Abstract
Background
The details of gastric cancer in young patients remain unclear because of the low prevalence of the disease. This study aimed to clarify the clinicopathological features and prognosis of gastric cancer in young patients.
Methods
From January 2007 to January 2016, patients in their 20s and 30s who were diagnosed with primary gastric cancer at 4 hospitals were enrolled. Their clinical characteristics and prognosis were evaluated.
Results
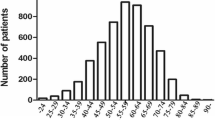
The total number of patients was 72. The median age was 36 years, and the ratio of males to females was 1:1. The dominant histological type was undifferentiated type (66/72, 92%). Helicobacter pylori (H. pylori) was positive in 81% (54/67). Although there were some asymptomatic patients in stages I–III, all stage IV patients had some clinical symptoms at the diagnosis. The percentage of stage IV was significantly higher in patients in their 20s than in those in their 30s (75% vs. 25%, P < 0.001). The Kaplan–Meier method showed that the overall survival of patients in their 20s was significantly lower than that of patients in their 30s (P = 0.037).
Conclusions
A high rate of H. pylori infection was revealed in young gastric cancer patients. The patients in their 20s had a worse prognosis than those in their 30s. We should consider examining the H. pylori infection status for young patients as well as older patients to identify high-risk populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is a major cause of cancer-related death worldwide [1]. The incidence of gastric cancer is usually predominant in patients 50–70 years of age [2], and is relatively low in younger patients. Indeed, the incidence of gastric cancer in patients < 40 years of age reportedly ranges from 4.6 to 6.2% [3,4,5]. Furthermore, gastric cancer in young patients is reported to have a poor prognosis compared with that in elderly patients. Factors associated with the poor prognosis of gastric cancer in the young include an undifferentiated histology, unresectability, the presence of lymphovascular invasion, and an advanced stage at presentation [6, 7]. However, recent studies have reported that the prognosis in young patients is comparable to that in middle-aged patients with cancers at the same stage [8, 9]. Thus, whether the prognosis of gastric cancer in young patients is better or worse than those in older patients is controversial.
The details of gastric cancer in young patients have not been fully clarified because the number of young patients with gastric cancer is small, resulting in insufficient data. Furthermore, the definition of “early onset” has varied in each study, and most previous studies have investigated the prognosis of gastric cancer in young patients who underwent curative gastric surgery [9,10,11]. Given this background, we investigated in detail the clinicopathological features and prognosis of gastric cancer in young patients with any treatment or at any clinical stage in a large-scale study.
Methods
Patients
This was a multicenter, retrospective, observational study. In this study, young patients were defined as those 20–39 years of age. From January 2007 to January 2016, patients in their 20s and 30s who had been diagnosed with primary gastric cancer at Okayama university hospital, Japanese Red Cross Society Himeji Hospital, Okayama Saiseikai General Hospital, and Tsuyama Chuo Hospital were enrolled in the present study. In all cases, the diagnosis was confirmed based on the results of a histological evaluation of biopsy or resected specimens from a primary gastric lesion.
The study procedures were implemented in accordance with the Declaration of Helsinki. The study protocol was approved by each hospital Ethics Committee, and informed consent was acquired by the opt-out method.
Assessment of Helicobacter pylori (H. pylori) infection status and endoscopic atrophy
The H. pylori infection status was examined by at least one of the following methods: serum H. pylori IgG antibodies, stool antigen test, a culture test, a histological examination, urea breath testing (UBT), or rapid urease test (RUT). The cut-off value of serum H. pylori IgG antibody was set at 10 U/mL, and the cutoff value for UBT was set at 2.5 ‰. When each of diagnostic examinations was negative, a patient was diagnosed as H. pylori infection negative. When any of diagnostic examinations was positive, a patient was diagnosed as H. pylori infection positive. None of endoscopic gastric atrophy was defined based on a positive regular arrangement of collecting venules (RAC). [12].
Evaluations
Clinical parameters of all patients were retrospectively recorded, including the age, body mass index, sex, family history of cancer within the first degree, Eastern Cooperative Oncology Group performance status (PS), symptoms at the diagnosis, H. pylori infection status, histological type of cancer, clinical stage (Union for International Cancer Control 7th), macroscopic type, tumor location, degree of endoscopic gastric atrophy based on the Kimura–Takemoto classification [13] and the presence of nodular gastritis by an endoscopic view [14], serum CEA and CA19-9 level, and treatment strategy.
