Abstract
Background
Peripherally inserted central catheters (PICCs) and midline catheters (MCs) may offer convenient intravenous access, but evidence to support their place in palliative care is limited. This review aimed to assess catheter indications, utilization, complications, dwell time, and patient experiences in cancer patients receiving palliative care.
Methods
A systematic search for studies on catheter utilization for supportive or symptom treatment was conducted in Medline, Embase, CINAHL, Web of Science, Cochrane, and CENTRAL databases. Studies with a study population or a subgroup of palliative care cancer patients were included. Study quality was assessed using the Effective Public Health Practice Quality assessment tool.
Results
Of 7631 unique titles, 17 articles were examined in detail, all published between 2002 and 2022. Median catheter dwell time varied from 15 to 194 days, the longest when utilized for home parenteral nutrition. For pain and symptom management, the typical duration was 2–4 weeks, often until the patient’s death. Complication rates were minimal, with thrombosis, infections, and occlusion ranging from 0 to 2.46 incidents per 1000 catheter days. In studies from palliative care services, patients reported minimal distress during procedures and high user satisfaction. Quality of life assessments post-procedure improved, possibly influenced by concurrent specialist palliative care provision. All studies were assessed to be of moderate or weak quality.
Conclusion
PICC and MC are safe and valuable tools in palliative care cancer patients who would benefit from intravenous access for symptom management. Further studies are needed to clarify indications for PICC or MC in palliative care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, the subcutaneous (SC) route has due to its simplicity, safety, and ubiquity been preferred for parenteral drug administration in palliative care patients. This is reflected in existing guidelines where the SC route is advocated as the preferred route for parenteral administration [1, 2]. Yet, the intravenous (IV) route, with its broader applicability, reduced incidence of local infiltrations and irritations, and more consistent pharmacokinetics, is recognized as a valuable alternative, especially when rapid drug action is paramount [3, 4].
Peripherally inserted central venous catheters (PICC) and midline catheters (MC) not only enable easier IV treatment in home care settings but also sidestep the discomforts associated with frequent venipuncture and cannulation [5, 6]. PICCs are inserted into the basilic, cephalic, or brachial vein by percutaneous cannulation. MCs, peripheral intravenous lines ranging between 3 and 8 inches, are inserted by the same technique without advancing the catheter beyond the distal axillary vein, primarily catering to shorter-term requirements.
While several studies address PICC placement in cancer patients undergoing chemotherapy, comprehensive guidelines from both European and American Societies of Oncology neither endorse nor discourage the use of a particular type of central venous catheter [7, 8]. Notably, recent randomized controlled trials support the preference of venous access ports over PICC for chemotherapy due to thrombosis concerns [9, 10]. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC), based on a combination of systematic reviews and expert opinions, positions PICCs as a viable option for cancer patients undergoing prolonged IV therapy [11]. However, it underscores a paucity of data concerning palliative care.
The aim of this systematic review was to comprehensively assess the use of PICC and MC in palliative care cancer patients. This entails identifying the indications and settings for catheter placement, treatments and procedures in which these catheters are applied, catheter dwell times, the timing of placement in relation to a patient’s death, and complications and reasons for catheter removal. Additionally, this review aimed to assess palliative care cancer patients’ experiences with PICC or MC.
Method
The study was conducted following PRISMA guidelines [12]. A systematic search was conducted in Medline, Embase, CINAHL, Web of Science, Cochrane, and CENTRAL databases. The search strategy to identify palliative care patients was adapted from a validated search filter for identifying this patient population [13]. The complete search strings (Appendix 1) were developed by an experienced biomedical librarian specialist with input from the researchers. The last day searched was December 23rd, 2022. Additionally, reference lists of the included articles were hand-searched for relevant papers.
Titles and abstracts of the retrieved citations were reviewed by one of the researchers (EG), and potentially relevant papers were read in full text by two researchers (EG, OMF). In cases of doubt or disagreement, papers were reassessed by EG and OMF to reach an agreement.
