Abstract
Purpose
Review the literature to update the MASCC guidelines from 2015 for controlling nausea and vomiting with systemic cancer treatment of moderate emetic potential.
Methods
A systematic literature review was completed using Medline, Embase, and Scopus databases. The literature search was done from June 2015 to January 2023 of the management of antiemetic prophylaxis for anticancer therapy of moderate emetic potential.
Results
Of 342 papers identified, 19 were relevant to update recommendations about managing antiemetic prophylaxis for systemic cancer treatment regimens of moderate emetic potential. Important practice changing updates include the use of emetic prophylaxis based on a triple combination of neurokinin (NK)1 receptor antagonist, 5-HT3 receptor antagonist, and steroids for patients undergoing carboplatin (AUC ≥ 5) and women < 50 years of age receiving oxaliplatin-based treatment. A double combination of 5-HT3 receptor antagonist and steroids remains the recommended prophylaxis for other MEC. Based on the data in the literature, it is recommended that the administration of steroids should be limited to day 1 in moderately emetogenic chemotherapy regimens, due to the demonstration of non-inferiority between the different regimens.
More data is needed on the emetogenicity of new agents at moderate emetogenic risk. Of particular interest would be antiemetic studies with the agents sacituzumab-govitecan and trastuzumab-deruxtecan. Experience to date with these agents indicate an emetogenic potential comparable to carboplatin > AUC 5. Future studies should systematically include patient-related risk assessment in order to define the risk of emesis with MEC beyond the emetogenicity of the chemotherapy and improve the guidelines for new drugs.
Conclusion
This antiemetic MASCC-ESMO guideline update includes new recommendations considering individual risk factors and the optimization of supportive anti-emetic treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The moderately emetogenic anticancer (MEC) agents are a large heterogeneous group in which patients receiving these treatments have a risk of developing emesis in 30 to 90% of cases. This wide range means that prophylaxis recommendations, based on the literature, need to be adapted to the different situations. For example, patients receiving carboplatin are considered to be at the high end of this group, with a higher risk of emesis than other agents in the same group.
This paper reviews the class of MEC agents that entail a risk of nausea and vomiting for 30 to 90% of patients treated, in the absence of prophylaxis [1].
Since the previous 2015 update of the MASCC-ESMO MEC recommendations [2], new anti-cancer drugs have come onto the market, within eight i.v. MEC and fourteen oral agents identified as high-moderate, making it necessary to include them in the table of recommendations for antiemetic prophylaxis [3]. In addition, several questions that had not been resolved due to a lack of data in the literature at the time of the previous MASCC-ESMO recommendations emerged and required a reassessment of the prophylactic proposals.
The primary research question was “In adults with solid cancers or hematological malignancies receiving MEC, what are the best antiemetic treatment combination strategies?”. Secondary questions were considered to refine the primary one. These secondary questions were as follows: (1) Are neurokinin (NK)1 receptor antagonists (RAs) required for MEC prophylaxis? (2) Can steroids be administered on day 1 of chemotherapy only? (3) What is the best recommended prophylaxis for acute (0–24 h after chemotherapy) MEC? (4) What is to be recommended for prophylaxis of delayed (24–120 h) MEC? (5) What is to be recommended for prophylaxis of overall (0–120 h) MEC? (6) Is there a place for olanzapine in MEC prophylaxis? (7) Is there a need for prophylaxis of very long delayed MEC (> 5 days)?
After a discussion, the authors decided to focus on five main specific topics:
-
Carboplatin—dose-dependent recommendations
-
Oxaliplatin—patient demographic risk factors
-
Other MEC antineoplastic agents
-
Steroid-sparing regimens
-
Olanzapine in MEC
In this systematic review, the recent literature was analyzed in order to update the previous MASCC guidelines of 2016 for controlling nausea and vomiting with systemic cancer treatment of moderate emetic potential [2].
Methods
A systematic literature review from June 1, 2015, through January 31, 2023, was done following the PRISMA guidelines for reporting [4].
The literature search used the Medline, Embase, and Scopus databases. Each database was searched individually using the platform listed in the previous items. We did not search any study registries and did not contact representatives from the manufacturers of antiemetics, corresponding or first authors of included trials. Many experts (including corresponding and/or first authors of included trials) were, however, available as members of the MASCC/ESMO 2023 Antiemetic Guideline Update Consensus Committee.
