Abstract
Background and aims
Body size-metabolic phenotype may help predict whether or not individuals with nonalcoholic fatty liver disease (NAFLD) develop advanced liver disease. We studied the association of body size-metabolic phenotype with nonalcoholic steatohepatitis (NASH) and significant fibrosis.
Methods
Our cross-sectional study included 559 subjects (mean age of 53 years; women 51%) with biopsy-proven NAFLD. Clinical, genetic, and histological characteristic features of NAFLD were evaluated. The metabolically unhealthy phenotype was defined by the presence of two or more metabolic components, while body size was categorized based on body mass index: obese (≥ 25 kg/m2) or non-obese (< 25 kg/m2). Body size-metabolic phenotypes were divided into four study groups: (1) non-obese metabolic syndrome (MS)−, (2) non-obese MS+ , (3) obese MS−, and (4) obese MS+.
Results
Obese MS− and non-obese MS+ groups demonstrated comparable levels of insulin resistance, adipose tissue insulin resistance indexes, and visceral adipose tissue (VAT) areas. The VAT area was significantly higher in the obese MS+ group versus obese MS− group. However, the VAT to subcutaneous adipose tissue (SAT) ratio was highest in the non-obese MS+ group. There was no difference in histology between the non-obese MS+, obese MS−, and obese MS+ groups. Multivariate analyses adjusted for age, sex, smoking status, PNPLA3, TM6SF2, and VAT/SAT areas demonstrated an independent and dose-dependent relationship between the body size-metabolic phenotype and NASH or significant fibrosis.
Conclusion
The non-obese MS+ group displayed similar degree of hepatic histological severity compared to their obese MS− counterparts. Metabolic milieu beyond obesity may play a pathogenic role in non-obese MS+ individuals who develop NASH with significant hepatic fibrosis.
Clinical trial number
NCT 02206841.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been recognized as the most prevalent chronic liver disease in the United States (US), affecting approximately 25% of the general population and up to 70% of diabetic and obese individuals [1]. NAFLD encompasses a wide spectrum of histologic phenotypes from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH), a subset of which can progress to advanced liver disease including cirrhosis and hepatocellular carcinoma [2]. Along with the rising prevalence of NAFLD, the prevalence of NASH has almost doubled during the recent years [1, 2]. The increasing prevalence of NASH and/or advanced hepatic fibrosis is a major concern because affected individuals experience higher overall mortality and cause-specific mortality from liver-related and non-liver-related etiologies [3].
The prevalence of obesity ranges around 35% in the US and poses a significant public health concern as well as a substantial economic burden [4]. On one hand, not all obese individuals develop cardiovascular disease and diabetes, while on the contrary not all non-obese individuals have a healthy cardio-metabolic profile [5,6,7]. There are several epidemiologic studies suggesting that a subset of individuals have reduced cardio-metabolic risk despite being obese [5, 6]. In contrast, a subset of non-obese individuals displayed a metabolic profile similar to the profile that is often associated with obesity [5, 8]. These subsets of individuals are referred to as ‘obese metabolic syndrome (MS)−’ and ‘non-obese MS+’, respectively [5,6,7]. Compared to individuals who are not obese and metabolically healthy, non-obese MS+ individuals have a higher risk of overall and/or cardiovascular mortality [9]. Therefore, it is important to consider both obesity and metabolic health status (so-called body size-metabolic phenotype) in evaluating cardiovascular and mortality risk [9].
Several studies have reported an increased prevalence of NAFLD in non-obese MS+ individuals [7]. However, data regarding the hepatic histologic differences between body size-metabolic phenotypes are still lacking. In addition, the concept of body size-metabolic phenotypes may be important in identifying individuals who need pharmacotherapy against NAFLD to prevent the progression to more advanced liver disease. Therefore, the aim of our study was to compare body size-metabolic phenotypes with a focus on (1) clinical, genetic, and histological characteristics in NAFLD, and (2) the risk for NASH and significant fibrosis.