We investigated the clinical characteristics of gastric cancer in young patients based on their background. We then divided the patients into age groups of 20s and 30s, and analyzed the difference in the details and overall survival (OS) rate between the two groups. Moreover, we analyzed the clinicopathological characteristics and OS rate in the patients at clinical stage IV.
Statistical analysis
Sequential data were expressed as the median and interquartile range (IQR). Categorical data were compared using a Chi-squared test or Fisher’s exact test. The OS rates were determined using the Kaplan–Meier method. The difference between survival curves was assessed using the log-lank test. All statistical calculations were carried out using the statistical software (JMP PRO, version 12; SAS institute Inc., Cary, North Carolina, United States). Data were considered to be statistically significant when P < 0.05.
Results
Patient’s characteristics
Table 1 shows the clinical characteristics of all patients. The total number of patients was 72. The median age was 36 years, and the ratio of males to females was almost 1:1. The proportion of patients who were in their 30s was higher than that of those in their 20s (78 vs. 22%). The proportion of patients who had a family history of gastric cancer within the first degree was 10%. Most of the patients were PS 0-1, but 78% had some clinical symptoms at the diagnosis. The histological type was mainly undifferentiated type (92%). The predominant tumor location was in the upper or middle third of the stomach.
The H. pylori infection status was examined in 67 patients (93%), and 81% (54/67) of patients who were examined H. pylori infection were positive for an infection. Among the H. pylori-positive patients (n = 54), 35 patients were diagnosed based on a positive histological examination, 6 patients were diagnosed based on the detection of serum H. pylori IgG antibodies (≥ 10 U/mL), 6 patients were diagnosed based on a UBT (≥ 2.5 ‰), 5 patients were diagnosed based on a positive RUT, 1 patient was diagnosed based on a positive culture test, and 1 patient was diagnosed based on a positive stool antigen test and the detection of serum H. pylori IgG antibodies (≥ 10 U/mL). Among the H. pylori-negative patients (n = 13), 6 patients were diagnosed based on a negative histological examination, 4 patients were diagnosed based on the detection of serum H. pylori IgG antibodies (< 3 U/mL), 1 patient was diagnosed based on the detection of serum H. pylori IgG antibodies (< 10 U/mL), 1 patient was diagnosed based on a negative stool antigen test, and 1 patient was diagnosed based on a negative stool antigen test and the detection of serum H. pylori IgG antibodies (< 3 U/mL).
Based on the endoscopic evaluation of atrophic gastritis, 10 patients (14%) had no gastric atrophy, 42 (58%) were classified as closed type, and 17 (24%) were classified as open type. Seven patients (9.7%) had nodular gastritis as comorbidity; all of whom were diagnosed with closed-type gastric atrophy. Among the H. pylori-positive patients (n = 54), 3 patients had no gastric atrophy, 35 patients had closed-type gastric atrophy, 13 patients had open-type gastric atrophy, and atrophic change was difficult to evaluate in 3 patients. Among the H. pylori-negative patients (n = 13), 6 patients had no gastric atrophy, 5 patients had closed-type gastric atrophy, and 2 patients had open-type gastric atrophy.
Regarding the levels of tumor marker associated with gastric cancer, such as CEA or CA 19-9, the values were normal in most cases. Various treatments were performed according to the clinical stage. Only one patient received the best supportive care due to a poor performance status. Among 32 patients with cStage I, endoscopic resection (ER) was performed in 7 patients, and surgical resection (SR) was performed in 25 patients. The pathological assessment after resection in ER group revealed stage IA in all cases, and the patients were followed without additional treatment. The stage of the SR group after surgery was stage IA in 21 patients, and stage IB in 4 patients. Although there were 6 patients with differentiated-type cancer in the ER group, all of the patients in the SR group had undifferentiated-type cancer.
Clinical characteristics and the OS rate in patients in their 20s and 30s
Table 2 shows the clinical characteristics of patients in their 20s and 30s. The proportion of stage IV disease was significantly higher in the patients in their 20s than in those in their 30s (75% vs. 25%, P < 0.001). Although the proportion of PS 0-1 patients in their 20s was significantly lower than that among patients in their 30s, 13 (81%) patients in their 20s were PS 0-1. The proportion of patients with high CEA and CA 19-9 levels was significantly higher in the patients in their 20s than in those in their 30s (31% vs. 5.5%, P = 0.012; and 44% vs. 13%, P = 0.011, respectively). Although the difference was not significant, the proportions of peritoneal metastasis and bone metastasis were higher in the patients in their 20s than in those in their 30s. The OS rate was significantly worse in the patients in their 20s than in those in their 30s (P = 0.037, Fig. 1).
