Abstract
Background
Endogenous opioids, including enkephalins, are fundamental regulators of pain. In inflammatory conditions, the local release of opioids by leukocytes at the inflammatory site inhibits nociceptor firing, thereby inducing analgesia. Accordingly, in chronic intestinal Th1/Th17-associated inflammation, enkephalins released by colitogenic CD4+ T lymphocytes relieve inflammation-induced visceral pain. The present study aims to investigate whether mucosal T-cell-derived enkephalins also exhibit a potent anti-inflammatory activity as described for exogenous opioid drugs in Th1/Th17-associated colitis.
Methods
The anti-inflammatory effects of endogenous opioids were investigated in both Th1/Th17-associated (transfer of CD4+CD45RBhigh T lymphocytes) and Th2-associated (oxazolone) colitis models in mice. Inflammation-induced colonic damage and CD4+ T cell subsets were compared in mice treated or not treated with naloxone methiodide, a peripheral antagonist of opioid receptors. The anti-inflammatory activity of T-cell-derived enkephalins was further estimated by comparison of colitis severity in immunodeficient mice into which naïve CD4+CD45RBhigh T lymphocytes originating from wild-type or enkephalin-knockout mice had been transferred.
Results
Peripheral opioid receptor blockade increases the severity of Th1/Th17-induced colitis and attenuates Th2 oxazolone colitis. The opposite effects of naloxone methiodide treatment in these two models of intestinal inflammation are dependent on the potency of endogenous opioids to promote a Th2-type immune response. Accordingly, the transfer of enkephalin-deficient CD4+CD45RBhigh T lymphocytes into immunodeficient mice exacerbates inflammation-induced colonic injury.
Conclusions
Endogenous opioids, including T-cell-derived enkephalins, promote a Th2-type immune response, which, depending on the context, may either attenuate (Th1/Th17-associated) or aggravate (Th2-associated) intestinal inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endogenous opioid neuropeptides are potent regulators of pain. Prototypical opioid peptides include β-endorphin, met-enkephalin, leu-enkephalin, and dynorphin A. Their physiological activities are mediated through the activation of three types of receptors, called μ, δ, and κ. All three opioid receptors are expressed on peripheral sensory neurons, and their activation by suitable agonists, including endogenous opioids produced by immune cells, induces a potent analgesia in models of inflammatory pain [1]. Although opioids are produced by both innate and adaptive immune cells, effector CD4+ T lymphocytes have been reported as the most substantial opioid producers [2, 3].
Inflammatory bowel diseases (IBDs) are chronic disorders characterized by an uncontrolled inflammatory response to colonic luminal content involving both innate and adaptive immune cells. An inappropriate regulation of the activity of proinflammatory Th1 and Th17 subsets of CD4+ T lymphocytes plays a pivotal role in the initiation and maintenance of intestinal inflammation. In the experimental colitis model induced by adoptive transfer of CD4+CD45RBhigh T lymphocytes into immunodeficient mice, we have previously reported that colitogenic Th1 and Th17 lymphocytes produce high levels of endogenous opioids [4]. The local release of their opioid content, triggered by the recognition of the cognate antigen, downmodulates afferent neuronal firing induced by inflammatory mediators, and thereby dampens the visceral hypersensitivity [4–6]. In addition to their antinociceptive properties, opioid ligands have also been described as potent anti-inflammatory drugs [7]. Administration of opioid receptor agonists results in a dramatic reduction of gut inflammation in Th1/ Th17 IBD-like colitis models [8–11]. In line with these observations, a few studies using experimental models of both somatic and intestinal chronic inflammation in mice have pointed out the anti-inflammatory properties of the endogenous opioid tone [10, 12, 13]. However, the nature as well as the cellular origin of the opioids produced in the inflammation context was not clearly established. Our study shows by both pharmacological and genetic approaches in the chronic Th1/ Th17-driven colitis model induced by transfer of T cells into immunodeficient mice that enkephalins produced by colitogenic CD4+ T lymphocytes decrease the severity of inflammation-induced colonic tissue damage. By contrast, and in agreement with opioid-mediated pro-Th2 differentiation [14, 15], peripheral opioid receptor neutralization attenuates the severity of Th2 oxazolone colitis.
Methods
Animals
C57BL/6 and BALB/c mice were provided by Janvier (Le Genest Saint Isle, France), severe combined immunodeficiency (SCID) mice were from Charles River Laboratories (Saint Germain sur l’Arbresle, France), and recombination-activating gene 2 deficient C57BL/6 (Rag2 −/−) mice were from ANEXPLO (Toulouse, France). The preproenkephalin-knockout (Penk −/−) mice were the B6.129-Penk-rstm1Pig/J strain with a C57BL/6 (MHC H-2 haplotype b) genetic background provided by Jackson Laboratory (Bar Harbor, ME, USA). All mice used in the study were 6–10-week-old male mice weighing 20–25 g. The mice were housed at a temperature between 20 and 22 °C and maintained under a 12-h light–dark cycle in sawdust-coated transparent cages. The mice were housed in groups of four in ventilated cages with chow and water ad libitum.