Methods
Subjects and study design
We performed our analyses utilizing the clinical parameters and pathological data from liver biopsy confirmed ‘Boramae NAFLD cohort’ (NCT 02206841) which has been described previously [10, 11]. In brief, we enrolled eligible subjects from January 2013 to February 2018 in a prospective manner. The inclusion criteria for this cohort were as follows: (1) adults at least 18 years old; (2) ultrasonographic findings confirming fatty infiltration of the liver, and (3) unexplained elevated alanine aminotransferase (ALT) levels within the past 6 months. In this cohort, we excluded the subjects with (1) significant alcohol consumption (> 30 g/day for men and > 20 g/day for women); (2) documented hepatitis B or C viral infection; (3) diagnosis of chronic liver disease other than NAFLD, or (4) diagnosis of cancer within the past year. Among the eligible subjects, a liver biopsy was performed if at least two of the following risk factors were present: high triglycerides level, low high-density lipoprotein (HDL)-cholesterol level, abdominal obesity, hypertension, presence of diabetes mellitus and/or insulin resistance, or clinically suspected NASH or hepatic fibrosis. This study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki for the participation of human subjects and was approved by the Institutional Review Board of Boramae Medical Center (IRB No.16-2014-86). We obtained written informed consent from each subject.
Clinical and laboratory evaluations
Methods used for clinical and laboratory assessments have been described in detail elsewhere [10, 11]. Briefly, each subject underwent laboratory testing and an anthropometric assessment. According to a standard protocol, a well-trained nurse collected anthropometric measurements. The body mass index (BMI) of the subjects was calculated with the following formula: BMI = weight (kg)/height squared (m2). Waist circumference was examined in accordance with a previously published protocol [12]. Blood pressure was assessed twice on the same day, and the mean of the two values was used. We defined hypertension as a systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg and/or the current use of anti-hypertensive medication. Current smokers were defined as those who had smoked at least one cigarette per day during the past year. Sarcopenia was defined as an appendicular skeletal muscle mass/weight beyond 2 standard deviations below the gender-specific mean for healthy young adults according to nationwide health examinations of the Korean population [10]. Venous blood samples were collected at the time of liver biopsy after a 12-h overnight fast. Using standard methods, we measured levels of serum ALT, serum aspartate aminotransferase (AST), serum gamma-glutamyl transpeptidase, serum total cholesterol, serum triglycerides, serum HDL-cholesterol, and fasting glucose. Fasting insulin levels were measured using immunoradiometric assays (DIAsource ImmunoAssays, Nivelles, Belgium). We calculated insulin resistance by utilizing the homeostasis model assessment (HOMA-IR) as follows: HOMA-IR = fasting glucose (mmol/L) × fasting insulin (mIU/mL)/22.5. We defined diabetes mellitus as fasting plasma glucose levels of ≥ 126 mg/dL and/or current treatment with anti-diabetic medication. We estimated adipose tissue insulin resistance index as follows: adipose tissue insulin resistance index = free fatty acid (mmol/L) × insulin (mIU/mL). Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [13].
Definition of obesity and metabolic phenotypes (body size-metabolic phenotypes)
We defined obesity as BMI ≥ 25 kg/m2 and used the harmonized International Diabetes Federation criteria for metabolic syndrome [14]. We used four metabolic components: (1) elevated fasting glucose, ≥ 100 mg/dL and/or drug treatment for elevated glucose; (2) elevated triglycerides, ≥ 150 mg/dL and/or drug treatment for elevated triglycerides; (3) reduced HDL-cholesterol, < 40 mg/dL in men or < 50 mg/dL in women, and (4) elevated blood pressure, systolic ≥ 130 and/or diastolic ≥ 85 mmHg. The criteria for waist circumference were not used because of known collinearity with BMI. On the basis of previous literature, the metabolically unhealthy phenotype was defined by the presence of two or more of the aforementioned metabolic components, and the metabolically healthy phenotype was defined by the absence of or presence of only one metabolic component [15,16,17]. Body size-metabolic phenotypes were defined using modified criteria previously described [8, 18]. Subjects were categorized into four phenotypes: (1) non-obese MS−; (2) non-obese MS+; (3) obese MS−, or (4) obese MS+ [19].