The symptoms at diagnosis according to the clinical stage
Table 3 shows the symptoms at the diagnosis according to the clinical stage. Sixteen (35%) patients in clinical stages I–III had no symptoms. In contrast, all patients at clinical stage IV had some symptoms at the diagnosis.
Clinicopathological characteristics and the OS rate in the patients with stage IV disease
Table 4 shows the clinicopathological characteristics of the patients with stage IV disease. The ratio of males to females was almost 1:1. Most of the patients were PS 0-1. The H. pylori infection status was examined in 21 patients (81%), and 67% (14/21) of patients who were examined H. pylori infection were positive for an infection. Eight patients (31%) had no gastric atrophy, 13 patients (50%) had closed-type gastric atrophy, and 4 patients (15%) had open-type gastric atrophy. The histological type was undifferentiated cancer in all patients, and the tumor was predominantly located in the upper or middle third of the stomach. Nineteen patients (73%) had peritoneum dissemination at the time of the diagnosis. Eighteen patients (69%) were CEA negative, and 15 (58%) were CA 19-9 negative. Twenty-four patients (92%) received chemotherapy. The median survival time (MST) of patients with stage IV disease was 184 days (Fig. 2).
Discussion
The present study investigated the details of gastric cancer in patients 20–39 years of age, and the following novelties were clarified. The rate of H. pylori infection positivity was 81% (54/67) in young gastric cancer patients. Similar to older gastric cancer patients, the incidence of H. pylori infection was high even in young patients in the present study. Although patients in their 20s had factors related to a poor prognosis, the OS of the patients in their 20s was significantly worse than that of the patients in their 30s. There were some asymptomatic patients with stage I–III disease, but all patients with stage IV disease had some clinical symptoms.
Several studies have been reported on the H. pylori infection rate in young patients with gastric cancer [15,16,17]. However, we bear in mind that a number of patients did not have their H. pylori infection status examined in those studies. In the present study, the H. pylori infection status was investigated in most of the patients (93%). These data allowed the relationship between gastric cancer and H. pylori infection at a young age to be assessed. The H. pylori infection rate was 81%, and was markedly higher than that of healthy Japanese subjects of the same generation (about 15–30% in 2007–2011) [18]. And recently, the morbidity rate of young age gastric cancer is decreasing in Japan [19]. This might be influenced by decreased rate of H. pylori infection in young age. Our data will help to clarify the influence of H. pylori infection on carcinogenesis even in young patients.
The predominant histological type of gastric cancer in older patients is considered to be differentiated type [20, 21]. In contrast, previous studies have reported that gastric cancer in young patients mainly tends to be undifferentiated histological type [7, 8, 22, 23]. Another study reported that gastric cancer of undifferentiated type in young patients was associated with H. pylori infection and antral lymphoid hyperplasia called “nodular gastritis”, and these patients had a lower degree of corporal atrophy than older gastric cancer patients [16]. Atrophic changes caused by H. pylori infection determine the development of differentiated-type gastric cancer, and the inflammation induced by H. pylori infection promotes the development of undifferentiated-type gastric cancer [24]. In the present study, most of the cases of gastric cancer were undifferentiated type, and the predominant tumor location was the upper or middle third of the stomach. Although only 7 patients (9.7%) had nodular gastritis, more than half of the patients in this study had gastric atrophy. The percentage of H. pylori positivity was also high. Our study suggested that H. pylori infection induced not only the inflammation but also the atrophic change, which was closely associated with the development of undifferentiated-type gastric cancer in young patients [25], as well as the development of differentiated-type gastric cancer in elderly patients.
Previous studies have reported on the prognosis of young gastric cancer patients. It was shown that the disease-free survival and OS of young patients with gastric cancer depended on the cancer stage at the diagnosis, as is the case with middle-aged patients with gastric cancer [10]. Peritoneal metastasis due to undifferentiated-type cancer was considered to be a poor prognostic factor [6, 26]. However, there have been no reports on the difference in the prognosis between gastric cancer patients in their 20s and 30s. In the present study, we investigated the difference in the clinicopathological features between gastric cancer patients in their 20s and 30s. The incidence of undifferentiated-type cancer was not significantly different between the two age groups. However, the incidence of stage IV disease was markedly higher in the patients in their 20s than in those in their 30s. This might be the main factor influencing the difference in the prognosis between the two age groups. We clarified that gastric cancer patients in their 20s had a worse prognosis than those in their 30s, possibly because younger patients tend not to visit a hospital with slight symptoms and instead only go after progression has occurred.