We did a systematic review based on the list of moderate emetic agents and included new and old agents, in the search strategy from the previous guideline including the new agents identified for this update [2, 5].
An overview of intravenous (classified as moderate) and oral (classified as high/moderate) anticancer agents is given in Table 1.
Search concepts were specific antiemetic agents (aprepitant, fosaprepitant, netupitant, fosnetupitant, rolapitant, ondansetron, granisetron, palonosetron, ramosetron, dexamethasone, methylprednisolone, prednisone, steroid, metoclopramide, domperidone, metopimazine, prochlorperazine, olanzapine, amisulpride) and moderately emetogenic chemotherapy regimens (amivantamab, dinutuximab, fam-trastuzumab deruxtecan, lurbinectedin, sacituzumab govitecan, trabectedin, oxaliplatin, carboplatin, ifosfamide, irinotecan, azacitidine, cytarabine, doxorubicin, epirubicin, daunorubicin, idarubicin, bendamustine, alemtuzumab). The search limited to randomized controlled trials (RCTs), systematic reviews, and meta-analyses. Filters restricted papers to humans and English language publications. It was decided to limit the work to adults 19 + years as pediatrics guidelines update were already performed by the POGO group (Pediatric Oncology Group of Ontario) (https://www.pogo.ca/wp-content/uloads/2023/01/4.3-Antiemetics.pdf).
The keywords used for the strategy of literature review, used the name of the anticancer drug (X) followed by the antiemetics selected by families:
-
X AND aprepitant OR netupitant OR rolapitant OR fosaprepitant OR fosnetupitant OR neurokinin antagonist.
-
X AND ondansetron OR granisetron OR palonosetron OR ramosetron OR serotonin antagonist.
-
X AND dexamethasone or methylprednisolone or prednisolone or steroid.
-
X AND metoclopramide OR domperidone OR metopimazine OR prochlorperazine OR olanzapine OR amisulpride OR dopamine antagonist.
The publications identified were divided into four subgroups to analyze topics related more specifically to serotonin (5-HT)3 receptor antagonists, NK1 receptor antagonists, dopamine receptor antagonists (focusing the multireceptor targeting agent, olanzapine) and steroids. All references were reviewed in duplicate and independently by two members of the assigned working group (chair and co-chair), in order to mitigate the risk of bias and identify the relevant papers for the review. All authors then met and analyzed the content of the publications identified to enable recommendations to be made.
Results
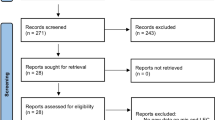
A total of 342 publications were screened. Among these, 41 were identified by the chair and co-chair as relevant for the update (Fig. 1) and divided in the four subgroups:
-
MEC AND NK1-RAs, 23 references
-
MEC AND steroids, 8 references
-
MEC AND olanzapine, 9 references (no other dopamine-receptor antagonist references qualified)
-
MEC AND 5-HT3-RAs, 1 reference
After review by all authors, 19 references were judged relevant and finally selected for the guidelines update to enable the answers to the prespecified questions (Fig. 1).
Recommendations herein were reviewed by all 34 members of the Guideline Update Committee and approved with minor changes.
Data are very limited as concerns prophylaxis of nausea and vomiting in patients treated with oral agents and consequently no precise recommendations can be given. In general, only on demand antiemetics are recommended.
Carboplatin—dose-dependent recommendations
Antiemetic regimens were specifically assessed in two double-blinded RCT and two meta-analyses. A sub-group analysis of a large RCT (1349 HEC and MEC patients) assessed the efficacy of an NK1-RA versus placebo in association with dexamethasone (DEX) 20 mg orally D1 and a 5-HT3-RA (oral granisetron 2 mg administered once daily on days 1–3), in patients receiving carboplatin) [6]. Of the 1332 patients who composed the intent‐to‐treat population, 401 received a first course of chemotherapy with a carboplatin-based regimen and were randomized in the NK1-RA cohort (n = 192) or the placebo cohort (n = 209). In patients of this cohort, the triplet regimen NK1-RA significantly improved complete response (CP) rates from 64.6 to 80.2% in the overall phase (P < 0.001) and 82.3% vs 65.6% (P < 0.001) in the delayed phase. No significant difference was seen in the acute phase.