Liver histology
For the liver histology, we utilized previously mentioned methods [10, 11]. Briefly, all liver biopsies were reviewed by an experienced liver pathologist. NAFLD was diagnosed as the presence of ≥ 5% macrovesicular steatosis. We defined NASH based on an overall pattern of histological hepatic injury consisting of macrovesicular steatosis, lobular inflammation, or hepatocyte balloon degeneration according to Brunt’s criteria [20, 21]. Additionally, we assessed the NAFLD activity score to determine NASH activity [22]. We assessed fibrosis with criteria proposed by Brunt and Kleiner et al.: F0 (absence of fibrosis); F1 (perisinusoidal or periportal fibrosis); F2 (perisinusoidal and portal/periportal fibrosis); F3 (bridging fibrosis), and F4 (cirrhosis) [22]. We defined significant fibrosis as F2–F4.
Measurement of abdominal adipose tissue areas
We used a previously described method for adipose tissue area measurement [23, 24]. Briefly, each subject was examined for quantification of abdominal fat at the level of the umbilicus with a 128-detector computed tomography (CT) scanner (Ingenuity CT; Philips Medical Systems, Cleveland, OH) in the supine position. The area at the umbilicus level was assessed with commercially available CT software (Rapidia 2.8; INFINITT, Seoul, Korea), which measured the adipose tissue area by setting the attenuation values within a range of − 250 to − 50 Hounsfield units.
Genotype
We used a previously described method for genotyping [25]. We selected established risk alleles of NASH and fibrosis for genotyping including patatin-like phospholipase domain-containing 3 (PNPLA3) [26], transmembrane 6 superfamily member 2 (TM6SF2) [27], sterol regulatory element-binding factor (SREBF) [28], and membrane-bound O-acyltransferase domain containing 7-transmembrane channel-like 4 (MBOAT7-TMC4) [29]. Single-nucleotide polymorphisms were genotyped by TaqMan 50-nuclease assays (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. We confirmed Hardy–Weinberg equilibrium using the Chi-squared test.
Statistical analysis
Continuous variables were summarized as the mean ± standard deviation (SD) or median (interquartile range) and categorical variables as numbers (percentage). Differences between groups were evaluated using the independent t test, the Mann–Whitney U test, analysis of variance (ANOVA), or the Kruskal–Wallis test for continuous variables as appropriate and the Chi-square test for categorical variables. To determine the independent risk factors for the presence of NASH or significant fibrosis, we performed logistic regression analyses. The multivariable-adjusted models were confined to variables that were based on clinical plausibility—including demographics (age and sex), smoking, genetic factors (PNPLA3 and TM6SF2), and visceral adipose tissue (VAT) area, and subcutaneous adipose tissue (SAT) area. Genetic data were described as frequency, and odds ratios for the existence of a respective risk allele were assessed using logistic regression. The VAT and SAT areas were standardized to a mean of 0 and a SD of 1. All analyses were performed using STATA 15.0 (StataCorp, College Station, TX, USA). Two-sided P values were used and considered statistically significant if P ≤ 0.05.
Results
Demographic, clinical, and biochemical characteristics according to obesity and metabolic phenotype status
Among the 559 subjects (mean age 53 ± 15 years; women 51%), 96 non-obese subjects were classified as non-obese MS−, and the remaining 79 non-obese subjects were classified as non-obese MS+ . Ninety-four obese subjects were enrolled as obese MS− and 290 obese subjects as obese MS+. The baseline characteristics are shown in Table 1. Non-obese MS+ was in between non-obese MS− and obese MS− with regard to BMI, waist circumference, sarcopenic proportions, and gamma-glutamyl transpeptidase levels. However, based on inclusion criteria, subjects in the non-obese MS+ group harbored components of metabolic syndrome with higher frequency than those in both non-obese MS− and obese MS− groups. Compared to obese MS− subjects, non-obese MS+ subjects demonstrated comparable levels of HOMA-IR, adipose tissue-insulin resistance index, and VAT area, but these values were significantly lower when compared to the obese MS+ group. However, the VAT-to-SAT ratio was highest in the non-obese MS+ group compared to the two obese groups. In contrast, high sensitivity C-reactive protein as an inflammatory marker did not differ between the non-obese MS+ , obese MS−, and obese MS+ groups. Despite both groups being categorized as non-obese, the non-obese MS+ group was consistently different in insulin resistance, adipose tissue insulin resistance, and the VAT-to-SAT ratio from the non-obese MS− group without exceptions. When comparing non-obese MS+ to obese MS+, a gradual increase in the prevalence of insulin resistance, higher VAT area, and higher levels of aminotransferase were observed in the obese MS+ group. In terms of eGFR, renal function was not significantly different according to body size-metabolic phenotype status except obese MS−. Baseline medications information regarding diabetes and hypertension is presented in Supplementary Table 1. No significant differences in the use of medications were found except angiotensin-2 receptor blockers and/or angiotensin converting enzyme inhibitors (P = 0.001).