Isolation and activation of CD4+ T lymphocytes in vitro
CD4+ T lymphocytes were isolated from splenocytes originating from wild-type C57BL/6 (Penk +/+) or preproenkephalin-knockout (Penk −/−) mice with use of cell negative isolation kits (Invitrogen Dynal, Oslo, Norway). Twenty-four-well cell culture plates (Corning, Life Sciences, Amsterdam, Netherlands) previously coated with anti-CD3 monoclonal antibody (clone 145-2C11) at 2.5 µg mL−1 and anti-CD28 (clone 37.51) monoclonal antibody at 2.5 µg mL−1 (BD Biosciences, San Jose, CA, USA) were seeded with 5 × 105 purified CD4+ T cells (more than 92% pure) in RPMI-1640 medium (GIBCO Life Technologies, Paisley, UK) supplemented with 10% heat-inactivated fetal calf serum (GIBCO Life Technologies), 1% nonessential amino acids, 4 mM l-glutamine, 1 mM sodium pyruvate, penicillin at 100 IU mL−1 and streptomycin at 100 µg mL−1 (GIBCO Life Technologies), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 2 × 10−5 M 2-mercaptoethanol. Cell proliferation was monitored by our labeling the cells with CellTrace™ Violet using cell proliferation CellTrace™ kits according to the manufacturer’s instructions (Molecular Probes, Life Technologies, Eugene, OR, USA).
T-cell induced colitis
Spleen cells from BALB/c, C57BL/6 (Penk +/+) wild-type mice, or preproenkephalin-knockout (Penk −/−) mice were first enriched for CD4+ T cells by use of a CD4+ negative isolation kit and then stained with peridinin–chlorophyll protein (PerCP)–Cy5.5-conjugated anti-CD4 (RM4-5), allophycocyanin-conjugated anti-CD25 (PC61.5), and fluorescein isothiocyanate conjugated anti-CD45RB (C363.16A) monoclonal antibodies (BD Biosciences). CD4+CD25−CD45RBhigh T lymphocytes were then separated by fluorescent cell sorting. Colitis was induced in 6-week-old immunodeficient recipient (SCID or Rag2 −/−) mice by intraperitoneal injection of 400,000 naive CD4+CD25−CD45RBhigh T lymphocytes isolated from immunocompetent genetically compatible (BALB/c or C57BL/6) mice.
Oxazolone-induced colitis
Eight-week-old C57BL/6 mice were swabbed on the abdomen with olive oil alone (noncolitis control group) or with olive oil containing 3% oxazolone (4-ethoxymemethylene-2-phenyl-2-oxazolin-5-one; Sigma). Seven days later, 50% ethanol alone (noncolitis control group) or a solution of 50% ethanol containing 1% oxazolone (colitis groups) was intracolonically injected into the mice. The mice were killed on day 11 of treatment [16].
Macroscopic assessment of inflammation-associated colon damage
Macroscopic colonic tissue damage was evaluated with a scale ranging from 0 to 11 as follows: erythema [absent (0), length of the area less than 1 cm (1), more than 1 cm (2)], edema [absent (0), mild (1), severe (2)], strictures [absent (0), one (1), two (2), more than two (3)], ulceration [absent (0), present (1)], mucus [present (0), absent (1)], and adhesion [absent (0), moderate (1), severe (2)]. Bowel wall thickness was measured with an electronic caliper in the proximal part of the colon, 0.5 cm below the cecum.
Histological assessment of inflammation-associated colon damage
Colonic tissue specimens were excised 2 cm proximal to the cecum and immediately transferred into 10% formol to be embedded in paraffin. Five-micrometer colonic sections were then stained with hematoxylin–eosin. Damage scoring was evaluated on a scale ranging from 0 to 8. Cellular infiltration and mucosal alteration (vasculitis, muscular thickening, and crypt abscesses) were graded from 0 to 3 (absent, mild, moderate and severe). Submucosal edema was scored from 0 to 2 (absent, moderate, and severe) [4].
Cytokine quantification in colonic tissue samples
Colonic samples were homogenized with a Precellys lysing kit (Bertin Technologies, Montigny-le-Bretonneux, France) at 4 °C in 500 µL of cell lysis buffer [20 mM tris(hydroxymethyl)aminomethane–HCl, pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM ethylene glycol bis(2-aminoethyl ether)tetraacetic acid, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and leupeptin at 1 µg/mL; Cell Signaling Technology, Ozymes, Saint-Quentin, France) supplemented with an antiprotease cocktail (Roche). After centrifugation (10,000g, 10 min, 4 °C), 20 µL of supernatant were used for cytokine quantification.
Interferon-γ (IFN-γ), tumor necrosis factor α (TNFα), interleukin (IL)-1β, IL-17A, CC chemokine ligand 2 (CCL2), and CC chemokine ligand 5 (CCL5) were quantified with a cytometric bead array (BD Biosciences). Cytokine concentrations were calculated from standard curves established with recombinant mouse cytokines (BD Biosciences). Quantitative analysis was performed with the software program FCAP Array (BD Biosciences). The amounts of proteins in each sample were quantified with a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Raw values obtained from cytometric bead array assays were then normalized to protein content (mg).
