H, Ledowski T, Linstedt U, Proppe D, Sitzlack D. Neuromuscular blocking effects of rocuronium during desflurane, isoflurane, and sevoflurane anaesthesia. Can J Anaesth. 1998;45:526–32.
Brock M, Kippel K, Nitsche B, Bach A, Martin E, Motsch J. Rocuronium potency and recovery characteristics during steady-state desflurane, sevoflurane, isoflurane or propofol anaesthesia. Br J Anaesth. 2000;84:43–7.
Acknowledgments
This study was supported by a Grant for research from Nihon Kohden.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Moriyama, T., Matsunaga, A., Nagata, O. et al. Effective method of continuous rocuronium administration based on effect-site concentrations using a pharmacokinetic/pharmacodynamic model during propofol–remifentanil anesthesia. J Anesth 29, 593–599 (2015). https://doi.org/10.1007/s00540-015-1991-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-015-1991-2