We clarified that patients with some clinical symptoms tended to be diagnosed with stage IV disease, which was in line with the results of previous reports [6, 7]. Moreover, all patients with stage IV disease had some symptoms and the MST was very poor. There were some cases of older patients with stage IV disease who showed no symptoms. However, in Japan, many such cases are detected by medical health checks. There is no medical health check system for younger individuals in Japan. This is one of the reasons why young gastric cancer patients were symptomatic and why their disease was detected at an advanced stage. For rescuing those patients, we need to perform esophagogastroduodenoscopy to the young generation with no symptoms as gastric cancer screening. However, it must be excessive. We need to identify young populations at high risk of developing gastric cancer. Our data suggest that H. pylori infection carries a high risk of gastric cancer, even in young populations. As such, an examination for H. pylori infection might be useful for identifying high-risk populations. Although there are no precise data available on young generation, we believe that the eradication of H. pylori in this population might help the risk of gastric cancer development.
Concerning about the correlation with prognosis and H. pylori status, it was reported that H. pylori-negative gastric cancer had poor prognosis compared with H. pylori-positive patients [27, 28]. However, the subjects in these studies were those after surgical resection. Moreover, the correlation with prognosis and H. pylori status in young gastric cancer patients was unknown. In our study, the prognosis of H. pylori-positive patients was comparable with that of H. pylori-negative patients by Kaplan–Meier method (data was not shown). Since the number of patients in this study was not enough, further study is needed about this issue.
Several limitations exist in the present study warrant mention. First, this was a retrospective study and the number of cases was small. However, we believe that this study is valuable because this was a multicenter observational study for a low-prevalence disease conducted over 10 years. Second, patients > 40 years of age were not included in this study, and we did not compare the clinicopathological features of young patients with those of older patients. Third, only one method for the diagnosis of H. pylori infection was used in most cases. Five (38%) of the H. pylori-negative patients (n = 13) had closed-type gastric atrophy, and 2 (15%) had open-type gastric atrophy. We should consider such cases to represent the insidious disappearance of H. pylori. In addition, there were 3 H. pylori-positive patients who had no gastric atrophy. The number of young patients with H. pylori infection and active gastritis was not insufficient; thus, endoscopic gastric atrophy was not observed or appeared unclear in some cases. Another diagnostic method was needed for these cases. Thus, it is possible that false-negative or false-positive results existed, making the incidence of H. pylori infection higher than we reported (81%).
In conclusion, we clarified the clinicopathological features of gastric cancer in patients 20–39 years of age in a multicenter study. The H. pylori infection rate was high, so H. pylori might be associated with carcinogenesis even in young patients. Patients in their 20s had factors related to a worse prognosis, and the prognosis of the patients in their 20s was significantly worse than that of the patients in their 30s. Patients who had some symptoms tended to be diagnosed with stage IV disease, and their prognosis was poor. To identify young populations at high risk of gastric cancer, examining the H. pylori infection status and its early eradication might be recommended.
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA. Cancer J Clin. 2011;61:69–90.
Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301–16.
Theuer CP, Kurosaki T, Taylor TH, et al. Unique features of gastric carcinoma in the young: a population-based analysis. Cancer. 1998;83:25–33.
Koea JB, Karpeh MS, Brennan MF. Gastric cancer in young patients: demographic, clinicopathological, and prognostic factors in 92 patients. Ann Surg Oncol. 2000;7:346–51.
Kulig J, Popiela T, Kolodziejczyk P, et al. Clinicopathological profile and long-term outcome in young adults with gastric cancer: multicenter evaluation of 214 patients. Langenbeck’s Archives surgery/Deutsche Gesellschaft fur Chirurgie. 2008;393:37–43.
Nakamura R, Saikawa Y, Takahashi T, et al. Retrospective analysis of prognostic outcome of gastric cancer in young patients. Int J Clin Oncol. 2011;16:328–34.
Park HJ, Ahn JY, Jung HY, et al. Clinical characteristics and outcomes for gastric cancer patients aged 18-30 years. Gastric Cancer. 2014;17:649–60.