A total of 324 patients were evaluated in a multicenter, placebo-controlled, double-blind, randomized study studying the addition of aprepitant to the antiemetic regimen in patients receiving a carboplatin-paclitaxel combination regimen for a gynecologic cancer [7]. All the patients received DEX 20 mg i.v. day 1 and a 5-HT3-RA (granisetron 1 mg or ondansetron 4 mg) orally day 1. The primary endpoint assessed hypersensitivity reaction (HSR) to paclitaxel, but secondary endpoints analyzed antiemetic efficacy (complete response, no vomiting and no nausea). Antiemetic efficacy as documented by the complete response rate was significantly higher in the group with aprepitant compared to the placebo group (61.6% versus 47.3%, p = 0.0073).
Sixteen trials (3848 patients) were identified in a systematic review with the intent to assess the use of NK1-RA in MEC non-AC regimen in which nine studies (1790 patients) received a carboplatin regimen [8]. The OR for achieving an overall CR was 1.96 (95% CI 1.57–2.45; p < 0.00001) in favor of the NK1-RA containing regimen.
A meta-analysis reviewed ten trials (2928 patients) for the efficacy of a triple regimen containing an NK1-RA versus a double regimen (DEX + a 5-HT3-RA) in patients receiving MEC. For the 1668 carboplatin-based chemotherapy patients, the triple regimen with an NK1-RA showed significant better CR (RR, 1.22; 95% CI, 1.14–1.32; p < 0.001) in the overall phase compared with the DEX + 5-HT3-RA regimen [9].
All of these data presented in the study used a standard dose of every 3-week carboplatin with a dosage clearly identified as AUC ≥ 5. No data were available in our systematic review for patients receiving a lower carboplatin dose (AUC < 5).
On the basis of the consistent findings presented above, it is recommended to use a three-drug regimen including single doses of a 5-HT3-RA, DEX, and an NK1-RA (aprepitant, fosaprepitant, fosnetupitant, netupitant, or rolapitant), given before chemotherapy for patients receiving carboplatin AUC ≥ 5.
There is no data to recommend the use of an NK1-RA for carboplatin AUC < 5 (I, A).
Oxaliplatin—patient demographic risk factors
Four hundred thirteen patients, enrolled in the SENRI trial, received an oxaliplatin-based regimen for colorectal cancer (neoadjuvant, adjuvant or recurrent-metastatic disease) and were randomized to antiemetic prophylaxis with a triple drug regimen including an NK1-RA or a double drug regimen (DEX + a 5-HT3-RA) [10]. Patients randomized to aprepitant/fosaprepitant also received i.v. DEX 6 mg on day 1 and oral DEX 2 mg twice daily on days 2 and 3. Patients in the control group received a 5-HT3-RA and DEX 9.9 mg i.v. day 1 followed by oral DEX (4 mg) twice daily on days 2 and 3. There was no difference in the characteristics of patients by age (> or < 60) or gender (male or female) between the two groups. The aprepitant group had significantly higher rates of complete response overall (85.0% versus 74.3%; p = 0.01).
An unplanned analysis identified risk factors for emesis and the differences in efficacy between antiemetic regimens in the previous study [11]. In women, the rate of no nausea, no vomiting, and total control (no nausea, no emesis, no rescue medication) was higher in the aprepitant group than in the control group. The benefit of triple antiemetic therapy was higher in the female cohort compared to males. The complete response rate increased from 65.3 to 78.1% in women receiving aprepitant, compared with 80.2 to 89.5% in men.
A total of 248 women were enrolled in a RCT to assess the prophylactic efficacy of a triple drug combination (NK1-RA + 5-HT3-RA and DEX) in young women (age ≤ 50 years) [12]. Patients receiving FOLFOX or FOLFIRI for a gastrointestinal cancer were randomized in the ratio 1:1 to intervention or control. Patients in the control group received placebo (day 1 through day 3), palonosetron 0.25 mg i.v., and DEX 12 mg orally 30 min before initiation of chemotherapy, while patients in the intervention group received aprepitant (125 mg orally on day 1 and 80 mg orally each morning of days 2 and 3) and palonosetron, 0.25 mg i.v. with DEX 6 mg orally on day 1. The primary efficacy endpoint was reached in the modified intention-to-treat population, with a CR rate of 87.0% in the NK1-RA group compared to 66.7% in the placebo group in the overall phase (P < 0.001). Results were also significantly superior in the acute and delayed phases. A subgroup analysis of patients in the oxaliplatin-based FOLFOX regimen, demonstrated a CR rate in the overall phase significantly higher in the NK1-RA vs placebo group (89.8% versus 66.3%; P < 0.001). The results were not statistically significant in the irinotecan-based FOLFIRI regimen group.