Histologic characteristics according to obesity and metabolic phenotype status
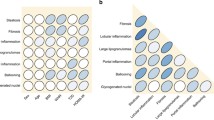
Figure 1 demonstrates the comparison of histological features among the four groups. The lobular inflammation, balloon degeneration, and presence of significant fibrosis (≥ F2) were similar among the four groups. In contrast, there was a significant difference in steatosis grade among groups. Compared to obese subjects, non-obese subjects were noted to have lower severity of hepatic steatosis. The presence of NASH was slightly higher in non-obese MS+ , obese MS−, and obese MS+ compared to non-obese MS−.
Genetic risk alleles for NASH according to obesity and metabolic phenotype status
As shown in Table 2, the proportion of subjects carrying PNPLA3 GG alleles was increased in non-obese MS+ , obese MS−, and obese MS+ compared with non-obese MS−, while there was no significant difference between non-obese MS+ , obese MS−, and obese MS+ . The carrier rate of subjects with at least one TM6SF2 T allele was increased in the metabolic healthy phenotype compared to obese MS+ with marginal significance. There were no significant differences in the proportion of SREBF T allele or MBOAT7 T allele between the groups.
Association between body size-metabolic phenotype status and NASH
We investigated which body size-metabolic phenotype is associated with the presence of NASH. As indicated in Table 3, age and sex-adjusted analysis showed that non-obese MS+ , obese MS−, and obese MS+ were significantly associated with 2.6-fold, 3.4-fold, and 4.0-fold increase in the risk for NASH, respectively. Multivariate analyses adjusted for age, sex, smoking status, and PNPLA3, TM6SF2 demonstrated an independent and dose-dependent relationship between the body size-metabolic phenotype and NASH (P for trend < 0.001). Because VAT and SAT areas play important roles in the development of NASH, the VAT and SAT areas were incorporated into additional analyses. A similar association was observed after adjusting for VAT and SAT areas; however, the association between non-obese MS+ and NASH was attenuated with marginal significance (P = 0.065), suggesting that VAT and SAT areas account for this association partially, but not completely. Increased VAT area demonstrated a significant association with NASH (61% increased risk for NASH [per 1-SD increase of VAT]). PNPLA3 and TM6SF2 polymorphisms were independently associated with an increased risk for NASH. When we performed multivariate analyses after adjustment for sarcopenia and chronic kidney disease, respectively, we found that body size-metabolic phenotype status remains a significant risk factor for NASH (Supplementary Table 2). When subjects with any features of body size-metabolic phenotypes were considered in the model, the impacts of non-obese MS+ , obese MS−, and obese MS+ on NASH were largely the same. In the multivariate model, there was no significant difference between obese MS− and obese MS+ compared to non-obese MS+ as the reference.