Kunisaki C, Akiyama H, Nomura M, et al. Clinicopathological features of gastric carcinoma in younger and middle-aged patients: a comparative study. J Gastrointest Surg. 2006;10:1023–32.
Pisanu A, Podda M, Cois A, et al. Gastric cancer in the young: is it a different clinical entity? A retrospective cohort study. Gastroenterol Res Pract. 2014;2014:125038.
Takatsu Y, Hiki N, Nunobe S, et al. Clinicopathological features of gastric cancer in young patients. Gastric cancer. 2015;19:472–8.
Liu S, Feng F, Xu G, et al. Clinicopathological features and prognosis of gastric cancer in young patients. BMC Cancer. 2016;16:478.
Watanabe K, Nagata N, Nakashima R, et al. Predictive findings for Helicobacter pylori-uninfected, -infected and -eradicated gastric mucosa: validation study. World J Gastroenterol. 2013;19:4374–9.
Kimura KTT. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97.
Kamada TTA, Yamanaka Y, Manabe N, et al. Nodular gastrits with Helicobacter pylori infection is strongly associated with diffuse-type gastric cacer in young patients. Dig Endosc. 2007;19:180–4.
Haruma K, Komoto K, Kamada T, et al. Helicobacter pylori infection is a major risk factor for gastric carcinoma in young patients. Scand J Gastroenterol. 2000;35:255–9.
Hirahashi M, Yao T, Matsumoto T, et al. Intramucosal gastric adenocarcinoma of poorly differentiated type in the young is characterized by Helicobacter pylori infection and antral lymphoid hyperplasia. Modern Pathol. 2007;20:29–34.
Ji T, Zhou F, Wang J, et al. Risk factors for lymph node metastasis of early gastric cancers in patients younger than 40. Medicine. 2017;96:e7874.
Shiota S, Murakawi K, Suzuki R, et al. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013;7:35–40.
Trends in Age-specific Incidence Rate (1980, 2013). In: Wakao F, Higashi T, Katanoda K,editors. Cancer Statistics in Japan 2017. Tokyo; 2018. pp. 50–53.
Bani-Hani KE. Clinicopathological comparison between young and old age patients with gastric adenocarcinoma. Int J Gastrointest Cancer. 2005;35:43–52.
Tavares A, Gandra A, Viveiros F, et al. Analysis of clinicopathologic characteristics and prognosis of gastric cancer in young and older patients. Pathol Oncol Res. 2013;19:111–7.
Kong X, Wang JL, Chen HM, et al. Comparison of the clinicopathological characteristics of young and elderly patients with gastric carcinoma: a meta analysis. J Surg Oncol. 2012;106:346–52.
Isobe T, Hashimoto K, Kizaki J, et al. Characteristics and prognosis of gastric cancer in young patients. Oncol Rep. 2013;30:43–9.
Tatemichi M, Sasazuki S, Inoue M, et al. Different etiological role of Helicobacter pylori (Hp) infection in carcinogenesis between differentiated and undifferentiated gastric cancers: a nested case-control study using IgG titer against Hp surface antigen. Acta oncologica (Stockh Swed). 2008;47:360–5.
Kikuchi S, Crabtree JE, Forman D, et al. Association between infections with CagA-positive or -negative strains of Helicobacter pylori and risk for gastric cancer in young adults research group on prevention of gastric carcinoma among young adults. Am J Gastroenterol. 1999;94:3455–9.
Hsieh FJ, Wang YC, Hsu JT, et al. Clinicopathological features and prognostic factors of gastric cancer patients aged 40 years or younger. J Surg Oncol. 2012;105:304–9.
Marrelli D, Pedrazzani C, Berardi A, et al. Negative Helicobacter pylori status is associated with poor prognosis in patients with gastric cancer. Cancer. 2009;115:2071–80.
Jung DH, Lee YC, Kim JH, et al. Postoperative Helicobacter pylori infection as a prognostic factor for gastric cancer patients after curative resection. Gut and liver. 2017;11:635–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We declare that there are no potential conflicts of interest relevant to this article.
Rights and permissions
About this article
Cite this article
Kono, Y., Kanzaki, H., Tsuzuki, T. et al. A multicenter observational study on the clinicopathological features of gastric cancer in young patients. J Gastroenterol 54, 419–426 (2019). https://doi.org/10.1007/s00535-018-1525-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-018-1525-4