Inclusion criteria
Papers were selected for further reading if the abstract contained any information related to the use of PICC or MC for supportive or symptom treatment in patients with cancer receiving palliative care. Full-text publications that identified a group or subgroup of cancer patients and described catheter utilization for palliative care interventions were included in the review. Palliative care interventions included any pain or other symptom treatment, transfusions, hydration, and parenteral nutrition. Studies on patients with a catheter primarily for administration of tumor-targeted treatment were not included. Studies with a heterogeneous study population were included if the majority of patients were cancer patients receiving palliative care or if data from a subgroup matching this description were presented separately. In line with the WHO definition of palliative care [14], studies on cancer patients receiving palliative care were included irrespective of expected survival time. Accordingly, study populations range from end-of-life care to patients with a longer life expectancy.
The review incorporated studies that employed either an experimental or quasi-experimental design (including clinical trials, cohorts, or case–control studies), as well as case reports, cross-sectional studies, and literature reviews. Studies addressing patient experiences were included. Any comparison groups, or the lack thereof, were accepted. Opinion pieces and conference abstracts were excluded. Studies were restricted to English language and publication date of January 2000 or later.
Quality assessment
The Effective Public Health Practice Quality (EPHPP) assessment tool for quantitative studies [15] was used to rate the 17 studies. This tool is applicable across a wide range of quantitative study designs. Studies were rated weak, moderate, or strong on the following six components: selection bias, study design, confounders, blinding, data collection, and withdrawal. The quality ratings across the six domains were aggregated to give a global rating for each study as follows: weak (two or more component weak ratings), moderate (one weak rating), or strong (no weak ratings). Quality assessments were scored independently by two authors (EG, OMF), and discrepancies were discussed until a consensus was reached. Bias was further discussed at an outcome level when considered relevant.
Results
Abstracts and papers
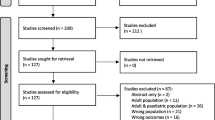
The search identified 4222 titles in Medline, 2628 in Embase, 1791 in Web of Science, 1291 in CINAHL, 687 in CENTRAL, and 22 in the Cochrane database (Fig. 1). After the removal of duplicates, a total of 7631 titles and abstracts were screened, yielding 192 articles that were selected for further reading according to the selection criteria described above. After examining the full papers, 17 articles were included in the study. Hand searching the reference lists of included papers did not identify additional relevant articles. Heterogeneity of studies did not allow for meta-analysis.
The 17 studies included in the analysis were published between 2002 and 2022 (Table 1). Eight were conducted in Europe (Italy, Poland, Spain, Switzerland, UK) [5, 6, 16,17,18,19,20,21], three in the USA [11, 22, 23], and six in Asia (China, Japan, South Korea, Taiwan) [24,25,26,27,28,29]. All studies were conducted either exclusively or predominantly in patients with a main diagnosis of cancer. One study was a randomized controlled trial [26], one a systematic review with guideline [11], four cohort studies [16, 18, 19, 22], nine case series [5, 6, 17, 20, 21, 23, 24, 27, 28], and two single case reports [25, 29]. Nine of the in total 13 case series and cohort studies were prospective surveys.
Six studies were carried out on patients in palliative care units or hospices [5, 6, 24, 26,27,28], of which two studies also encompassed home care settings [5, 24]. Five studies were conducted on cancer patients receiving home parenteral nutrition (HPN) [16, 18, 19, 21, 22]. Three studies were undertaken on patients from several hospital wards, or hospitals with mixed patient populations where 56–100% had cancer [17, 20, 23]. The two single case studies described hospitalized palliative care cancer patients with catheter-related complications [25, 29]. The number of participants per study ranged from 1 to 1250. In two studies, the use of both PICC and MC was investigated [5, 6]; in nine studies, exclusively PICC was addressed [17, 20, 23,24,25,26,27,28,29]; and in six studies, PICC was examined in comparison to other central venous catheters [11, 16, 18, 19, 21, 22].
Quality assessment
The EPHPP global rating scores are presented in Table 1. None of the included articles were rated “strong.” Seven were rated “moderate” and nine as “weak.” The most common factors contributing to a weak rating were the lack of blinding, failure to control for confounders, and the use of unvalidated data collection methods.
Indications for catheter placement and catheter utilization
The six studies conducted primarily in palliative care units and hospices [5, 6, 24, 26,27,28] all documented pain or symptom management as an indication for PICC or MC. Additional indications reported in these studies included hydration, blood sampling, blood product transfusions, and nutrition.
