Abstract
Background
Traditional serrated adenoma (TSA) is the least common type of colorectal serrated polyp, which exhibits considerable morphological and molecular diversity.
Methods
We examined the spectra of alterations in MAPK and WNT pathway genes and their relationship with clinicopathological features in 128 TSAs.
Results
Sequencing analyses identified BRAF V600E, BRAF non-V600E, KRAS, and NRAS mutations in 77, 3, 45, and 1 lesion, respectively. Collectively, 124 lesions (97%) had mutations in MAPK pathway genes. Alterations in WNT pathway genes were identified in 107 lesions (84%), including RSPO fusions/overexpression, RNF43 mutations, ZNRF3 mutations, APC mutations, and CTNNB1 mutations in 47, 45, 2, 13, and 2 lesions, respectively. Ten lesions (8%) harbored GNAS mutations. There was significant interdependence between the altered MAPK and WNT pathway genes. RSPO fusions/overexpression was significantly associated with KRAS mutations (31/47, 66%), whereas most RNF43 mutations coexisted with the BRAF V600E mutation (40/45, 89%). Histologically, extensive slit-like serration was more common in lesions with the BRAF V600E mutation (71%) and those with RNF43 mutations (87%). Prominent ectopic crypt formation was more prevalent in lesions with RSPO fusions/overexpression (58%) and those with GNAS mutations (100%).
Conclusions
Our observations indicate that TSAs mostly harbor various combinations of concurrent WNT and MAPK gene alterations. The associations between genetic and morphological features suggest that the histological diversity of TSA reflects the underlying molecular heterogeneity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies have suggested that approximately 20–30% of colorectal cancers are derived from serrated lesions [1, 2]. Serrated lesions include hyperplastic polyps, sessile serrated adenoma/polyps (SSA/Ps), and traditional serrated adenomas (TSAs) [3]. Among these, TSA is the least common subtype and is associated with premalignant potential, along with sessile serrated adenoma/polyp [4]. The histological features of TSA include abundant eosinophilic cytoplasm, elongated nuclei, ectopic crypt formation, and slit-like serration [5,6,7]. It is well established that TSA represents a distinct entity and the histological diagnosis is usually straightforward. However, it is also true that TSAs exhibit significant morphological heterogeneity. For instance, the presence of ectopic crypt formation is characteristic but not a consistent feature of TSAs [6]. Although still not widely accepted, some authors have suggested some morphological subtypes of TSAs, including mucin-rich and filiform variants [8,9,10].
Serrated polyps mostly harbor genetic alterations leading to MAPK pathway activation, mostly KRAS or BRAF mutations [6, 11,12,13,14]. KRAS mutations are common in goblet cell-rich hyperplastic polyps whereas microvesicular hyperplastic polyps and SSA/Ps frequently harbor the BRAF V600E mutation [15,16,17]. TSAs also frequently have MAPK pathway gene mutations, but unlike others, both BRAF and KRAS mutations are detected in TSAs, indicating their molecular variability [6, 11,12,13,14]. Furthermore, our previous studies showed that the majority of TSAs also harbor alterations in WNT pathway genes, including RSPO fusions/overexpression, RNF43 mutations, and APC mutations, introducing another layer of genetic heterogeneity [18, 19].
Based on these previous observations, we postulated that the morphological variability of TSAs might reflect their molecular heterogeneity. The present study aimed to investigate the spectrum of genetic alterations in a larger series of TSAs and assessed their clinicopathological correlations.
Materials and methods
Samples
This study was approved by the Ethics Committee of the National Cancer Center, Tokyo, Japan. Tissue samples were obtained by endoscopic resection at the National Cancer Center Hospital, Tokyo, Japan, or at the National Cancer Center Hospital East, Chiba, Japan. We analyzed 128 TSAs, which were examined in our previous studies [18, 19]. Samples with insufficient quality of DNA and/or RNA for next-generation sequencing or reverse transcription PCR were excluded from the present study.
Histological analysis
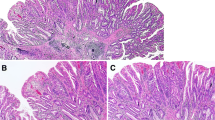
All TSAs were confirmed to exhibit at least two of the following three histological features: (1) typical cytology, (2) slit-like epithelial serrations, and (3) ectopic crypt formation, with at least one feature present in > 50% of the polyps excluding the precursor component (Fig. 1a–d) [6]. The areas exhibiting typical cytology, slit-like serrations and ectopic crypt formation were semi-quantitatively evaluated as < 10%, 10–50%, or > 50% (regarded as extensive/prominent). The proportion of goblet cells/mucin-rich cells was also semiquantitatively evaluated as < 10%, 10–50%, or > 50% (Fig. 1e, f). The presence of high-grade components and the association of hyperplastic polyp, SSA/P, and superficially serrated adenoma were determined (Figs. 1g, 2a–d). The cecum to the transverse colon was defined as the right colon and the descending colon to the sigmoid colon was defined as the left colon.
