Abstract
Purpose
Paclitaxel is associated with an acute pain syndrome (P-APS- and chronic chemotherapy-induced peripheral neuropathy (CIPN). P-APS is associated with higher risk of CIPN. Omega-3 fatty acids have well-established anti-inflammatory and neuroprotective properties. The primary purpose of this pilot study was to assess whether omega-3 fatty acids could decrease P-APS and thus CIPN.
Methods
Patients scheduled to receive weekly paclitaxel for breast cancer were randomized to receive 4 g of omega-3 acid ethyl esters (Lovaza) or placebo, beginning 1 week prior and continued until paclitaxel was stopped. Patients completed acute pain questionnaires at baseline, daily after each treatment, and 1 month after completion of therapy.
Results
Sixty patients (49 evaluable) were randomized to treatment versus placebo. Seventeen (68.0%) patients receiving the omega-3 fatty acids intervention experienced P-APS, compared to 15 (62.5%) of those receiving placebo during the first week of treatment (p = 0.77). Over the full 12-week study, 21 (84.0%) patients receiving the omega-3 fatty acid intervention experienced P-APS, compared to 21 (87.5%) of those receiving placebo (p = 1.0). Secondary outcomes suggested that those in the intervention arm used more over-the-counter analgesics (OR: 1.65, 95% CI: 0.72–3.78, p = 0.23), used more opiates (OR: 2.06, 95% CI: 0.55–7.75, p = 0.28), and experienced higher levels of CIPN (12.8, 95% CI: 7.6–19.4 vs. 8.4, 95% CI: 4.6–13.2, p = 0.21).
Conclusions
The results of this pilot study do not support further study of the use of omega-3 fatty acids for the prevention of the P-APS and CIPN.
Trial registration
Number: NCT01821833
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paclitaxel, a widely used chemotherapeutic agent, is associated with several well-known side effects, including peripheral neuropathy and generalized body aches [1]. The latter has been described as paclitaxel-associated acute pain syndrome (P-APS) and often occurs in the first 3–4 days after chemotherapy administration, affecting 58–90% of patients [2, 3]. Previously, these symptoms have been described as myalgias and arthralgias. However, data support that P-APS may be an early manifestation of neuropathy, as opposed to being musculoskeletal in origin, as it was noted that patients with higher P-APS pain scores with the first dose of paclitaxel are at higher risk for developing subsequent chronic peripheral neuropathy [4]. The P-APS can be severe, particularly with higher doses of paclitaxel, and may require use of potent analgesics for symptom control. Non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used as frontline therapy [5]. However, there is concern over their side effects including gastrointestinal bleeding and acute renal injury [6]. Many other therapies have been studied including steroids [7], glutamine [8], antihistamine [9], opioid analgesics [5], integrative therapy [10], and herbal supplements [11, 12], with variable results [13]. Currently, there is no standard of care for the treatment or prevention of P-APS. As paclitaxel remains one of the most frequently used chemotherapeutic agents and P-APS is common and can be debilitating to some patients, an agent with a favorable toxicity profile that may prevent P-APS, and the development of peripheral neuropathy would have a tremendous clinical impact.
Because the subacute aches and pains associated with paclitaxel were described as myalgias and arthralgias based on patient-reported symptoms and were self-limited, this led to a hypothesis that symptoms are the result of an inflammatory reaction to paclitaxel. Loprinzi et al. described the natural history of P-APS [2]. In the study, P-APS symptoms were assessed in 94 patients using patients’ daily symptom logs with 10 items assessing pain symptoms and the use of pain medication. The most common manifestation was a diffuse achiness occurring in a truncal/hip distribution evolving over 1–4 days. Interestingly, in the weekly paclitaxel treatment group, the initial pain experienced with the first treatment did not predict the severity of pain with additional doses, but appeared to predict the severity of symptoms associated with peripheral neuropathy, predominantly sensory, which evolved over the 12-week period of treatment. This was the first study suggesting that P-APS may be an early manifestation of neuropathy and is associated with the subsequent development of chronic peripheral neuropathy.
One mechanism proposed for P-APS is an early inflammatory process characterized by macrophage activation in both the dorsal root ganglia (DRG) and peripheral nerves, occurring shortly after paclitaxel therapy [14]. Morphologic alterations in DRG satellite cells and upregulation of proinflammatory cytokines have been hypothesized as early events in the development of neuropathy. Therefore, it is possible that paclitaxel-induced neuropathic pain may be mediated by proinflammatory cytokines. If P-APS and chronic neuropathy are indeed part of a continuum, inflammatory pathways would be a reasonable target for therapy. The mechanism of chronic chemotherapy-induced peripheral neuropathy (CIPN) similarly remains poorly understood. Taxanes promote stabilization of microtubules leading to disruption of normal cell division eventually resulting in cell death. Short-term administration of paclitaxel induces mainly reversible changes in the peripheral nerves and spinal roots, which, over time, may result in permanent damage [15].
Long-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are common dietary supplements. They have well-established anti-inflammatory properties that serve as the basis for their use in therapeutic trials in inflammatory conditions [16]. EPA competes with arachidonic acid (AA) for enzymatic metabolism, thereby decreasing inflammatory derivatives. Additionally, several clinical studies have described the pain-relieving effects of omega-3 fatty acids for inflammatory joint diseases [16, 17]. Omega-3 fatty acid consumption may attenuate the production of pro-inflammatory metabolites and accelerate nerve regeneration [18]. In addition, they generate local mediators that facilitate resolution of inflammation [19,20,21]. These medications overall have a minimal side effect profile but do carry a small risk of increased LDL cholesterol [22].