Isolation of lamina propria mononuclear cells
The intestine was longitudinally opened, cut into small pieces, and washed. Intestine pieces were incubated twice with predigestion buffer consisting of RPMI-1640 medium, 5% fetal calf serum, and 5 mM EDTA at 37 °C for 15 min. After they had been, colonic tissues were digested with 0.02% collagenase VIII (Sigma, St Louis, MO, USA) for 1 h at 37 °C. Supernatant was then passed through a 70-µm cell strainer and centrifuged. Mononuclear cells were then isolated with a Percoll gradient.
Flow cytometry analysis of intracellular cytokines
In vitro activated CD4+ T lymphocytes, mesenteric lymph node cells, or mononuclear cells purified from lamina propria were stimulated with phorbol myristate acetate at 50 ng mL−1 and ionomycin at 500 ng mL−1 (Sigma) for 4 h. The protein transport inhibitor brefeldin A (eBioscience) was added to the cells for the last 2 h. After they had been washed, the cells were first incubated with blocking buffer (phosphate-buffered saline with 1% fetal calf serum, 3% normal mouse serum, 3% normal rat serum, 5 mM EDTA, and 0.1% NaN3) containing anti-CD16/CD32 (2.4G2; American Type Culture Collection) at 5 µg mL−1 for 15 min at room temperature. CD4 T-cell surface antigen was stained with fluorescein isothiocyanate conjugated rat anti-mouse CD4 (RM4-5) monoclonal antibody diluted at the optimal concentration in fluorescence-activated cell sorting buffer (phosphate-buffered saline with 1% fetal calf serum, 5 mM EDTA, and 0.1% NaN3) for 30 min on ice. Cells were then fixed with 2% paraformaldehyde in phosphate-buffered saline and permeabilized with 0.5% saponin. Intracellular cytokine staining was performed with Alexa Fluor® 488 conjugated rat anti-IFN-γ (XMG1.2), allophycocyanin-conjugated rat anti-granulocyte–macrophage colony-stimulating factor (GM-CSF) (MP1-22E9), phycoerythrin-conjugated rat anti-IL-17 (TC11-18H10), Alexa Fluor® 488 conjugated rat anti-IL-13 (eBio13A), allophycocyanin-conjugated rat anti-IL-5 (TRFK5) and PerCP–Cy5.5 rat anti-IL-4 (11B11) monoclonal antibodies (all from BD Biosciences). Data were acquired with a LSRFortessa instrument (BD Biosciences) and further analyzed with the software program FlowJo (Tree Star, Ashland, OR, USA).
Colorectal distension and electromyographic recording
Visceral sensitivity was measured by colorectal distension as already described [4]. Briefly, two electrodes implanted in the abdominal external oblique musculature of the mice were connected to an electromyogram acquisition system (ADInstruments, Colorado Springs, CO, USA). A 10.5-mm-diameter balloon catheter (Fogarty catheter for arterial embolectomy, 4F; Edwards Lifesciences, Nijmegen, Netherlands) was gently inserted into the colon 5 mm proximal to the anus. Distensions were performed by inflation of the balloon, and visceromotor responses were calculated with the software program Chart 5 (ADinstruments).
Because of the skewed distribution of the response, nonparametric tests were applied on longitudinal data with use of a ranked-based approach on factorial experiments. Statistics tests were computed with modified ANOVAs with the R software package nparLD for longitudinal data [17]. Mann–Whitney–Wilcoxon tests were used for the post hoc analysis with R 3.0.2. We considered p < 0.05 as significant. The abdominal responses over the different pressures were explained in the statistical model by treatment groups (Penk +/+ and Penk −/−), an interaction term between treatment groups, and distension pressures as fixed effects and a random effect to take into account the repeated measures of distension pressure on mice. Post hoc analysis by a Mann–Whitney–Wilcoxon test was performed at each distension pressure.
Ethical considerations
All experiments involving mice were performed in accord with ethical guidelines (Institut National de la Santé et de la Recherche Médicale), and were approved by the Midi-Pyrénées Ethics Committee (application number MP/06/73/10/12).
Results
Peripheral opioid receptor blockade increases the severity of colonic inflammation in SCID mice into which with CD4+CD45RBhigh T lymphocytes have been transferred
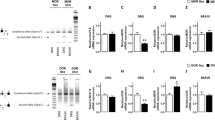
Given that colitogenic T lymphocytes are the main producers of opioids in the inflamed intestinal mucosa [4, 18], we used the T-cell-induced colitis model to investigate the role of endogenous opioids in the regulation of intestinal inflammation. Colitis was induced by transfer of naïve CD4+CD45RBhigh T lymphocytes isolated from immunocompetent BALB/c mice into immune-deficient SCID mice. Naloxone methiodide, an antagonist of the three opioid receptor classes unable to cross the blood–brain barrier, was intraperitoneally injected into the mice three times a week from the third week after T-cell transfer until they were killed. As shown in Fig. 1, the blockade of peripheral opioid receptors by naloxone methiodide significantly increased inflammation-induced colonic injury. Although treatment with naloxone methiodide significantly increased macroscopic and histopathological scores (Fig. 1b, c), the weight loss was similar to that of untreated mice (Fig. 1a). The antagonization of peripheral opioid receptors by naloxone methiodide was associated with an almost twofold increase in the levels of proinflammatory cytokines (IFN-γ, tumor necrosis factor α, IL-1β) and chemokines (CCL2, CCL5) within the inflamed mucosa (Fig. 1d). Thus, activation of opioid receptors by endogenous opioids locally released within the inflamed intestine reduces colitis severity.