Five studies were primarily concerned with PICC utilized for HPN in cancer patients [16, 18, 19, 21, 22]. In two of these studies [16, 21], PICCs or tunneled PICCs were by local tradition preferred to centrally inserted catheters and ports in patients with low-performance status, palliative treatment intention, and expected period of HPN shorter than 6 months. In the other three studies on patients with HPN, either no selection criteria were presented, or clinicians’ and institutional preferences were described as motivating factors for the choice of PICC or other central venous access options. In the three studies from mixed hospital wards [17, 20, 23], catheter indications such as antibiotics, blood sampling and blood products, nutrition, hydration, pain management, and palliative care were reported.
PICC and MC dwell times and patient survival after catheter placement
Median dwell times for PICCs were documented in 11 studies and ranged from 15 to 194 days across studies. PICC dwell time of less than 20 days was reported in four studies [23, 26,27,28]. Three of these studies were conducted in palliative care units where death was the reason for catheter removal in 72–82% of cases. In a study mainly on patients receiving chemotherapy, but with a small subgroup of two patients who received a PICC primarily for palliative care [20], the PICC dwell times for these two patients were 8 and 11 days, respectively.
Three studies demonstrated a median PICC dwell time of more than 100 days [5, 18, 21]. Two of these studies were conducted on cancer patients with PICCs utilized for HPN. The third study was from a palliative care network of hospices and home care services and included 13 patients with PICCs that had a median dwell time of 102 (range 13–462) days [5]. Removal reasons were not specified, but at the end of the 17-month study period, 11 of 13 patients with PICC had died. This study was also the only one that presented an MC median dwell time, which for the 42 participants was 50.5 (range 8–231) days. Five of the 42 patients were still alive at the end of the study.
The other study including MCs was performed within a palliative care department [6] and encompassed eight patients with MCs and 58 patients with short (9 cm) MCs. At the end of the study, 44% of the participants had dropped out. The proportion of patients who died during the 14-day study period is not disclosed, but already at follow-up day three, 9% of participants were deceased.
Patient survival after catheter placement was reported explicitly in only one study [19]. In this British study on HPN for patients with advanced cancer, the median overall survival after PICC or centrally inserted tunneled catheters was 80 days (2.64 months). In addition, two studies from South Korea reported median survival of 16 and 39 days after hospital admittance for PICC [26, 27]. Both studies were conducted on terminally ill cancer patients admitted to hospice or palliative care departments.
Reasons for removal of PICC and MC
Four studies on patients in hospices and palliative care units [5, 26,27,28] and one study on cancer patients receiving HPN [18] described death as the main reason for catheter removal. The latter study also had the longest median PICC dwell time of the included studies. Additionally, the three studies on mixed patient populations [17, 20, 23] reported death as the reason for PICC removal in 13.3–54.1% of cases. In the same three studies, completed therapy was the removal reason in 36.7–63.7% of cases. The Italian study on cancer patients receiving HPN [18] and the Taiwanese study comparing PICC for palliative care with PICC for chemotherapy [24] reported completed therapy as reason for PICC removal in 31% and 22.9% of cases, respectively.
All of the clinical studies on PICC described complications as a reason for catheter removal, accounting for 1–33% of cases. Accidental removal was documented in eight studies [17, 19, 21, 23, 24, 26,27,28] with a frequency of 0.7–12.3%. Occlusion as removal reason was outlined in four studies [5, 20, 24, 28] with 0.7–8% frequency. Infection-related PICC removal was reported in nine studies with a frequency range of 0–8% [5, 6, 16, 19,20,21,22, 25, 28]. Catheter removal due to catheter-related thrombosis was noted in two studies, with 1.5 and 4% frequencies [20, 23]. One study reported catheter removal in 5.2% of patients due to catheter tip migration [20], and another study reported catheter damage in 6% of PICCs [21]. One study mentioned patients’ requests as the reason for 0.5% of PICC removals [24]. In the RCT from South Korea conducted in a palliative care unit, PICC removal due to patient transfer to home or other hospitals was reported in 14% of PICC users [26].