Association between body size-metabolic phenotype status and significant fibrosis
We determined the effects of body size-metabolic phenotypes on significant fibrosis (Table 4). Age and sex-adjusted analysis showed that non-obese MS+ , obese MS−, and obese MS+ were associated with a 2- to 2.5-fold increase in the risk for significant fibrosis. This association remained the same after adjusting for smoking status and genetic factors with a marginal significance in the non-obese MS+ group (P = 0.065). When we further considered VAT and SAT areas in the multivariate model, the overall trend remained the same. However, non-obese MS+ was no longer statistically significantly associated with significant fibrosis, indicating that the association between non-obese MS+ and significant fibrosis is partly mediated by VAT and SAT areas. When the analysis was repeated without non-obese MS−, overall results were identical to those obtained utilizing the risk factors for NASH. There was no significant difference between the obese MS−, obese MS+ , and non-obese MS+ groups in terms of an increased risk of significant fibrosis. With reference to body fat distribution, subjects with a 1-SD increase of VAT area had an almost 30% greater odds of significant fibrosis. Regarding genetic features, PNPLA3 and TM6SF2 polymorphisms demonstrated a close relationship with an increased risk for significant fibrosis. When we performed multivariate analyses after adjustment for sarcopenia and chronic kidney disease, respectively, we found that body size-metabolic phenotype status remains a significant risk factor for significant fibrosis (Supplementary Table 2).
Discussion
We report an independent and dose-dependent relationship between body size-metabolic phenotypes and NASH and significant fibrosis. When the analysis was repeated without non-obese MS−, there was a strong association of NASH and significant fibrosis with the metabolically unhealthy phenotype irrespective of obesity status. In addition, the presence of NASH and significant fibrosis did not differ significantly between non-obese MS+ and obese MS− groups. Our findings demonstrated comparable severity of hepatic histology in non-obese MS+ and obese MS− groups. In other words, metabolic phenotypic features in the absence of obesity can promote the progression of NAFL to NASH and significant fibrosis. Furthermore, our results also provided supporting data that the VAT area in non-obese MS+ is more likely to mediate progression to NASH and significant fibrosis from NAFL.
Obesity is typically a heterogeneous condition caused by the regional distribution of body fat. A subset of non-obese MS+ individuals display a metabolic profile similar to the profile that is often associated with being obese [8, 30]. In light of the well-known association of insulin resistance and visceral adiposity with NAFLD, non-obese NAFLD may represent a subset of phenotype with NAFLD in non-obese MS+ individuals [31]. Our study shows that non-obese MS+ comprises individuals who are non-obese, have insulin resistance and an increased VAT area. The presence of non-obese MS+ may be a harbinger of insulin resistance and adipose tissue insulin resistance that portends worse NAFLD-related outcomes in these subjects. Our data have demonstrated that non-obese MS+ had a significant effect on the pathogenesis of NASH and significant fibrosis, and these findings in the non-obese MS+ group were largely comparable to the obese MS− group. A recent meta-analysis reported that, compared to non-obese MS−, individuals with obese MS− do not have increased overall mortality; however, individuals with non-obese MS+ have a threefold increased risk for mortality [9]. In the setting of MS, the liver is responsible for the overproduction of glucose and triglycerides, the two main components of MS [32]. An increase in the expression of inflammatory genes in VAT along with the progression of histologic liver damage has been reported [33]. In metabolically unhealthy individuals in whom adipose tissue has difficulty in expanding in a normal healthy fashion by increasing cellularity, metabolic abnormalities are triggered and induce a stressful environment [34, 35]. The adipose tissue expandability hypothesis proposed that once adipocytes reached a threshold capacity for fat storage, they begin to promote insulin resistance with lipo-toxicity and adipokine release [36, 37]. While subjects with obese MS− maintain a proper storage capacity, the loss of expansion capacity could occur in non-obese subjects resulting in the non-obese MS+ phenotype [35]. A recent study showed that the severity of NASH and significant fibrosis does not differ significantly in non-obese and obese NAFLD [2, 38]. Consistent with our results, others have also shown that obesity is not an independent risk factor for cardiovascular disease and mortality when both MS and obesity were evaluated simultaneously [39, 40]. Our study shows that the associations between the non-obese MS+ phenotype and the risk of NASH and significant fibrosis were attenuated when we adjusted for VAT and SAT areas. These results indicate that changes in regional body fat distribution may provide a mechanistic basis for the putative pathogenetic pathway in the induction and progression of NAFL to advanced liver disease in the non-obese MS+ phenotype. Therefore, general obesity may not be the only driver for NASH and fibrosis [2]. Our study suggests that the metabolic milieu due to ectopic fat deposits in individuals with non-obese MS+ may play a role in the progression from NAFL to NASH and significant fibrosis.