Peripheral opioid receptor blockade increases T-cell-induced colonic inflammation. Naïve CD4+CD45RBhigh lymphocytes isolated from wild-type BALB/c mice were passively transferred into severe combined immunodeficiency (SCID) mice. From the third week after cell transfer until they were killed 3 weeks later, 200 µL of either phosphate-buffered saline (PBS; white bars, n = 10) or naloxone methiodide at 10 mg mL−1 (2 mg per mouse) (NLX-meth; black bars, n = 10) was intraperitoneally injected three times a week into recipient SCID mice [13]. a The time course of weight loss, expressed as the percentage of the initial weight (day 0); the arrow denotes when treatment started. b The severity of intestinal inflammation was assessed by measurement of the wall thickness (left), macroscopic colonic damage (middle), and microscopic colonic damage (right). c Representative histopathological analysis performed with an eight-point scale on hematoxylin–eosin-stained colon sections. Mice treated with PBS show moderate cellular infiltration and moderate edema (left), whereas those treated with NLX-meth show massive cellular infiltration, vasculitis, and, in agreement with increased wall thickness, massive submucosal edema (right). d The effect of NLX-meth treatment on the production of proinflammatory cytokines and chemokines by colon tissue. Data are expressed as the mean ± standard error of the mean. Statistical analysis was performed with the Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001, CCL2 CC chemokine ligand 2, CCL5 CC chemokine ligand 5, IFN interferon, IL interleukin, TNF tumor necrosis factor
Enkephalin-deficient CD4+ T lymphocytes display a proinflammatory profile on activation in vitro
Considering that T-cell-induced colitis is dependent on the potency of mucosal effector CD4+ T lymphocytes to respond to the intestinal microbiota, we first assessed whether the lack of enkephalins altered or did not their proliferative properties. In these experiments, naïve CD4+ T lymphocytes purified from wild-type (Penk +/+) or enkephalin-deficient (Penk −/−) mice were stimulated with anti-CD3 and anti-CD28 antibodies under nonpolarizing conditions for 6 days. As depicted in Fig. 2a, the proliferation and activation status (i.e., CD69 and CD25 upregulation) of Penk +/+ and Penk −/− CD4+ T lymphocytes in response to T-cell receptor (TCR) triggering were similar. However, the frequency of CD4+ T lymphocytes producing proinflammatory cytokines, including IFN-γ and GM-CSF, was significantly increased when lymphocytes originated from enkephalin-knockout mice (Fig. 2b). Inversely, the percentage of CD4+ T lymphocytes producing IL-13 was significantly decreased (Fig. 2b). Under these nonpolarizing conditions of TCR-mediated activation, Penk +/+ as well as Penk −/− CD4+ T lymphocytes did not produce IL-17 (not shown). Neither IL-4 nor IL-5 was detected (not shown).
Enkephalin deficiency in CD4+ T lymphocytes favors commitment toward a pro-inflammatory phenotype without altering T-cell-receptor-induced proliferative response under nonpolarizing conditions. Naïve CD4+ T lymphocytes isolated from C57BL/6 (Penk +/+) mice (upper panels) or from preproenkephalin-knockout (Penk −/−) mice (lower panels) were activated with a cocktail of anti-CD3 and anti-CD28 antibodies for 6 days. The purity of the CD4+ T cell preparations was assessed by cytofluorometry after staining with anti-CD4 antibody; proliferation was monitored by analysis of CellTrace Violet dispersion in live CD4+ T cells, and the activation status was estimated by the upregulation of CD69 and CD25 on CD4-gated cells. One representative experiment of three performed is shown in a. b The frequencies of CD4+ T lymphocytes producing interferon-γ (IFNγ), granulocyte–macrophage colony-stimulating factor (GM-CSF), both IFNγ and GM-CSF, or interleukin-13 (IL-13) were analyzed by cytofluorometry (right, n = 3 × 2 per group). A representative cytofluorometric analysis of intracellular production of IFNγ and GM-CSF by activated Penk +/+ and Penk −/− CD4+ T lymphocytes is shown on the left. Data are expressed as the mean ± standard error of the mean. Statistical analysis was performed with the Mann–Whitney U test. *p < 0.05, **p < 0.01
The lack of enkephalin in colitogenic T lymphocytes increases colitis severity
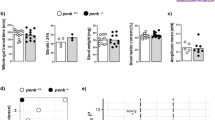
The regulatory role of T-cell-derived enkephalins in intestinal inflammation was investigated by comparison of the severity of colitis in Rag2 −/− mice into which CD4+CD45RBhigh lymphocytes from wild-type (Penk +/+) or enkephalin-deficient (Penk −/−) mice had been transferred. As shown in Fig. 3a–d, macroscopic and histological colon tissue injuries were severer in Rag2 −/− mice into which T lymphocytes from Penk −/− CD4+CD45RBhigh mice had been transferred than in Rag2 −/− mice into which T lymphocytes from Penk +/+ CD4+CD45RBhigh mice had been transferred. The aggravation of the colitis was associated with an increase in the absolute numbers of colitogenic CD4+ T lymphocytes, characterized by the secretion of the proinflammatory cytokines IFN-γ, IL-17, and GM-CSF within mesenteric lymph nodes (Fig. 3e). The enhancement of inflammatory effector CD4+ T lymphocytes within the lymph nodes draining the inflamed intestine was associated with a significant increase in the absolute numbers of mucosal colitogenic CD4+ T lymphocytes, in particular those producing IFN-γ and GM-CSF (Fig. 4). As expected, the intrinsic inability of colitogenic CD4+ T lymphocytes to produce enkephalins also resulted in an increase in the colonic sensitivity to colorectal distension (Fig. 5).