Based on the above studies, a two-drug regimen including single doses of a 5-HT3-RA and DEX, given before chemotherapy is recommended for patients receiving oxaliplatin.
Addition of an NK1-RA (aprepitant, fosaprepitant, netupitant, fosnetupitant, or rolapitant) is suggested for oxaliplatin CINV prophylaxis in women aged < 50 years old.
There is no evidence that an NK1-RA should be routinely used as first line in women > 50 years old (III, B).
It should be noted that the European Medicines Agency has withdrawn marketing authorization for rolapitant. It remains available in the USA as an oral agent.
Other MEC antineoplastic agents
In the 21 selected RCT, meta-analysis, or systematic review publications, none revealed significant data to change the previous recommendation for other MEC antineoplastic agents than carboplatin- and oxaliplatin-based regimens.
No data were available on new anticancer drugs. New treatments considered as MEC should then receive the same prophylaxis as other MEC. Exceptions may be the new agents sacituzumab-govitecan and trastuzumab-deruxtecan which appear to have an emetogenic potential comparable to carboplatin, at the high end of the moderate category. While prospective studies are needed it is suggested to prevent emesis as for carboplatin AUC ≥ 5.
However, in the absence of clinical trials evaluating antiemetic approaches for these agents, definitive antiemetic prophylaxis recommendations cannot be made at this time.
A two-drug regimen including single doses of a 5-HT3-RA and DEX, given before chemotherapy, is recommended for patients receiving other MEC (II, C).
Steroid-sparing regimens
In a randomized controlled open label study, 320 chemotherapy-naïve patients receiving a first-line regimen of mFOLFOX6 (oxaliplatin, leucovorin, and 5-fluorouracil) were randomized in a 1:1 ratio to aprepitant 125 mg orally on day 1, 80 mg daily on days 2–3 or to DEX 10 mg i.v. on day 1, followed by 5 mg daily on days 2 and 3. Both groups in addition received palonosetron 0.25 mg i.v. on day 1 [13]. The overall (0–120 h) complete response rate was superior in the aprepitant (+ palonosetron) arm compared to the DEX (+ palonosetron) arm (88.8% versus 74.2%, p = 0.0010) and also in the delayed (25–120 h) phase (90.6% versus 75.5%, p < 0.0001). No significant difference was found in the acute phase.
A large meta-analysis including 4534 patients (17 trials) compared the antiemetic efficacy of 3 days of DEX combined with an NK1-RA (3-DEX + NK1-RA) to 1 day of DEX also combined with an NK1RA (1-DEX + NK1-RA). Complete response rate in the delayed phase was used as the primary endpoint [14]. There was no significant difference between the two cohorts with an absolute risk difference of 9% (95% CI, − 2.3 to 21.1).
Another large systematic review including 1194 patients (5 RCT) assessed the complete response rate in the overall phase (day 1 through 5) in chemotherapy-naïve adult patients undergoing either MEC or an anthracycline plus cyclophosphamide (AC)–containing regimen [15]. The non-inferiority margin was set at − 8.0%. The non-inferiority of the DEX-sparing regimen (1 day versus 3 days) was demonstrated with a risk difference between the two cohorts at − 1.5% (95% CI, − 7.1% to 4.0%). No significant difference was highlighted between the different chemotherapy regimens (AC vs MEC).
In a randomized, controlled phase III, open label study, non-inferiority was assessed between two regimens of DEX prophylaxis; DEX day 1 only versus DEX day 1 through day 3 [16]. Each of the 305 patients enrolled in the trial received palonosetron (0.75 mg, i.v.) and DEX (9.9 mg i.v.) prior to MEC. Patients in the 3-day DEX regimen received DEX 8 mg (i.v. or p.o.) on days 2–3. The primary endpoint was the overall complete response rate (0–120 h), and the non-inferiority margin was set at − 15%. Non-inferiority was reached with a 2.5% difference (95% confidence interval (CI): − 7.8–12.8%; p = 0.0004).