MC removal due to complications was reported in two Italian studies [5, 6]. In the study on palliative care patients in hospice or home care [5], one of the 35 study participants had the MC removed due to occlusion. In the 14-day follow-up study including eight patients with MC and 58 patients with short MC in a palliative care unit [6], four patients had an accidental removal of a short MC, and five patients with a short MC placed in a superficial vein had phlebitis.
The two single case studies described complications to PICC in palliative care patients with cancer [25, 29]. One was a case of persistent hiccups, in which the PICC had migrated to and was bent and folded in the right subclavian vein. The symptom resolved after catheter removal. The other case was a patient with intestinal obstruction after abdominal cancer surgery and PICC-related infection with Kocuria marina, which was resolved after catheter removal and antimicrobial therapy.
PICC and MC complication rates
The overall complication rate for PICC was documented in eight studies and ranged from 0.69 to 14.1 per 1000 catheter days [5, 17, 18, 20, 21, 23, 24, 27]. There were no reports on complication rates for MC, but in one study on 13 PICCs and 35 MCs, the overall complication rate was 3.6 per 1000 catheter days.
In some of the studies, only complications leading to catheter removal were counted [17, 23, 24]. In one study, only the complication of catheter-related bloodstream infections was investigated [22]. Two studies omitted any mentioning of (accidental) catheter dislocation as a complication [16, 20]. It is possible that either no instances of accidental catheter removal occurred, or this issue was not regarded as a complication in those studies.
Catheter-related bloodstream infections were reported in eleven studies [5, 16,17,18,19,20,21,22,23,24, 26], with a rate between 0 and 2.46 per 1000 catheter days. A catheter-related infection was commonly defined as a clinically suspected bloodstream infection verified by positive peripheral and central blood culture or catheter tip culture [5, 16, 18, 21, 22, 26]. Two studies registered suspected catheter-related infections unverified by blood or tip culture [20, 23], one study only registered suspected exit site infections [17], and two studies did not provide a definition [19, 24].
The highest complication rate was from the 2002 US study, which included 351 PICCs in cancer patients of all ages [23]. This study included both primary and secondary catheter-related bloodstream infections and the highest rates were found among patients with hematological malignancies and bone marrow transplants. Eight studies reported a rate below 0.5 per 1000 catheter days, including four studies primarily focusing on HPN [16, 18, 19, 21], two studies on palliative care cancer patients with PICC for symptom treatment [5, 24], and two studies with mixed populations and indications for PICC [17, 20]. Neither of the two studies on MC identified MC-related bloodstream infections [5, 6].
The rate of PICC-related thrombosis ranged from 0 to 1.14 per 1000 catheter days [16,17,18, 20, 21, 23, 26]. Typically, a radiology-verified diagnosis of thrombosis required the participant to exhibit symptoms. However, in the Polish study on HPN, routine ultrasound investigations detected both symptomatic and asymptomatic deep vein thrombosis at a rate of 0.17 and 0.09 per 1000 catheter days, respectively [21]. MC-related thrombosis was not recognized in any of the studies.
PICC occlusion varied from 0 to 2.34 per 1000 catheter days [5, 17, 20, 23, 24, 27]. Occlusion was commonly acknowledged as a lumen occlusion resistant to attempts of regaining flow. In one of the larger retrospective case reviews with an occlusion rate of 1.08 per 1000 catheter days, both complete and partial occlusion and withdrawal occlusion were considered [17].
Accidental removal occurred at a rate of 0.04 to 6.1 per 1000 PICC days [5, 17, 21, 23, 24, 26, 27]. The lowest rate was reported in the Polish study where PICCs were tunneled [21]. The highest rates of 3.1 and 6.1 were documented in the two South Korean studies from palliative care units where median patient survival was 16 and 39 days, respectively [26, 27]. In both studies, an association with terminal delirium was suggested.
Palliative care cancer patients’ experiences with PICC or MC
Patient-related outcome measures were only documented in studies from hospice/palliative care departments [5, 26,27,28]. In these studies, 79–94% of patients reported little or no distress during PICC procedure [27, 28], and 83–96% of patients reported a favorable satisfaction with PICC compared to before PICC [27, 28] or compared to peripheral vascular access [26]. In three of these studies [26,27,28], assessments of patient satisfaction were conducted in an unblinded manner with study-specific questionnaires. Participants were asked after catheter placement, “Did you experience distress because of the procedure?” They could respond with “distressing,” “slightly distressing,” or “not distressing.” In two of these studies [26, 27], participants were also asked 5 days after catheter placement, “Are you more comfortable after placement of parenteral access?” Participants could answer on a verbal rating scale ranging from “much comfort,” to “much discomfort.”