Enkephalin deficiency in colitogenic CD4+ T lymphocytes worsens T-cell-induced colonic inflammation. Spleen cells from wild-type (Penk +/+) or preproenkephalin-knockout (Penk −/−) mice were enriched for CD4+ T cells and then stained with anti-CD4, anti-CD25, and anti-CD45RB monoclonal antibodies. a Lymphocytes gated on CD4 expression (CD4+ cells) were collected on the basis of their expression level of CD45RB and CD25. b CD4+ T lymphocytes that do not express CD25 and highly express CD45RB were intraperitoneally injected into immunodeficient Rag2 −/− mice. Weight loss, expressed as the percentage of the initial weight (day 0), was monitored for 5 weeks and then the mice were killed. c Colitis severity was compared between mice into which Penk +/+ (white bars; n = 28) or Penk −/− (black bars; n = 28) CD4+CD45RBhigh T lymphocytes had been transferred by measurement of wall thickness (left), macroscopic colonic tissue damage (middle), and microscopic colonic tissue damage (right). d Representative histopathological analysis. Mice into which Penk +/+ T lymphocytes had been transferred show moderate cellular infiltration and moderate edema; the overall epithelial structure is still identifiable (left). Mice into which Penk −/− T lymphocytes had been transferred show massive cellular infiltration, crypt abscess, massive submucosal edema, and quasi-complete loss of the overall epithelial structure (right). e The absolute numbers of CD4+ T lymphocytes (top left), the percentage of CD4+ T lymphocytes expressing interferon-γ (IFNγ), interleukin-17 (IL-17), or granulocyte–macrophage colony-stimulating factor (GM-CSF) (top right) and their absolute numbers (lower panels) in mesenteric lymph nodes of Rag2 −/− mice into which Penk +/+ (white bars and open symbols) or Penk −/− T lymphocytes (black bars and closed symbols) had been transferred (n = 4–5 per group). Data are expressed as the mean ± standard error of the mean. Statistical analysis was performed with the Mann–Whitney U test. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant
Rag2 −/− mice into which enkephalin-deficient CD4+ T lymphocytes have been transferred display an increased number of interferon-γ (IFNγ)- and granulocyte–macrophage colony-stimulating factor (GM-CSF)-producing CD4+ T lymphocytes within inflamed intestinal mucosa. a Lamina propria mononuclear cells were isolated from colon of Rag2 −/− mice 5 weeks after the transfer of Penk +/+ (white bars) or Penk −/− (black bars) CD4+CD45RBhigh T lymphocytes. The absolute numbers of the subsets of CD4+ T lymphocytes producing IFNγ, interleukin-17 (IL-17), or GM-CSF were calculated according to the cytofluorometric analysis. Data are expressed as the mean ± standard error of the mean (n = 4–5 per group). Statistical analysis was performed with the Mann–Whitney U test. *p < 0.05. b Representative cytofluorometric analysis of the frequencies of CD4+ T lymphocytes producing IFNγ, IL-17, and/or GM-CSF
The lack of enkephalin in colitogenic CD4+ T lymphocytes exacerbates inflammatory visceral pain. Colonic sensitivity of Rag2 −/− mice was measured by colorectal distension 5 weeks after the transfer of Penk +/+ (open circles; n = 25) or Penk −/− (closed circles; n = 22) CD4+CD45RBhigh T lymphocytes. Abdominal muscle contraction was recorded in response to distension pressures of 15, 30, 45, and 60 mmHg. Data are expressed as the mean ± standard error of the mean. Statistical analysis was performed as described in “Methods.” *p < 0.05, **p < 0.01
Peripheral opioid receptor blockade attenuates the severity of colonic inflammation in Th2-type oxazolone colitis
To assess whether the protective effect of endogenous opioids in Th1/Th17-driven colitis might be dependent on an opioid-mediated shift toward a Th2-type immune response, we investigated the effect of peripheral opioid receptor blockade by naloxone methiodide in the Th2-associated oxazolone colitis model. Colitis was induced by rectal instillation of 3% oxazolone in presensitized naïve C57BL/6 mice. Naloxone methiodide was then intraperitoneally injected into the mice 1 day before and on days 1 and 3 after oxazolone treatment. As shown in Fig. 6, the blockade of peripheral opioid receptors by naloxone methiodide significantly reduces the severity of the disease (Fig. 6a–d). The antagonization of peripheral opioid receptors by naloxone methiodide was associated with a decrease in the absolute number of mononuclear cells infiltrating the colonic mucosa, in particular CD4+ T lymphocytes producing Th2-related cytokines IL-13- and IL-5 (Fig. 6e, f).