Representative histology of traditional serrated adenomas. a Slit-like serration. Narrow slits in the epithelium exhibiting eosinophilic cytoplasm. b Ectopic crypt formations. Epithelial buds along the villous projection. c Tumor cells showing abundant eosinophilic cytoplasm and prominent slit-like serration. d TSA with prominent ectopic crypt formation. e TSA containing numerous goblet cells. f TSA showing a focal goblet cell-rich area (left). g A lesion associated with a high-grade component (right)
Traditional serrated adenoma associated with precursor polyps. TSAs with a hyperplastic polyp (a, left) and a sessile serrated polyp (b, arrowheads), respectively. TSA with a superficially serrated adenoma (c, arrowheads). The superficially serrated adenoma component show adenomatous glands with mild serration confined to the superficial layer (d)
Molecular characterization
The detailed methods and part of the results of molecular analyses were described in our previous studies [18, 19]. Outlines of the analytical methods are as follows.
Sections of formalin-fixed paraffin-embedded specimens were dissected under a microscope using sterilized toothpicks to enrich for tumor content and were used for DNA and RNA extraction. In the analysis of precursor polyp-associated lesions, we subjected only TSA components to DNA and RNA extraction.
For next-generation sequencing, amplicon libraries, targeting frequently mutated regions of APC, BRAF, CTNNB1, GNAS, KRAS, and NRAS, and the entire coding regions of RNF43 and ZNRF3, were prepared using the Ion AmpliSeq™ custom panel (ThermoFisher Scientific, Waltham, MA, USA). Sequencing was performed using an Ion Proton Sequencer, an Ion PI Chip, and an Ion PI Hi-Q Sequencing 200 Kit (ThermoFisher Scientific). The sequences obtained were mapped onto the human reference genome hg19, and sequence variations with variant frequencies > 10% for single-nucleotide variants and 15% for insertions/deletions were identified as candidate mutations. Synonymous mutations and common single nucleotide polymorphism, based on the Single Nucleotide Polymorphism Database build 137, were excluded. All resulting mutation candidates were confirmed using Sanger sequencing. Part of the sequencing results was previously reported [18].
The presence of overexpression and fusions of RSPO2 and RSPO3 was tested using quantitative PCR and the specific fusions were detected by conventional reverse transcription PCR. The results of RSPO fusion and overexpression analyses were described previously [19].
CpG island methylator phenotype (CIMP) status was assessed by the quantitative methylation-specific PCR using the panel of Weisenberger et al. (NEUROG1, CACNA1G, IGF2, RUNX3, and SOCS1) [20]. Lesions were classified by the number of markers showing a percentage of the methylated reference ≥ 10 as follows: CIMP-high, ≥ 3; CIMP-low, 1 or 2; and CIMP-negative, no markers. Part of the methylation analysis was previously reported [18].
Immunohistochemistry
Immunohistochemical staining for MLH1 was performed as described previously [18]. Part of the staining results was previously reported [18].
Statistical analysis
Fisher’s exact test and Welch’s t-test were used to analyze categorical variables and continuous variables, respectively. P values < 0.01 were considered to indicate statistical significance.
Results
The TSAs were obtained from 80 male and 48 female patients who were aged 34–85 years (median, 68 years). The distribution of the polyps was as follows: 22 lesions (17%) in the right colon, 51 (40%) in the left colon, and 55 (43%) in the rectum. They were 2–58 mm in size (median, 7 mm). A high-grade component was seen in 21 lesions (16%). The association of hyperplastic polyp, SSA/P, and superficially serrated adenoma was observed in 22 (17%), 11 (9%), and 15 lesions (12%), respectively. Typical cytology was observed in all lesions and extensive in 118 lesions (92%). Slit-like serration, ectopic crypt formation, and goblet cells were prominent (> 50% of areas or cells) in 72 (56%), 49 (38%), and 19 lesions (15%), respectively. Twenty-four lesions (19%) showed the CIMP-high phenotype.