In a study on PICC and MC for palliative care patients in hospice or home care [5], quality of life was assessed with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core15-Palliative (EORTC QLQ-C15-PAL) [30], which was completed at baseline and 1 week after catheter placement. The study demonstrated improved global health score 1 week after the procedure, along with a trend of improved scores on physical symptoms. In another study on PICC or MC impact on quality of care for patients at a palliative care unit, the Palliative Care Outcome Scale (POS) indicated a favorable impact on quality of care 3-day post-catheter placement [6]. It is worth noting that these improvements might also be attributed to the simultaneous initiation of specialist palliative care. There were no differences between PICC and MC subgroups.
Discussion
This systematic review included 17 articles concerning the application of PICC and MC for supportive interventions and symptom treatment in palliative care cancer patients, with the majority of studies being case series and cohort studies. Strikingly, only three of the included studies actually incorporated MCs. A mere six of the included studies were based in specialist palliative care settings, emphasizing the limited evidence surrounding both the use and the complications of PICCs in this setting.
The PICC dwell times exhibited considerable variation across studies, which also reflects when in the patient’s disease trajectory the catheter was placed: The duration of the PICC usage was protracted when used for HPN, while the typical duration for pain and symptom management was 2–4 weeks, with the catheter often maintained until death. This pattern may underscore the heightened need for parenteral symptom treatment as patients near the end of life. Moreover, the broad variation in dwell times of PICCs, including shorter durations than an MC typically could accommodate, suggests the absence of clear guidelines on choosing the most fitting vascular access for these scenarios. Notably, even in the study where the median dwell time for MCs was 50.2 days—surpassing the recommended 30-day lifespan—the complication rates remained low [5].
Our review found PICCs and MCs to have a generally low complication rate. Notably, one of the highest rates was from the oldest study, which saw more frequent infections and phlebitis [23]. In this study, diverse catheter placement methods, often without ultrasound guidance, were practiced. One important reason for variation in complication rates across studies was discrepancies in what was considered a complication. Some studies focused solely on complications leading to catheter removal, and complications such as accidental catheter dislocation and bleeding were inconsistently recorded. Notably, trivial bleeding during catheter insertion was reported only in the two South Korean studies [26, 27], contributing to their elevated complication rates.
The rates for catheter-related thrombosis, infections, and occlusion were all low (0–2.46 per 1000 catheter days), underlining the safety of these catheters. In terminally ill patients, diagnostic procedures might be skipped to spare discomfort, leading to underreporting of complications. However, in several of the included studies, there were attempts to compensate this through routine PICC culture after catheter removal [26,27,28] or routine ultrasound to detect asymptomatic catheter-related thrombosis [21].
Short catheter dwell times might influence observed complications, as risk factors for complications, such as infections, amplify with extended use of central venous catheters. However, in the included studies, no discernible trend of rising infection rates was evident with extended dwell times.
Cancer patients with PICCs utilized primarily for chemotherapy were not encompassed in this review. These patients face, especially when immunocompromised, an elevated risk of catheter-related infections [31]. This assertion is substantiated by one of the studies within our review which demonstrated a higher infection rate in PICCs used for chemotherapy compared to PICCs used for symptom management [24].
Previous studies have indicated an association between catheter-related thrombosis and cancer, particularly hematological cancers, when using PICCs [32]. However, advancements in insertion techniques in recent research appear to mitigate this risk [33]. Furthermore, it is suggested that catheter diameter, especially multi-lumen catheters, may elevate thrombosis risk [34]. The majority of studies in our review exclusively utilized single-lumen PICCs. In the reviewed studies, ports or other central venous catheters (CVCs) were typically favored over PICCs for chemotherapy and anticipated longer catheter dwell time, which might influence the observed complication rates.