In general, Asians have a higher percentage of VAT area compared to Hispanics and Caucasians of the same age, gender, and BMI [41, 42]. In this respect, there appears to be a greater percentage of subjects with non-obese MS+ phenotype among Asians compared with their European counterparts [42, 43], because BMI does not reflect regional body fat distribution. A recent study demonstrated a wide variation in the prevalence of non-obese MS+ between major ethnic groups, with a rate of 21% in whites, 31% in African American, 36% in Hispanics, 32% in Chinese American, and 44% in South Asians [15]. Our data indicate that impaired expansion of SAT area may predispose individuals to a metabolically unhealthy phenotype [44]. In addition, we found that there are similarities between non-obese MS+ and obese MS− for adipose tissue insulin resistance, which leads to enhanced lipolysis and increasing the influx of non-esterified fatty acids into the liver [45]. Therefore, we suggest that the clinical use of components of MS may be advantageous in predicting and profiling subjects at increased risk for NASH and significant fibrosis.
Risk variants in PNPLA3 (GG allele) are noted in 13–19% in Asians, 4% in Caucasians, 2% in African Americans, and 25% in Hispanics [26]. A recent study showed that the impact of PNPLA3 on NAFLD is more pronounced in subjects without MS [46]. Additionally, PNPLA3 risk variants are more prevalent in non-obese NAFLD and are also an independent risk factor for non-obese NAFLD [47]. The TM6SF2 risk gene (T allele) is associated with NAFLD and fibrosis. However, the low prevalence in Asians may underestimate the impact of TM6SF2 polymorphism on NAFLD-related advanced liver disease [48]. It is well established that genetic risk variants involve the histological progression of NAFLD regardless of obesity status [49]. Our study also demonstrated that non-obese MS+ is an independent risk factor for NASH and significant fibrosis even after adjusting for well-known genetic risk factors. Subjects with non-obese MS+ phenotype have an increased risk for NASH and significant fibrosis and are therefore candidates for closer monitoring and pre-emptive treatment with a focus on avoiding weight gain. Recent data have suggested that preventative lifestyles such as a healthy dietary pattern and being physically active were more preferable for obese MS− phenotype compared to subject who were obese MS+ [37]. Interestingly, a healthy lifestyle index may determine the transition from obese MS− to obese MS+ [37, 50]. Although lifestyle modification remains the mainstay in the management of subjects with non-obese MS+ and NAFLD, pharmaceutical agents may also be effective in this patient population when indicated to optimize the management of individual components of MS.
One strength of this study is that an expert pathologist who specialized in liver pathology reviewed the histological diagnoses of NASH and other histological features. The large sample size, wealth of genetic data, CT-based body fat assessment, and laboratory data allowed for the comparison between obesity and metabolic phenotypes with sufficient statistical power. This study also has several limitations. First, we could not confirm the causality of the observed relationships due to the cross-sectional nature of the study design. Moreover, it is possible that subjects who used to be obese could be non-obese at the entry of this study due to the therapies including body weight control and medications. Second, the number of normal weight subjects (BMI < 23 kg/m2) remains relatively small. This precludes further analyses of the association between metabolic healthy normal weight and NASH or significant fibrosis. However, the BMI cut-off points for observed risk varies from 22 to 25 kg/m2 in diverse Asian populations; for high risk, it varies from 26 to 31 kg/m2 [51, 52]. In the cohort of 1 million East Asians, the lowest risk of mortality was seen among subjects with a BMI in the range of 22.6–27.5 and the risk was elevated among subjects with a BMI higher than 27.5 [53]. Additionally, the WHO Expert Consultation recommended that the WHO BMI cut-off points should be retained as international classifications in Asians [51, 52]. Third, as we only included subjects of East Asian ethnicity, these results may not be generalizable to other ethnic populations. Fourth, according to a recent report from Japan, a combination of PNPLA3 and TLL1 polymorphisms can accurately predict advanced fibrosis in patients with NAFLD [54]. However, genetic data regarding polymorphisms in TLL1 were not available in our study. Fifth, because we were unable to obtain nutritional data including dietary pattern or coffee consumption for all subjects in our cohort, we were unable to evaluate the impact of nutrition on the association between body size-metabolic phenotype and NASH and significant fibrosis. Finally, we performed liver biopsy if clinically suspected NASH or fibrosis or metabolic abnormalities were present. This may have resulted in that the metabolically healthy groups in the current study may have more severe cardio-metabolic derangements than the metabolically healthy groups in other studies. Thus, our inclusion criteria might lead to biased results. However, due to the invasive nature of liver biopsy and ethical considerations, we could not perform liver biopsy for all subjects with NAFLD, especially for those with NAFL and low risk of NASH or significant fibrosis.