Two phase II, randomized, controlled trials (including 82 and 109 patients, respectively) investigated a DEX-sparing regimen in patients receiving carboplatin (AUC5 or AUC6). In the AUC5 carboplatin regimen study [17], all patients received DEX 20 mg i.v. on day 1 associated with palonosetron 0.75 mg i.v. Patients in the non-sparing group received oral DEX 8 mg daily, days 2 and 3. The primary endpoint (CR in the delayed phase) was not statistically significantly different between the two groups (3-day group, 76.9% [30/39]; 1-day group 69.8% [30/43]; p = 0.4652).
In the AUC6 carboplatin regimen study [18], patients were treated for a gynecologic cancer with a standard paclitaxel-carboplatin (PC) regimen or a dose dense regimen PC regimen. All patients received palonosetron at 0.75 mg i.v. day 1 and were randomized to additional antiemetic therapy with 1 day of DEX versus 3 days of DEX. Complete response in the overall phase (0–120 h) was the primary end-point. CR was observed in 67.9% (95% CI, 53.7–80.1) of patients in the 3-day DEX arm, and 60.7% (95% CI, 46.8–73.5) of patients in the 1-day DEX arm.
Based on these trials, it is recommended that no steroid (or other antiemetic) should be routinely administered after day 1 MEC administration (II, B).
No steroid (or other antiemetic) should be routinely administered after day 1 carboplatin administration (II, B).
No steroid (or other antiemetic) should be routinely administered after day 1 oxaliplatin administration (II, B).
Olanzapine in MEC
A single study was reviewed out of nine screened in order to define the place of olanzapine (OLZ) in MEC antiemetic prophylaxis [19].
In this randomized open-label study, 81 patients received palonosetron, DEX, and OLZ before start of chemotherapy and were randomized to either OLZ 10 mg as a single oral dose days 2–3 or OLZ 10 mg + DEX 4 mg days 2–3 or DEX 8 mg daily days 2–3 for delayed emesis protection. The primary endpoint was total control (no vomiting, no rescue treatment + no nausea) on days 2–5. No significant difference was found between the three cohorts.
There is no evidence supporting the use of OLZ as primary prophylaxis following MEC (II, C).
A summary of the recommendations for MEC are presented in Table 2.
Discussion
The major changes introduced in this guideline update are to consider triple association of an NK1-RA combined with a 5-HT3-RA and DEX on day 1 for patients receiving a carboplatin-based regimen and for females below 50 years of age, receiving an oxaliplatin-based regimen.
The evidence to use this triple antiemetic regimen was clearly demonstrated for patients receiving carboplatin at a dose AUC > 5 with a classic 3-week schedule. No data were available in our systematic review to recommend the use of a triple regimen in patients receiving lower doses of carboplatin.
Several randomized controlled trials evaluated adding an NK1-RA in patients receiving oxaliplatin. A triple antiemetic prophylaxis demonstrates a significant impact in young women without superior efficacy in other situations (male and older female).
The 2022 NCCN guidelines proposes three different options including the triple association of 5-HT3-RA + DEX + NK1-RA [20]. The ASCO guidelines for adults treated with moderate-emetic-risk antineoplastic agents (excluding carboplatin AUC ≥ 4 mg/mL/min, but including oxaliplatin) recommend a 2-drug combination of a 5-HT3-RA and DEX. In oxaliplatin-based regimens, ASCO recommends that a steroid should be offered for two additional days [21].
The Takemoto et al. [11] and Wang et al. [12] studies specifically evaluated the impact related to risk factors and in a young women specific cohort with benefit favoring the NK1-RA addition in younger women treated with oxaliplatin.
Lack of evidence excluded any recommendation for adding an NK1-RA outside of the defined younger women population, but this MASCC-ESMO guidelines’ update is practice changing with the upgrading of prophylaxis in women < 50 years of age.
Further trials are needed to investigate if other populations treated with an oxaliplatin-based regimens or other MEC including new drugs such as antibody drug conjugates (e.g., sacituzumab-govitecan or trastuzumab-deruxtecan defined as MEC agents) could benefit form addition of an NK1-RA.
The question of sparing steroids after day 1 administration is heavily debated, due to the adverse events of multiple-day steroids, but we only discovered a poor level of rigorous clinical trials limiting the ability to adopt a strong statement.