Patient satisfaction regarding PICC or MC was uniformly high. This is in contrast to findings from a study involving a broader cohort of hospitalized patients, who, when receiving PICC or MC for various reasons, reported higher levels of distress from both the procedure and the indwelling catheter [35]. The consistent positive response in our reviewed studies might be influenced by the non-blinded nature of evaluations and potentially further biased by feedback being provided directly to the treating clinician. In the included studies, patient satisfaction with the catheter was assessed in comparison to peripheral venipuncture, and the timing of evaluation often coincided with admittance to specialist palliative care. Furthermore, introducing a comparison to optimized subcutaneous symptom management could alter perceptions.
Despite the detailed review, there were inherent limitations. Relevant papers may have been published after the literature search was completed. Defining the palliative care cancer population is a challenge. To counteract this, we employed a previously published search strategy specifically curated to have a high sensitivity for identifying palliative care patients and cast a wide net by including both “cancer” and “palliative care” patients in our search strategy. Palliative care is applicable at different stages of the cancer disease trajectory, which may result in large variations in catheter utilization, dwell times, and complications within this heterogeneous population. Parenteral nutrition, if indicated, is typically provided earlier in the disease trajectory and may present different catheter complications from those seen with pain and symptom management. Including studies with parenteral nutrition could therefore be questioned. However, the complication rates remained low for this intervention in palliative care patients. The few relevant hits, low quality of evidence, and the broad spectrum of indications and settings highlight a need for additional research to elucidate the role of PICC and MC in the management of palliative care cancer patients.
Conclusion
PICC and MC are safe tools with high user satisfaction and can be utilized for extended periods. However, we still lack clarity on optimal deployment for pain and symptom management in palliative care cancer patients, specifically in relation to preferential use over peripheral IV or SC administration routes. Questions also remain regarding the optimal timing in a patient’s disease trajectory to introduce these catheters and when to favor PICC over MC, especially if a catheter is only required for a few weeks. Further studies are needed to clarify indications for PICC or MC in palliative care.
Data availability
No datasets were generated or analyzed during the current study.
References
Fallon M, Giusti R, Aielli F, Hoskin P, Rolke R, Sharma M, et al. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(Suppl 4):iv166-iv91. https://doi.org/10.1093/annonc/mdy152
National Clinical Guideline Centre (UK) (2015) Care of dying adults in the last days of life. (NICE Guideline, No. 31). National Institute for Health and Care Excellence (NICE), London. https://www.ncbi.nlm.nih.gov/books/NBK338140/
Dale O, Thoner J, Nilsen T, Tveita T, Borchgrevink PC, Klepstad P (2007) Serum and cerebrospinal fluid morphine pharmacokinetics after single doses of intravenous and intramuscular morphine after hip replacement surgery. Eur J Clin Pharmacol 63(9):837–842. https://doi.org/10.1007/s00228-007-0329-x
Tveita T, Thoner J, Klepstad P, Dale O, Jystad A, Borchgrevink PC (2008) A controlled comparison between single doses of intravenous and intramuscular morphine with respect to analgesic effects and patient safety. Acta Anaesthesiol Scand 52(7):920–925. https://doi.org/10.1111/j.1399-6576.2008.01608.x
Bortolussi R, Zotti P, Conte M, Marson R, Polesel J, Colussi A et al (2015) Quality of life, pain perception, and distress correlated to ultrasound-guided peripherally inserted central venous catheters in palliative care patients in a home or hospice setting. J Pain Symptom Manage 50(1):118–123. https://doi.org/10.1016/j.jpainsymman.2015.02.027