We conclude that non-obese MS+ phenotype displays a similar degree of histological severity compared to their obese MS− counterparts. These results suggest that metabolic phenotypes beyond obesity could play a role in the progression to NASH and significant fibrosis. While the pathophysiology of non-obese MS+ remains undetermined, our data suggest that a high index of clinical suspicion is needed to promptly diagnose patients at risk for NASH or significant fibrosis.
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- MS:
-
Metabolic syndrome
- ALT:
-
Alanine aminotransferase
- HDL:
-
High-density lipoprotein
- AST:
-
Aspartate aminotransferase
- CT:
-
Computed tomography
- PNPLA3 :
-
Patatin-like phospholipase domain-containing 3
- TM6SF2 :
-
Transmembrane 6 superfamily member 2
- SREBF :
-
Sterol regulatory element-binding factor
- MBOAT7-TMC4 :
-
Membrane-bound O-acyltransferase domain containing 7-transmembrane channel-like 4
- SD:
-
Standard deviation
- VAT:
-
Visceral adipose tissue
- SAT:
-
Subcutaneous adipose tissue
References
Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20.
Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65.
Kim DD, Basu A. Estimating the medical care costs of obesity in the United States: systematic review, meta-analysis, and empirical analysis. Value Health. 2016;19:602–13.
Badoud F, Perreault M, Zulyniak MA, et al. Molecular insights into the role of white adipose tissue in metabolically unhealthy normal weight and metabolically healthy obese individuals. FASEB J. 2015;29:748–58.
Stefan N, Haring HU, Hu FB, et al. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62.
Stefan N, Schick F, Haring HU. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 2017;26:292–300.
Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med. 2008;168:1617–24.
Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta-analysis. Ann Intern Med. 2013;159:758–69.
Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–31.
Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16(123–31):e1.
Obesity: preventing and managing the global epidemic. In: Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1–253.
Mauss S, Berger F, Filmann N, et al. Effect of HBV polymerase inhibitors on renal function in patients with chronic hepatitis B. J Hepatol. 2011;55:1235–40.
Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
Gujral UP, Vittinghoff E, Mongraw-Chaffin M, et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the united states: a cross-sectional analysis of two cohort studies. Ann Intern Med. 2017;166:628–36.
Heianza Y, Arase Y, Tsuji H, et al. Metabolically healthy obesity, presence or absence of fatty liver, and risk of type 2 diabetes in Japanese individuals: Toranomon Hospital Health Management Center Study 20 (TOPICS 20). J Clin Endocrinol Metab. 2014;99:2952–60.
Hashimoto Y, Hamaguchi M, Fukuda T, et al. Fatty liver as a risk factor for progression from metabolically healthy to metabolically abnormal in non-overweight individuals. Endocrine. 2017;57:89–97.
Gasull M, Castell C, Pallares N, et al. Blood concentrations of persistent organic pollutants and unhealthy metabolic phenotypes in normal-weight, overweight, and obese individuals. Am J Epidemiol. 2018;187:494–506.
Dobson R, Burgess MI, Sprung VS, et al. Metabolically healthy and unhealthy obesity: differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes (Lond). 2016;40:153–61.
Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74.
Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–20.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Kim D, Choi SY, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–13.
Yim JY, Kim D, Lim SH, et al. Sagittal abdominal diameter is a strong anthropometric measure of visceral adipose tissue in the Asian general population. Diabetes Care. 2010;33:2665–700.