Next-generation sequencing followed by confirmation by Sanger sequencing identified 57 RNF43, 10 ZNRF3, 18 APC, 2 CTNNB1, 80 BRAF, 46 KRAS, 1 NRAS, and 10 GNAS mutations (Supplementary Table 1). Among the tumor suppressor genes examined, eight RNF43, eight ZNRF3, and three APC mutations were missense. Since the functional consequences of these missense mutations were unclear, only protein-truncating mutations have been considered as inactivating mutations hereafter. Mutations detected in CTNNB1, KRAS, NRAS, and GNAS have been previously reported in diverse tumors and regarded as oncogenic. A total of six lesions had two RNF43, APC, or KRAS mutations. Quantitative and conventional reverse transcription-PCR detected PTPRK-RSPO3 fusions, an NRIP1-RSPO2 fusion, RSPO2 overexpression, and RSPO3 overexpression in 42, 1, 2, and 2 lesions, respectively.
Collectively, alterations in MAPK pathway genes were identified in 124 lesions (97%; Fig. 3). BRAF V600E, KRAS, and NRAS mutations were mutually exclusive to each other. In contrast, two of the three TSAs with BRAF non-V600E mutations also had a KRAS or NRAS mutation; therefore, lesions with the BRAF V600E and non-V600E mutations were analyzed as separate groups. Alterations in WNT pathway genes were detected in 107 lesions (84%). RSPO fusions/overexpression, RNF43 mutations, APC mutations, and CTNNB1 mutations were mutually exclusive to each other. In contrast, the two lesions with truncating ZNRF3 mutations concurrently harbored RNF43 mutations, consistent with their cooperative roles with RNF43 mutations [18, 21]. Overall, 80% of TSAs possessed alterations in both WNT and MAPK pathways.
Since the numbers of lesions with BRAF non-V600E and those lacking MAPK gene mutations were too small for statistical analyses, we compared TSAs with the BRAF V600E and KRAS mutations for their clinicopathological characteristics (Table 1). There was significant interdependence between alterations in MAPK and WNT pathway genes. KRAS mutations were associated with RSPO fusions/overexpression (31/44, 70%). More than half of the lesions with the BRAF V600E mutation also harbored RNF43 mutations (40/77, 52%); conversely, most RNF43 mutations coexisted with the BRAF V600E mutation (40/45, 89%). TSAs with the BRAF V600E mutations were more likely to be located in the proximal colon and less frequently had high-grade components. The association with hyperplastic polyps and SSA/Ps was common in lesions with the BRAF V600E mutations. As previously reported [22], superficially serrated adenoma exclusively coexisted with KRAS-mutated TSAs. Slit-like serration and ectopic crypt formation were more extensive in BRAF-mutated and KRAS-mutated TSAs, respectively. The CIMP-high phenotype was more common in BRAF-mutated TSAs.
With regard to WNT pathway gene mutations, clinicopathological features associated with RSPO fusions/overexpression, RNF43 mutations, and APC mutations were examined (Table 2). TSAs with RSPO fusions/overexpression were rare in the proximal colon, and were slightly larger in size than others. The presence of a high-grade component was more frequent in TSAs with RSPO fusions, but was rare in TSAs with RNF43 mutations. Hyperplastic polyps and SSA/Ps were more commonly associated with RNF43-mutated TSAs, whereas superficially serrated adenoma was almost exclusively associated with TSAs with RSPO fusions/overexpression. Slit-like serration and ectopic crypt formation were more extensive in TSAs with RNF43 mutations and RSPO fusions/overexpression, respectively.
GNAS mutations were significantly associated with older age, the presence of high-grade components, less slit-like serration, and extensive ectopic crypt formation (Table 3).
MLH1 expression was retained in all lesions, except for one lesion in which a section for immunohistochemical staining was unavailable (Supplementary Table 1).
Discussion
Our analysis identified concurrent alterations in MAPK and WNT pathway genes in four-fifths of TSAs. The frequency of MAPK pathway-related mutations was higher than those reported in most previous studies, which is partly because these studies focused on mutations in codon 600 of BRAF and codon 12 and 13 of KRAS [6, 11,12,13,14]. Our analysis identified three BRAF and seven KRAS mutations outside these most commonly mutated residues and one NRAS mutation. Most MAPK pathway gene alterations were mutually exclusive of each other, but two of the three TSAs with a BRAF non-V600E mutation concurrently harbored a KRAS or NRAS mutation. This finding is similar to our previous observation on SSA/P, demonstrating that all three lesions with BRAF non-V600E mutations also had a KRAS or NRAS mutation [17], and is consistent with the fact that many BRAF non-V600E mutations are loss-of-function mutations but enhance MAPK signaling in cooperation with active RAS [23, 24].