In one phase III RCT and one phase II RCT, non-inferiority was tested and significantly reached between two regimens of DEX prophylaxis: DEX day 1 only versus DEX day 1 to day 3 [16, 17]. These results were confirmed in the Okada et al. systematic review [15]. These studies led to our recommendation for sparing steroids after day 1.
The place of olanzapine in MEC could not be defined because of a paucity of data addressing its use in this setting.
These findings (or missing data) are a reminder of the need to carry out further research in order to develop future recommendations, particularly with regard to other patient populations or other anti-cancer drugs. Reassessment before each course of treatment and vigilance with regard to the individual risk factors of each patient are essential if these recommendations are to be applied as effectively as possible, and to avoid any emetic events during the course of treatment for patients undergoing treatment for cancer [21, 22].
Conclusions
These antiemetic guidelines for moderately emetogenic chemotherapy, based on a systematic review of the current literature, have undergone some important changes, particularly with regard to the use of emetic prophylaxis based on a triple combination of NK1-RA, 5-HT3-RA and DEX for patients undergoing carboplatin (AUC ≥ 5) and women < 50 years of age receiving oxaliplatin-based treatment. A double combination of a 5-HT3-RA and steroids remains the recommended prophylaxis for other MEC. Based on the data in the literature, it has been recommended that steroids be spared after day 1, in MEC regimens, due to the demonstration of non-inferiority in antiemesis control for single-day steroids compared to multi-day regimens.
More data are needed on the emetogenicity of new agents of moderate emetogenic risk. Of particular interest would be antiemetic studies with the agents sacituzumab-govitecan and trastuzumab- deruxtecan. Experience to date with these agents indicate an emetogenic potential comparable to carboplatin in a dose of AUC > 5. Future studies should systematically include patient-related risk assessment in order to define the risk of emesis with MEC beyond the emetogenicity of the chemotherapy and improve the guidelines for new drugs.
Change history
12 February 2024
The correct family name should be: Iihara
References
Grunberg SM, Osoba D, Hesketh PJ, Gralla RJ, Borjeson S, Rapoport BL, Du Bois A, Tonato M (2005) Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity—an update. Support Care Cancer 13:80–84. https://doi.org/10.1007/s00520-004-0718-y
Roila F, Warr D, Hesketh PJ, Gralla R, Herrstedt J, Jordan K, Aapro M, Ballatori E, Rapoport B (2017) 2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following moderately emetogenic chemotherapy. Support Care Cancer 25:289–294. https://doi.org/10.1007/s00520-016-3365-1
Jordan K, Chan A, Gralla RJ, Jahn F, Rapoport B, Ruhlmann CH, Sayegh P, Hesketh PJ (2023) Emetic risk classification and evaluation of the emetogenicity of antineoplastic agents –Updated MASCC/ESMO consensus recommendation. Support Care Cancer 21:xx-yy
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzieJE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372: n71 https://doi.org/10.1136/bmj.n71:10.1136/bmj.n71)
Herrstedt J, Clark-Snow R, Ruhlmann CH, Molassiotis A, Olver I, Rapoport BL, Aapro M, Dennis K, Hesketh PJ, Navari RM, Schwartzberg L, Affronti ML, Garcia-Del-Barrio MA, Chan A, Celio L, Chow R, Fleury M, Gralla RJ, Giusti R, Jahn F, lihara H, Maranzano E, Radhakrishnan V, Saito M, Sayegh P, Bosnjak S, ZhangL, Lee J, Ostwal V, Smit T, Zilic A, Jordan K, Scotté F. 2023 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting. ESMO OPEN XXX
Hesketh PJ, Schnadig ID, Schwartzberg LS, Modiano MR, Jordan K, Arora S, Powers D, Aapro M. Efficacy of the neurokinin-1 receptor antagonist rolapitant in preventing nausea and vomiting in patients receiving carboplatin-based chemotherapy. Cancer 2016;122:2418-25. Erratum: Cancer 2016;122:3579.https://doi.org/10.1002/cncr.30054