Koo BK, Joo SK, Kim D, et al. Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2018;33:1277–85.
Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5.
Kozlitina J, Smagris E, Stender S, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–6.
Musso G, Cassader M, Bo S, et al. Sterol regulatory element-binding factor 2 (SREBF-2) predicts 7-year NAFLD incidence and severity of liver disease and lipoprotein and glucose dysmetabolism. Diabetes. 2013;62:1109–20.
Mancina RM, Dongiovanni P, Petta S, et al. The MBOAT7-TMC4 Variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of european descent. Gastroenterology. 2016;150(1219–30):e6.
Karelis AD, St-Pierre DH, Conus F, et al. Metabolic and body composition factors in subgroups of obesity: what do we know? The Journal of clinical endocrinology and metabolism. 2004;89:2569–75.
Kim D, Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15:474–85.
Hojland Ipsen D, Tveden-Nyborg P, Lykkesfeldt J. Normal weight dyslipidemia: is it all about the liver? Obesity (Silver Spring). 2016;24:556–67.
du Plessis J, van Pelt J, Korf H, et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology. 2015;149(635–48):e14.
Arner E, Westermark PO, Spalding KL, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59:105–9.
Munoz-Garach A, Cornejo-Pareja I, Tinahones FJ. Does metabolically healthy obesity exist? Nutrients. 2016;8:320.
Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65(1):73–80. https://doi.org/10.1016/j.metabol.2015.10.019
Phillips CM. Metabolically healthy obesity across the life course: epidemiology, determinants, and implications. Ann N Y Acad Sci. 2017;1391:85–100.
Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65:54–64.
Mongraw-Chaffin M, Foster MC, Anderson CAM, et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2018;71:1857–65.
Fan J, Song Y, Chen Y, et al. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;168:4761–8.
Alberti KG, Zimmet P, Shaw J, et al. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–62.
Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775–7.
Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:1389–97.
Stefan N, Haring HU, Schulze MB. Metabolically healthy obesity: the low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018;6(3):249–58.
Feldman A, Eder SK, Felder TK, et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects With Non-alcoholic Fatty Liver. Am J Gastroenterol. 2017;112:102–10.
Shen J, Wong GL, Chan HL, et al. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment Pharmacol Ther. 2014;39:532–9.
Wei JL, Leung JC, Loong TC, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015;110:1306–14 (quiz 15).
Pirola CJ, Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology. 2015;62:1742–56.
Honda Y, Yoneda M, Kessoku T, et al. Characteristics of non-obese non-alcoholic fatty liver disease: effect of genetic and environmental factors. Hepatol Res. 2016;46:1011–8.
Schroder H, Ramos R, Baena-Diez JM, et al. Determinants of the transition from a cardiometabolic normal to abnormal overweight/obese phenotype in a Spanish population. Eur J Nutr. 2014;53:1345–53.
Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–73.
Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63.
Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med. 2011;364:719–29.
Seko Y, Yamaguchi K, Mizuno N, et al. Combination of PNPLA3 and TLL1 polymorphism can predict advanced fibrosis in Japanese patients with nonalcoholic fatty liver disease. J Gastroenterol. 2018;53:438–48.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (2016R1D1A1B04934590), and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, Republic of Korea (H I17C0912). The funding organization played no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
DK: study concept and design, analysis and interpretation of data, drafting the manuscript, and approval of the final draft manuscript. WK: study concept and design, obtained funding, acquisition of data, analysis and interpretation of data, drafting the manuscript, and approval of the final draft manuscript. SKJ, JH, and JHK: acquisition of data, interpretation of data, critical revision of the manuscript for important intellectual content, and approval of the final draft manuscript. SAH and ZMY: interpretation of data, critical revision of the manuscript for important intellectual content, and approval of the final draft manuscript. AA: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, and approval of the final draft manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, D., Kim, W., Joo, S.K. et al. Association between body size-metabolic phenotype and nonalcoholic steatohepatitis and significant fibrosis. J Gastroenterol 55, 330–341 (2020). https://doi.org/10.1007/s00535-019-01628-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-019-01628-z