Magnani C, Calvieri A, Giannarelli D, Espino M, Casale G (2019) Peripherally inserted central catheter, midline, and “short” midline in palliative care: patient-reported outcome measures to assess impact on quality of care. J Vasc Access 20(5):475–481. https://doi.org/10.1177/1129729818814732
Sousa B, Furlanetto J, Hutka M, Gouveia P, Wuerstlein R, Mariz JM, et al (2015) Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann Oncol 26 Suppl 5(suppl 5):v152–68. https://doi.org/10.1093/annonc/mdv296
Schiffer CA, Mangu PB, Wade JC, Camp-Sorrell D, Cope DG, El-Rayes BF et al (2013) Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 31(10):1357–1370. https://doi.org/10.1200/JCO.2012.45.5733
Moss JG, Wu O, Bodenham AR, Agarwal R, Menne TF, Jones BL et al (2021) Central venous access devices for the delivery of systemic anticancer therapy (CAVA): a randomised controlled trial. Lancet 398(10298):403–415. https://doi.org/10.1016/S0140-6736(21)00766-2
Taxbro K, Hammarskjold F, Thelin B, Lewin F, Hagman H, Hanberger H, Berg S (2019) Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth 122(6):734–741. https://doi.org/10.1016/j.bja.2019.01.038
Chopra V, Flanders SA, Saint S, Woller SC, O’Grady NP, Safdar N et al (2015) The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med 163(6 Suppl):S1-40. https://doi.org/10.7326/M15-0744
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Rietjens JA, Bramer WM, Geijteman EC, van der Heide A, Oldenmenger WH (2019) Development and validation of search filters to find articles on palliative care in bibliographic databases. Palliat Med 33(4):470–474. https://doi.org/10.1177/0269216318824275
World Health Organization (2002) National cancer control programmes: policies and managerial guidelines, 2nd edn. World Health Organization, Geneva. https://www.who.int/publications/i/item/national-cancer-control-programmes
McMaster Evidence Review & Synthesis Team: Quality assessment tool for quantitative studies. https://merst.healthsci.mcmaster.ca/ephpp/ (2010). Accessed.
Botella-Carretero JI, Carrero C, Guerra E, Valbuena B, Arrieta F, Calanas A et al (2013) Role of peripherally inserted central catheters in home parenteral nutrition: a 5-year prospective study. JPEN J Parenter Enteral Nutr 37(4):544–549. https://doi.org/10.1177/0148607112457422
Campagna S, Gonella S, Berchialla P, Rigo C, Morano G, Zerla PA et al (2019) A retrospective study of the safety of over 100,000 peripherally-inserted central catheters days for parenteral supportive treatments. Res Nurs Health 42(3):198–204. https://doi.org/10.1002/nur.21939
Cotogni P, Mussa B, Degiorgis C, De Francesco A, Pittiruti M (2021) Comparative complication rates of 854 central venous access devices for home parenteral nutrition in cancer patients: a prospective study of over 169,000 catheter-days. JPEN J Parenter Enteral Nutr 45(4):768–776. https://doi.org/10.1002/jpen.1939
Kopczynska M, Teubner A, Abraham A, Taylor M, Bond A, Clamp A, et al (2022) Home parenteral nutrition in patients with advanced cancer: quality outcomes from a centralized model of care delivery. Nutrients 14(16). https://doi.org/10.3390/nu14163379
Lo Priore E, Fliedner M, Heverhagen JT, Novak U, Marschall J (2017) The role of a surveillance programme for intro-ducing peripherally inserted central catheters: a 2-year observational study in an academic hospital. Swiss Med Wkly 147:w14441. https://doi.org/10.4414/smw.2017.14441
Matysiak K, Szewczuk M, Sobocki J, Zdziarska M, Siatkowski I (2021) Complications of tunneled peripherally inserted and tunneled-cuffed central catheters in home parenteral nutrition. Nutrition 91–92:111354. https://doi.org/10.1016/j.nut.2021.111354
Vashi PG, Virginkar N, Popiel B, Edwin P, Gupta D (2017) Incidence of and factors associated with catheter-related bloodstream infection in patients with advanced solid tumors on home parenteral nutrition managed using a standardized catheter care protocol. BMC Infect Dis 17(1):372. https://doi.org/10.1186/s12879-017-2469-7
Walshe LJ, Malak SF, Eagan J, Sepkowitz KA (2002) Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 20(15):3276–3281. https://doi.org/10.1200/JCO.2002.11.135
Lin S, Wen K, Liu C, Wei C (2010) The use of peripherally inserted central catheters in cancer patients. J Assoc Vasc Access 15(1):16–19. https://doi.org/10.2309/java.15-1-4