Peripheral opioid receptor blockade relieves oxazolone colitis. C57BL/6 mice were swabbed on the abdomen with olive oil alone (noncolitis control, n = 15) or presensitized with 3% oxazolone in olive oil (colitis groups, n = 7–15). Seven days later, 50% ethanol was intracolonically injected into the mice in the absence (noncolitis control group; white square) or the presence of 1% oxazolone (colitis groups). Two hundred microliters of either phosphate-buffered saline (PBS; open circles) or naloxone methiodide (NLX-meth; 10 mg mL−1; closed circles) was intraperitoneally injected into oxazolone-treated mice 1 day before and on days 1 and 3 after the administration of oxazolone. The mice were killed 1 day after the last injection of PBS or NLX-meth. a Weight loss, expressed as the percentage of the initial weight (day 0); the arrow denotes when oxazolone was intrarectally injected. b The severity of intestinal inflammation was assessed by measurement of colon length, wall thickness (2 cm proximal to the anus), and macroscopic and microscopic colonic damage. c, d Representative morphological and histopathological images. Mice treated with oxazolone and into which PBS has been injected exhibit a shortened colon with hemorrhagic edema in the distal half (c, middle). Oxazolone-treated mice into which PBS has been injected show strong cellular infiltration, submucosal edemas, massive epithelial disruption, and muscle thickening (d, middle), whereas those treated with NLX-meth show cellular infiltration, submucosal edema, and vasculitis (d, right). The absolute numbers of mononuclear cells and subsets of CD4+ T lymphocytes producing interleukin (IL)-13, IL-4, or IL-5 (calculated according to the cytofluorometric analysis) isolated from lamina propria are depicted in e and f respectively. Data are expressed as the mean ± standard error of the mean. Statistical analysis was performed with the Kruskal–Wallis test and subsequent Dunn’s multiple comparison tests when appropriate. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant
Discussion
Opioid neuropeptides are expressed in the gut, where they play a role in the regulation of gastrointestinal physiology, including motility and water and electrolyte fluxes [11]. Their receptors are widely distributed throughout the gastrointestinal tract, mainly in enteric neurons but also in intestinal epithelium and immune cells. Components of the endogenous opioid system, including both ligands and receptors, are upregulated on intestinal inflammation [11]. The level of met-enkephalin, the endogenous opioid peptide predominantly produced by T lymphocytes on intestinal inflammation in mice [4, 18], has been shown to be increased in inflamed mucosa from IBD patients [19].
A defect in the regulation of the immune response to the intestinal flora may result in the development of chronic intestinal inflammation. Indeed, aberrant responses of CD4+ Th1 and Th17 lymphocytes to the microbiota highly contribute to the development of IBD as Crohn’s disease. Colitis induced by a failure in the regulation of the T-cell response was mimicked by transfer of naïve CD4+ CD25−CD45RBhigh T lymphocytes in the absence of regulatory CD4+CD25+CD45RBlow T lymphocytes into immunodeficient SCID or Rag2 −/− recipient mice. In the absence of regulatory T lymphocytes, naïve CD4+CD45RBhigh T lymphocytes not yet producing enkephalins are activated and differentiate into proinflammatory Th1 and Th17 effector CD4+ T lymphocytes [2, 4]. While acquiring their proinflammatory properties, colitogenic T lymphocytes upregulate enkephalins, the main opioid peptides produced by lymphocytes in mice [3, 20]. Because of the local release of their enkephalin content, colitogenic Th1 as well as Th17 lymphocytes insidiously render painless inflammation-induced colonic injuries they initiated [4, 18, 21]. SCID mice into which CD4+CD45RBhigh T lymphocytes are transferred develop colitis but are less sensitive to colorectal distension than healthy SCID mice in which no transfer has occurred [4]. This peripheral analgesic effect of T-cell-derived opioids was reversed by treatment of mice with naloxone methiodide 30 min before visceral pain assessment [4]. In contrast with long-lasting treatment, naloxone methiodide does not worsen inflammation-induced colonic damage within 30 min after its injection [4], thereby indicating, in this case, that analgesia is dependent on the effect of T-cell-derived opioids on peripheral sensory neurons.
In addition to their analgesic properties, we have shown here that mucosal T-cell-derived enkephalins exhibit anti-inflammatory effects in the T-cell-induced colitis model. However, the significant reduction of colonic tissue injuries associated with the local release of enkephalins by colitogenic T cells is not sufficiently potent to alter the colitis-associated weight loss. Indeed, as reported in other studies [22], the weight loss commonly related to both the digestive tract dysfunction and the reduction of food uptake is a clinical sign of the disease that does not discriminate subtle differences in gross intestinal injuries.
Given the wide expression of opioid receptors in hematopoietic cells, including neutrophils, macrophages, dendritic cells, and B and T lymphocytes, and nonhematopoietic cells, including epithelial and neuronal cells in the colon, a number of nonexclusive mechanisms may explain the anti-inflammatory effects of enkephalins in the T-cell-induced colitis model. These mechanisms include inhibition of inflammatory cell activity [23], the shift in cytokine pattern from type 1 to type 2 [14, 15], the inhibition of sensory nerve firing [18], and the enhancement/triggering of colon wound healing [9].