Yahata H, Kobayashi H, Sonoda K, Shimokawa M, Ohgami T, Saito T, Ogawa S, Sakai K, Ichinoe A, Ueoka Y, Hasuo Y, Nishida M, Masuda S, Kato K (2016) Efficacy of aprepitant for the prevention of chemotherapy-induced nausea and vomiting with a moderately emetogenic chemotherapy regimen: a multicenter, placebo-controlled, double-blind, randomized study in patients with gynecologic cancer receiving paclitaxel and carboplatin. Int J Clin Oncol 21:491–497. https://doi.org/10.1007/s10147-015-0928-y
Jordan K, Blättermann L, Hinke A, Müller-Tidow C, Jahn F (2018) Is the addition of a neurokinin-1 receptor antagonist beneficial in moderately emetogenic chemotherapy? -a systematic review and meta-analysis. Support Care Cancer 26:21–32. https://doi.org/10.1007/s00520-017-3857-7
Zhang Y, Hou X, Zhang R, Chen G, Huang Y, Yang Y, Zhao Y, Fang W, Hong S, Kang S, Zhou T, Zhang Z, Chen X, Zhang L (2018) Optimal prophylaxis of chemotherapy-induced nausea and vomiting for moderately emetogenic chemotherapy: a meta-analysis. Future Oncol 14:1933–1941. https://doi.org/10.2217/fon-2017-0712
Nishimura J, Satoh T, Fukunaga M, Takemoto H, Nakata K, Ide Y, Fukuzaki T, Kudo T, Miyake Y, Yasui M, Morita S, Sakai D, Uemura M, Hata T, Takemasa I, Mizushima T, Ohno Y, Yamamoto H, Sekimoto M, Nezu R, Doki Y, Mori M, Multi-center Clinical Study Group of Osaka, Colorectal Cancer Treatment Group(MCSGO) (2015) Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy (SENRI trial): a multicentre, randomised, controlled phase 3 trial. Eur J Cancer 51:1274–1282. https://doi.org/10.1016/j.ejca.2015.03.024
Takemoto H, Nishimura J, Komori T, Kim HM, Ota H, Suzuki R, Ikenaga M, Ikeda M, Yamamoto H, Satoh T, Hata T, Takemasa I, Mizushima T, Doki Y, Mori M. Multicenter Clinical Study Group of Osaka, Colorectal Cancer Treatment Group (MCSGO) (2017) Combination antiemetic therapy with aprepitant/fosaprepitant in patients with colorectal cancer receiving oxaliplatin-based chemotherapy in the SENRI trial: analysis of risk factors for vomiting and nausea. Int J Clin Oncol 22:88–95. https://doi.org/10.1007/s10147-016-1022-9
Wang DS, Hu MT, Wang ZQ, Ren C, Qiu MZ, Luo HY, Jin Y, Fong WP, Wang SB, Peng JW, Zou QF, Tan Q, Wang FH, Li YH (2021) Effect of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in women: a randomized clinical trial. JAMA Netw Open 4(4):e215250. https://doi.org/10.1001/jamanetworkopen.2021.5250
Cheng Y, Wu Z, Shi L, Shen C, Zhang J, Hu H, Li W, Cai Y, Xie X, Ling J, Zheng Q, Deng Y (2022) Aprepitant plus palonosetron versus dexamethasone plus palonosetron in preventing chemotherapy-induced nausea and vomiting in patients with moderate-emetogenic chemotherapy: a randomized, open-label, phase 3 trial. EclinicalMedicine 49:101480. https://doi.org/10.1016/j.eclinm.2022.101480
Watanabe D, Iihara H, Fujii H, Makiyama A, Nishida S, Suzuki A (2022) One-day versus three-day dexamethasone with NK1RA for patients receiving carboplatin and moderate emetogenic chemotherapy: a network meta-analysis. Oncologist 27:e524–e532. https://doi.org/10.1093/oncolo/oyac060
Okada Y, Oba K, Furukawa N, Kosaka Y, Okita K, Yuki S, Komatsu Y, Celio L, Aapro M (2019) One-day versus three-day dexamethasone in combination with palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a systematic review and individual patient data-based meta-analysis. Oncologist 24:1593–1600. https://doi.org/10.1634/theoncologist.2019-0133
Komatsu Y, Okita K, Yuki S, Furuhata T, Fukushima H, Masuko H, Kawamoto Y, Isobe H, Miyagishima T, Sasaki K, Nakamura M, Ohsaki Y, Nakajima J, Tateyama M, Eto K, Minami S, Yokoyama R, Iwanaga I, Shibuya H, Kudo M, Oba K, Takahashi Y (2015) Open-label, randomized, comparative, phase III study on effects of reducing steroid use in combination with palonosetron. Cancer Sci 106:891–895. https://doi.org/10.1111/cas.12675