We used the Weisenberger panel to determine the CIMP status in the present study [20]. Although this panel was originally designed to correlate with the presence of BRAF mutations in colorectal cancers, recent studies have shown the association between the BRAF V600E mutation and the CIMP-high phenotype also in the precursor lesions using this panel [6, 14]. Consistently, the CIMP-high phenotype was more common in lesions with the BRAF V600E mutation also in the present study.
Mutations related to the WNT pathway are virtually ubiquitous in colorectal cancers and are thought to be the initial genetic alterations in the conventional pathway of tumorigenesis [25, 26]. In the serrated pathway of tumorigenesis, MAPK pathway gene alterations are the initial event and alterations in WNT pathway genes are acquired during the transition from non-dysplastic to dysplastic lesions [27,28,29]. Consistently, alterations in WNT pathway genes were detected in the majority of TSAs; however, the mutation spectrum was different from that of conventional adenomas. Most conventional adenomas possess inactivating APC mutations and less frequently, CTNNB1 mutations [18, 30, 31]. In contrast, RSPO fusions/overexpression and RNF43 mutations were predominant mutations in TSAs [18, 19]. Indeed, similar to the BRAF V600E mutation, these two genetic alterations are specific to serrated lesions [18, 32]. Interestingly, RSPO fusions/overexpression was significantly associated with KRAS mutations and most RNF43 mutations coexisted with the BRAF V600E mutation, indicating the interdependence between MAPK and WNT pathway gene mutations.
GNAS mutations were identified in 8% of the lesions, a frequency similar to those reported by other studies [14, 33]. While the number of GNAS-mutated lesions was limited, they correlated with several clinicopathological features, including the presence of extensive ectopic crypt formation. On the other hand, there was no apparent association between GNAS and other mutations.
MLH1 expression was retained in all TSAs examined, in agreement with previous studies [6, 11]. This observation suggests that mismatch repair deficiency does not play a role in the development of TSA unlike tumorigenesis via SSA/P with dysplasia [27, 34, 35].
Slit-like serration and ectopic crypt formation are characteristic histological features of TSAs [5,6,7]. Although both these features are observed in the majority of TSAs and commonly coexist, their extent varies among lesions [6]. Our results showed the correlations between genetic alterations and these histological observations. Extensive slit-like serration was associated with the BRAF V600E mutation and RNF43 mutations whereas it was less common in lesions with RSPO fusions/overexpression and APC mutations. Prominent ectopic crypt formation was frequent in lesions with RSPO fusions/overexpression and GNAS mutations. The correlations between morphology and genetic alterations suggest that although each of the alterations in MAPK and WNT pathway genes activates common signaling pathways, they have different biological activities. However, lesions with different genotypes exhibited overlapping histological features, supporting the validity to regard TSA as a single entity despite its genetic diversity.
Recent studies have suggested that the mucin-rich variant of TSA, which is defined as lesions containing ≥ 50% goblet cells, should be regarded as a distinct histological subtype [9, 10]. The prevalence of mucin-rich TSA was reported to be 15–28%, which is consistent with our findings. However, there was considerable variability in the number of goblet cells among the polyps, and the distribution of goblet cells showed notable heterogeneity within the respective lesions. Therefore, although it is true that some TSAs are rich in goblet cells, we are hesitant to regard them as a histologically distinct variant. A previous study reported that mucin-rich TSAs were more likely to have BRAF mutations [10]; however, this finding was not reproduced in our analysis.
The presence of a high-grade component was more frequent in lesions with KRAS mutations, RSPO fusions/overexpression, and GNAS mutation. This implies that TSAs with these genetic alterations may have a higher risk of malignant progression, but this hypothesis requires confirmation by analysis of lesions associated with adenocarcinoma. Consistent with our finding, the correlation between KRAS mutations and higher-grade dysplasia was also described in a previously study [12]. In contrast, high-grade dysplasia was rare among lesions with BRAF and/or RNF43 mutations, including those associated with SSA/P. It is generally believed that once SSA/Ps acquire dysplasia, they quickly progress to adenocarcinoma [34]; however, considering the rarity of high-grade dysplasia in SSA/P-associated TSA, it may not always be the case.
The present study demonstrated the common coexistence of mutations leading to WNT and MAPK pathway activation in TSAs. Importantly, various genetic alterations are involved in the activation of the respective pathways and each genetic alteration is significantly associated with different clinical and morphological features. These findings suggest that the histological variability of TSAs reflects their mutational diversity.
References
Bettington M, Walker N, Clouston A, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–86.
Rosty C, Hewett DG, Brown IS, et al. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287–302.
Snover DC, Ahnen DJ, Burt RW, et al. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC; 2010. p. 160–165.
Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29 (quiz 4, 30).
Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol. 2008;32:21–9.