Mori N, Nishihara Y, Tayama H, Higuchi A, Aoki Y (2017) Peripherally inserted central catheter-related bloodstream infection caused by Kocuria marina in an elderly man. Infection 45(5):683–686. https://doi.org/10.1007/s15010-017-0980-9
Park EJ, Park K, Kim JJ, Oh SB, Jung KS, Oh SY et al (2021) Safety, Efficacy, and patient satisfaction with initial peripherally inserted central catheters compared with usual intravenous access in terminally ill cancer patients: a randomized phase II study. Cancer Res Treat 53(3):881–888. https://doi.org/10.4143/crt.2020.1008
Park K, Jun HJ, Oh SY (2016) Safety, efficacy, and patient-perceived satisfaction of peripherally inserted central catheters in terminally ill cancer patients: a prospective multicenter observational study. Support Care Cancer 24(12):4987–4992. https://doi.org/10.1007/s00520-016-3360-6
Yamada R, Morita T, Yashiro E, Otani H, Amano K, Tei Y, Inoue S (2010) Patient-reported usefulness of peripherally inserted central venous catheters in terminally ill cancer patients. J Pain Symptom Manage 40(1):60–66. https://doi.org/10.1016/j.jpainsymman.2009.11.327
Zhang Y, Jiang H, Wei L, Yu H (2011) Persistent hiccup caused by peripherally inserted central catheter migration. J Anesth 25(4):625–626. https://doi.org/10.1007/s00540-011-1146-z
Groenvold M, Petersen MA, Aaronson NK, Arraras JI, Blazeby JM, Bottomley A et al (2006) The development of the EORTC QLQ-C15-PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer 42(1):55–64. https://doi.org/10.1016/j.ejca.2005.06.022
Larcher R, Barrigah-Benissan K, Ory J, Simon C, Beregi JP, Lavigne JP, Sotto A (2023) Peripherally inserted central venous catheter (PICC) related bloodstream infection in cancer patients treated with chemotherapy compared with noncancer patients: a propensity-score-matched analysis. Cancers (Basel) 15(12). https://doi.org/10.3390/cancers15123253
Mitbander UB, Geer MJ, Taxbro K, Horowitz JK, Zhang Q, O’Malley ME et al (2022) Patterns of use and outcomes of peripherally inserted central catheters in hospitalized patients with solid tumors: a multicenter study. Cancer 128(20):3681–3690. https://doi.org/10.1002/cncr.34410
Balsorano P, Virgili G, Villa G, Pittiruti M, Romagnoli S, De Gaudio AR, Pinelli F (2020) Peripherally inserted central catheter-related thrombosis rate in modern vascular access era-when insertion technique matters: a systematic review and meta-analysis. J Vasc Access 21(1):45–54. https://doi.org/10.1177/1129729819852203
Chopra V, Anand S, Hickner A, Buist M, Rogers MA, Saint S, Flanders SA (2013) Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 382(9889):311–325. https://doi.org/10.1016/s0140-6736(13)60592-9
Leonardsen AL, Lunde EM, Smith ST, Olsen GL (2020) Patient experiences with peripherally inserted venous catheters- a cross-sectional, multicentre study in Norway. Nurs Open 7(3):760–767. https://doi.org/10.1002/nop2.448
Acknowledgements
The authors acknowledged Helene Lie Biomedical librarian specialist Akershus University Hospital.
Funding
Open access funding provided by Akershus University Hospital (AHUS) This study was supported by the South-Eastern Norway Regional Health Authority.
Author information
Authors and Affiliations
Contributions
Eva Gravdahl (E.G.), Dagny Faksvåg Haugen (D.F.H), Olav Magnus Fredheim (O.M.F) Eva Gravdahl (EG) Dagny Faksvåg Haugen (DFH), Olav Magnus Fredheim (OMF) OMF, DFH and EG planned and designed the study. Titles and abstracts of the retrieved citations were reviewed by EG and potentially relevant papers were read in full text by EG, DFH and OMF. In cases of doubt or disagreement, papers were reassessed by EG and OMF to reach agreement. EG, OMF, and DFH critically revised the manuscript and analyses strategy, and approved the submission of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gravdahl, E., Haugen, D.F. & Fredheim, O.M. Use of peripherally inserted central venous catheters and midline catheters for palliative care in patients with cancer: a systematic review. Support Care Cancer 32, 464 (2024). https://doi.org/10.1007/s00520-024-08664-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08664-3