Furukawa N, Kanayama S, Tanase Y, Ito F (2015) Palonosetron in combination with 1-day versus 3-day dexamethasone to prevent nausea and vomiting in patients receiving paclitaxel and carboplatin. Support Care Cancer 23:3317–3322. https://doi.org/10.1007/s00520-015-2760-3
Matsuura M, Satohisa S, Teramoto M, Tanaka R, Iwasaki M, Nishikawa A, Mizunuma M, Tanaka S, Hayakawa O, Saito T (2015) Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following paclitaxel and carboplatin in patients with gynecologic cancers: a randomized, multicenter, phase-II trial. J Obstet Gynaecol Res 41:1607–1613. https://doi.org/10.1111/jog.12748
Celio L, Saibene G, Lepori S, Festinese F, Niger M, Raspagliesi F, Lorusso D (2019) Short-course olanzapine to prevent delayed emesis following carboplatin/paclitaxel for gynecologic cancer: a randomized study. Tumori 105:253–258. https://doi.org/10.1177/0300891619839301
Antiemesis. Version 2.2022. National Comprehensive Cancer Network® (NCCN).
Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Lyman GH (2020) Antiemetics: ASCO Guideline Update. J Clin Oncol 38:2782–2797. https://doi.org/10.1200/JCO.20.01296
Dranitsaris G, Molassiotis A, Clemons M, Roeland E, Schwartzberg L, Dielenseger P, Jordan K, Young A, Aapro M (2017) The development of a prediction tool to identify cancer patients at high risk for chemotherapy induced nausea and vomiting. Ann Oncol 28:1260–1267. https://doi.org/10.1093/annonc/mdx100
Author information
Authors and Affiliations
Contributions
FS and LS did the literature search. All authors reviewed the selected publications. The initial draft was written by FS, and all authors revised it critically for its intellectual content.
All authors reached agreement on the recommendations, are accountable for the accuracy and integrity of the work, and have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Florian Scotté declares he has received honoraria from Sanofi, Sandoz, Roche, MSD, Prostrakan, Leo Pharma, Janssen, AMGEN, Pierre Fabre Oncologie, Vifor Pharma, Arrow, Pfizer, BMS, Bayer, Thermo Fisher, Pharmanovia, Gilead, Viatris, and Helsinn.
Lee Schwartzberg declares he has received honoraria from Helsinn, GlaxoSmithKline, and Pfizer.
Hirotoshi Iihara declares that he has received personal fees from Taiho, Chugai, Yakult, Astellas, Eli Lilly, Daiichi Sankyo, AstraZeneca, Nippon Kayaku, Ono, and Nippon Boehringer Ingelheim and consulting fees for their institution from Taiho and Eisai outside the submitted work.
Matti Aapro declares the following interests relevant to this manuscript: has received honoraria from Berlin-Chemie, Fosun, Helsinn Healthcare SA, Juniper Biologics, Knight Therapeutics, Mundipharma International Limited, and Vifor Pharma.
Richard Gralla declares the following interests relevant to this manuscript: has received honoraria from Fosun, Helsinn Healthcare SA, Juniper Biologics, Knight Therapeutics, Mundipharma International Limited, and Vifor Pharma.
Paul J Hesketh declares that he has no financial interests.
Karin Jordan reports personal fees as an invited speaker from Amgen, Art Tempi, Helsinn Healthcare SA, Hexal, Med Update GmbH, MSD, Mundipharma, Onkowissen, Esteve, Roche, Shire (Takeda), and Vifor; personal fees for advisory board membership from Amgen, AstraZeneca, BD Solutions, Hexal, Karyopharm, and Voluntis; and personal fees as author for UpToDate.
Ronald Chow declares that he has no financial interests.
Jørn Herrstedt declares he has received honoraria from Pharmathen S.A.
The other authors have no relevant financial or non-financial interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scotté, F., Schwartzberg, L., Iihara, H. et al. 2023 updated MASCC/ESMO Consensus recommendations: Prevention of nausea and vomiting following moderately emetic risk antineoplastic agents. Support Care Cancer 32, 45 (2024). https://doi.org/10.1007/s00520-023-08222-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08222-3