Bettington ML, Walker NI, Rosty C, et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod Pathol. 2015;28:414–27.
Chetty R. Traditional serrated adenoma (TSA): morphological questions, queries and quandaries. J Clin Pathol. 2016;69:6–11.
Yantiss RK, Oh KY, Chen YT, et al. Filiform serrated adenomas: a clinicopathologic and immunophenotypic study of 18 cases. Am J Surg Pathol. 2007;31:1238–45.
Kalimuthu SN, Serra S, Hafezi-Bakhtiari S, et al. Mucin-rich variant of traditional serrated adenoma: a distinct morphological variant. Histopathology. 2017;71:208–16.
Hiromoto T, Murakami T, Akazawa Y, et al. Immunohistochemical and genetic characteristics of a colorectal mucin-rich variant of traditional serrated adenoma. Histopathology. 2018;73:444–53.
O'Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–501.
Kim KM, Lee EJ, Kim YH, et al. KRAS mutations in traditional serrated adenomas from Korea herald an aggressive phenotype. Am J Surg Pathol. 2010;34:667–75.
Tsai JH, Liau JY, Lin YL, et al. Traditional serrated adenoma has two pathways of neoplastic progression that are distinct from the sessile serrated pathway of colorectal carcinogenesis. Mod Pathol. 2014;27:1375–85.
Wiland HOT, Shadrach B, Allende D, et al. Morphologic and molecular characterization of traditional serrated adenomas of the distal colon and rectum. Am J Surg Pathol. 2014;38:1290–7.
O'Brien MJ, Yang S, Clebanoff JL, et al. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28:423–34.
Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–7.
Cho H, Hashimoto T, Yoshida H, et al. Reappraisal of the genetic heterogeneity of sessile serrated adenoma/polyp. Histopathology. 2018;73:672–80.
Sekine S, Yamashita S, Tanabe T, et al. Frequent PTPRK-RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J Pathol. 2016;239:133–8.
Sekine S, Ogawa R, Hashimoto T, et al. Comprehensive characterization of RSPO fusions in colorectal traditional serrated adenomas. Histopathology. 2017;71:601–9.
Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93.
de Lau W, Peng WC, Gros P, et al. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305–16.
Hashimoto T, Tanaka Y, Ogawa R, et al. Superficially serrated adenoma: a proposal for a novel subtype of colorectal serrated lesion. Mod Pathol. 2018;31:1588–98.
Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21.
Nieto P, Ambrogio C, Esteban-Burgos L, et al. A Braf kinase-inactive mutant induces lung adenocarcinoma. Nature. 2017;548:239–43.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67 (Epub 1990/06/01).
Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7.
Hashimoto T, Yamashita S, Yoshida H, et al. WNT pathway gene mutations are associated with the presence of dysplasia in colorectal sessile serrated adenoma/polyps. Am J Surg Pathol. 2017;41:1188–97.
Borowsky J, Dumenil T, Bettington M, et al. The role of APC in WNT pathway activation in serrated neoplasia. Mod Pathol. 2018;31:495–504.
Hashimoto T, Ogawa R, Yoshida H, et al. Acquisition of WNT pathway gene alterations coincides with the transition from precursor polyps to traditional serrated adenomas. Am J Surg Pathol. 2019;43:132–9.
Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–7 (Epub 1992/09/17).
Borras E, San Lucas FA, Chang K, et al. Genomic landscape of colorectal mucosa and adenomas. Cancer Prev Res (Phila). 2016;9:417–27.
Tsai JH, Liau JY, Yuan CT, et al. RNF43 is an early and specific mutated gene in the serrated pathway, with increased frequency in traditional serrated adenoma and its associated malignancy. Am J Surg Pathol. 2016;40:1352–9.
Liu C, McKeone DM, Walker NI, et al. GNAS mutations are present in colorectal traditional serrated adenomas, serrated tubulovillous adenomas and serrated adenocarcinomas with adverse prognostic features. Histopathology. 2017;70:1079–88.
Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97–106.
Liu C, Walker NI, Leggett BA, et al. Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry. Mod Pathol. 2017;30:1728–38.
Acknowledgements
This work was supported by JSPS KAKENHI Grant numbers 17K08711 and 18K07925. We thank Ms. Sachiko Miura, Ms. Toshiko Sakaguchi, Ms. Chizu Kina, and Yuka Nakamura for their skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sekine, S., Yamashita, S., Yamada, M. et al. Clinicopathological and molecular correlations in traditional serrated adenoma. J Gastroenterol 55, 418–427 (2020). https://doi.org/10.1007/s00535-020-01673-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-020-01673-z