On activation, Penk −/− CD4+ T lymphocytes are more prone to produce the proinflammatory cytokines IFN-γ and GM-CSF than are Penk +/+ CD4+ T lymphocytes. Further, δ opioid receptors selective for enkephalins virtually absent in resting CD4+ T lymphocytes are upregulated on TCR-induced activation in vivo and in vitro [13, 24]. Thus, it is tempting to speculate that enkephalins autoregulate the proinflammatory activity of T lymphocytes by modulating their cytokine expression profile.
The expression of opioid receptors differs between the immune cell subsets and, as described in neurons, is highly dependent on their activation status and the local cytokine environment [25–27]. Thus, the endogenous regulatory (direct or indirect) mechanisms of intestinal inflammation by endogenous opioids may differ depending on the physiopathological context.
The propensity of enkephalin-deficient T lymphocytes to produce proinflammatory cytokines such as GM-CSF, which has recently been shown to be crucial for the pathogenesis of T-cell-induced colitis [28], resulted in an exacerbation of inflammation-induced colon injury. As a consequence of both the increase in inflammation-induced injuries and the absence of any inhibitory effect of the enkephalins on the nociceptors (Fig. S1) [3, 4], the colonic sensitivity was significantly higher in Rag2 −/− mice into which lymphocytes from Penk −/− mice had been transferred than in Rag2−/− mice into which lymphocytes from Penk +/+ mice had been transferred. Our data are consistent with data from previous studies demonstrating the anti-inflammatory potency of opioids, including drugs derived from enkephalins [29], in several Th1/Th17-associated colitis models in mice. Indeed, in experimental intestinal inflammation induced by dextran sodium sulfate, 2,4,6-trinitrobenzenesulfonic acid (TNBS), or T-cell transfer, administration of exogenous opioids dramatically improves disease outcome [8, 10, 29, 30]. As reported here for mucosal T-cell-derived enkephalins, the anti-inflammatory effects of opioid drugs were associated with a downregulation of proinflammatory cytokines and reduction of bowel injuries [8, 10, 30]. Accordingly, it can be assumed that the aggravation of intestinal inflammation observed by transfer of enkephalin-deficient T lymphocytes will be reversed by injection of exogenous opioid ligands as already reported in the same model of colitis [10]. It was also reported that the antagonization by naloxone methiodide of peripheral opioid receptors in mice with TNBS-induced colitis worsened inflammation, with more numerous and deeper colon ulcerations [10]. Although the cellular origin of the opioids was not identified, their role in the endogenous regulation of intestinal inflammation was further argued by the increased susceptibility of µ opioid receptor knockout mice to TNBS-induced colitis [10].
In contrast with Th1/Th17-driven colitis models resembling Crohn’s disease, Th2-type oxazolone colitis displays histological resemblance to ulcerative colitis. In agreement with both previous studies reporting opioid-induced Th2 skewing and our results indicating that enkephalin-deficient CD4+ T lymphocytes exhibit a higher propensity to differentiate into IFN-γ-producing cells, naloxone methiodide treatment attenuates the severity of Th2 oxazolone-induced colitis. It is well recognized that IL-4 and IL-13 may induce µ opioid receptor in activated CD4+ T lymphocytes via a signal transducer and activator of transcription 6 dependent pathway [27]. In turn, the activation of µ opioid receptor in activated lymphocytes downregulates TCR signaling pathway [31, 32] and leads to the inhibition of IFN-γ and the induction of IL-4 [14, 33, 34].
Taken together, our data, in line with previous findings, clearly establish the role of endogenous opioid tone in the regulation of intestinal inflammation. They also point out an (auto)regulatory role of enkephalins in the activity of colitogenic CD4+ T lymphocytes. Furthermore, our results highlight the relevance of the therapeutic use of opioid drugs with peripheral activity to reduce both visceral pain and inflammation in chronic Th1/Th17-driven intestinal inflammatory disease [5].
References
Stein C. Opioids, sensory systems and chronic pain. Eur J Pharmacol. 2013;716:179–87.
Boue J, Blanpied C, Brousset P, et al. Endogenous opioid-mediated analgesia is dependent on adaptive T cell response in mice. J Immunol. 2011;186:5078–84.
Basso L, Boue J, Mahiddine K, et al. Endogenous analgesia mediated by CD4+ T lymphocytes is dependent on enkephalins in mice. J Neuroinflammation 2016;13:132.
Boue J, Basso L, Cenac N, et al. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4+ T cells in mice. Gastroenterology. 2014;146:166–75.
Basso L, Bourreille A, Dietrich G. Intestinal inflammation and pain management. Curr Opin Pharmacol. 2015;25:50–5.
Boue J, Blanpied C, Djata-Cabral M, et al. Immune conditions associated with CD4+ T effector-induced opioid release and analgesia. Pain. 2012;153:485–93.
Stein C, Kuchler S. Non-analgesic effects of opioids: peripheral opioid effects on inflammation and wound healing. Curr Pharm Des. 2012;18:6053–69.
Anselmi L, Huynh J, Duraffourd C, et al. Activation of mu opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol Motil. 2015;27:509–23.
Goldsmith JR, Uronis JM, Jobin C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving Stat3 signaling. Am J Pathol. 2011;179:673–83.
Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu-opioid receptor support its use in the treatment of colon inflammation. J Clin Investig. 2003;111:1329–38.
Sobczak M, Salaga M, Storr MA, et al. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. 2014;49:24–45.
Benard A, Boue J, Chapey E, et al. Delta opioid receptors mediate chemotaxis in bone marrow-derived dendritic cells. J Neuroimmunol. 2008;197:21–8.
Jaume M, Laffont S, Chapey E, et al. Opioid receptor blockade increases the number of lymphocytes without altering T cell response in draining lymph nodes in vivo. J Neuroimmunol. 2007;188:95–102.
Roy S, Wang J, Charboneau R, et al. Morphine induces CD4+ T cell IL-4 expression through an adenylyl cyclase mechanism independent of the protein kinase A pathway. J Immunol. 2005;175:6361–7.
Sacerdote P, Manfredi B, Gaspani L, et al. The opioid antagonist naloxone induces a shift from type 2 to type 1 cytokine pattern in BALB/cJ mice. Blood. 2000;95:2031–6.
Cenac N, Cellars L, Steinhoff M, et al. Proteinase-activated receptor-1 is an anti-inflammatory signal for colitis mediated by a type 2 immune response. Inflamm Bowel Dis. 2005;11:792–8.
Noguchi K, Gel YR, Brunner E, et al. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw 2012; 50(12). doi:10.18637/jss.v050.i12
Valdez-Morales E, Guerrero-Alba R, Ochoa-Cortes F, et al. Release of endogenous opioids during a chronic IBD model suppresses the excitability of colonic DRG neurons. Neurogastroenterol Motil. 2013;25:39–46.
Owczarek D, Cibor D, Mach T, et al. Met-enkephalins in patients with inflammatory bowel diseases. Adv Med Sci. 2011;56:158–64.
Baddack-Werncke U, Busch-Dienstfertig M, Gonzalez-Rodriguez S, et al. Cytotoxic T cells modulate inflammation and endogenous opioid analgesia in chronic arthritis. J Neuroinflammation. 2017;14:30.
Basso L, Boue J, Bourreille A, et al. Endogenous regulation of inflammatory pain by T-cell-derived opioids: when friend turns to foe. Inflamm Bowel Dis. 2014;20:1870–7.
Zimmermann J, Kuhl AA, Weber M, et al. T-bet expression by Th cells promotes type 1 inflammation but is dispensable for colitis. Mucosal Immunol. 2016;9:1487–99.
Wang J, Barke RA, Charboneau R, et al. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–34.
Nguyen K, Miller BC. CD28 costimulation induces delta opioid receptor expression during anti-CD3 activation of T cells. J Immunol. 2002;168:4440–5.
Benard A, Cavailles P, Boue J, et al. µ-Opioid receptor is induced by IL-13 within lymph nodes from patients with Sezary syndrome. J Investig Dermatol. 2010;130:1337–44.
Borner C, Woltje M, Hollt V, et al. STAT6 transcription factor binding sites with mismatches within the canonical 5′-TTC…GAA-3′ motif involved in regulation of delta- and mu-opioid receptors. J Neurochem. 2004;91:1493–500.
Kraus J, Borner C, Giannini E, et al. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J Biol Chem. 2001;276:43901–8.
Griseri T, Arnold IC, Pearson C, et al. Granulocyte macrophage colony-stimulating factor-activated eosinophils promote interleukin-23 driven chronic colitis. Immunity. 2015;43:187–99.
Sobczak M, Pilarczyk A, Jonakowski M, et al. Anti-inflammatory and antinociceptive action of the dimeric enkephalin peptide biphalin in the mouse model of colitis: new potential treatment of abdominal pain associated with inflammatory bowel diseases. Peptides. 2014;60:102–6.
Goldsmith JR, Perez-Chanona E, Yadav PN, et al. Intestinal epithelial cell-derived mu-opioid signaling protects against ischemia reperfusion injury through PI3 K signaling. Am J Pathol. 2013;182:776–85.
Borner C, Kraus J. Inhibition of NF-κB by opioids in T cells. J Immunol. 2013;191:4640–7.
Borner C, Warnick B, Smida M, et al. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 2009;183:882–9.
Roy S, Wang J, Gupta S, et al. Chronic morphine treatment differentiates T helper cells to Th2 effector cells by modulating transcription factors GATA 3 and T-bet. J Neuroimmunol. 2004;147:78–81.
Wang J, Barke RA, Charboneau R, et al. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003;278:37622–31.
Acknowledgements
The authors thank the ANEXPLO (UMR 006) animal care facility (Y. Barreira and S. Appolinaire), Aninfimip, an EquipEx (Equipement d’Excellence) supported by the French government through the Investments for the Future program (ANR-11-EQPX-0003), and the U1043 flow cytometry facility (F. L’Faqihi-Olive and V. Duplan-Eche). This work was supported by the Institut National de la Santé et de la Recherche Médicale, Université Paul Sabatier, Toulouse III, and the Association François Aupetit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Basso, L., Garnier, L., Bessac, A. et al. T-lymphocyte-derived enkephalins reduce Th1/Th17 colitis and associated pain in mice. J Gastroenterol 53, 215–226 (2018). https://doi.org/10.1007/s00535-017-1341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1341-2