The nervous system is richly endowed with omega-3 fatty acids, primarily DHA. Omega-3 fatty acids may act as natural neuroprotective agents, as well as key components in nerve hemostasis. Several studies have reported the role of omega-3 fatty acids in maintaining nerve integrity and function [23, 24]. Long-chain n-3 polyunsaturated fatty acids (PUFAs) such as DHA have been shown to be promising in the treatment of a broad range of neurodegenerative conditions and spinal cord injuries [25,26,27]. These preclinical models have shown the neuroprotective effect of DHA in models of spinal cord injury. A mouse model has recently been used comparing groups with different fatty acid profiles. One group expressed the fat-1 gene from Caenorhabditis elegans, which encodes a fatty acid desaturase, the enzyme that converts n-6 into n-3 PUFAs, leading to enrichment in tissue n-3 PUFA levels (low n-6/n-3 ratio). This was compared to the group that had a high n-6/ n-3 ratio as well as to wild-type strain. Utilizing in vitro and in vivo injury models, neurite growth, axonal regeneration, and sensory and motor functions were assessed and compared. Interestingly, the dorsal root ganglion neurons from fat-1 mice (n-3 enriched) had significant increase in neurite outgrowth and improved neuronal survival after injury. The omega-3-enriched group did not exhibit an increase in neuronal cell death after mechanical or hypoxic injury, in contrast to the 2 groups where hypoxia resulted in neuronal cell death in the order of 2.3–2.6-fold increase. The mice expressing the fat-1 gene showed a more complex neurite outgrowth phenotype, with neurons displaying both longer neurites and branches, supporting the notion that n-3 PUFA level had improved neuroregenerative potential. Interestingly, the in vivo experiments also showed that the higher tissue n-3 PUFA group also exhibited a faster recovery of sensory loss, as assessed by mechanical stimulation thresholds. Even after a severe form of injury such as a crush injury, recovery of sensation was faster in the group that was enriched for omega-3. Alzheimer’s disease has also been reported to strongly correlate with decreases in omega-3 fatty acid levels in the brain and peripheral tissue, compared to age-matched controls [28]. Epidemiological studies have also shown a correlation between lower levels of dietary omega-3 fatty acids and the development of Alzheimer’s disease, suggesting a neuroprotective effect [29].
With both anti-inflammatory and neuroprotective properties, omega-3 fatty acids were an attractive candidate for the prevention of P-APS and peripheral neuropathy. We report here the results of a double-blinded placebo-controlled pilot trial to assess if omega-3 acid supplementation impacted the development of pain and use of pain medication in breast cancer patients receiving weekly paclitaxel.
Methods
Study design and participants
This was a randomized double-blinded, placebo-controlled pilot trial enrolling patients receiving weekly paclitaxel chemotherapy for breast cancer randomized to omega-3 fatty acid supplementation vs placebo with the goal of preventing P-APS. This study was approved by the University of New Mexico Health Sciences Center Institutional Review Board and monitored by the Data Safety Monitoring Board. Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. Patients must had been diagnosed with breast cancer, stage I–IV, were ≥ 18 years old, male or female, had an Eastern Cooperative Oncology Group performance status of 0–2, had not taken omega-3 fatty acid supplementation for the previous 1 month, and had not taken analgesics for the week prior to enrollment. Patients were permitted to receive concurrent HER2-directed therapies, carboplatin, and/or bevacizumab. Patients with previously reported peripheral neuropathy, fibromyalgia, planned use of granulocyte-stimulating factor, or prior paclitaxel within the previous 6 months were excluded. Sixty patients who were scheduled to initiate weekly paclitaxel (70–90 mg/m2) were recruited and were randomized by pharmacy staff 1:1 to receive 4 g of omega-3 acid ethyl esters (Lovaza, 38% DHA and 47% EPA) given as either 4 1-g pills once a day or 2 1-g pills twice a day per patient preference or matching placebo beginning 1 week prior to starting paclitaxel. The intervention was continued until paclitaxel was discontinued, for a maximum of 12 weeks. A dose of at least 2.7 g/day of EPA and DHA has been reported to have analgesic effects in inflammatory conditions [16]. The dose of 4 g/day is an FDA-approved dose of omega-3 fatty acids (Lovaza) for the treatment of hypertriglyceridemia and has a well-documented toxicity profile. On the basis of this, a dose of 4 g/day was selected. The placebo excipient material will be microcrystalline cellulose.
Assessments
The five outcome measures used were five questionnaires. Questionnaires evaluating the natural history of P-APS were utilized for all participants: (1) a baseline/pretreatment questionnaire; (2) a daily paclitaxel-induced acute pain syndrome symptom log from day 2 to 7 each cycle; (3) a day-8 P-APS symptom summary; (4) a CIPN questionnaire administered monthly and after the last paclitaxel cycle [2]; and (5) for CIPN neurotoxicity, the EORTC-QLQ CIPN 20 questionnaire was completed prior to each dose of paclitaxel and 1 month after completion of last dose [30] (All questionnaires are included in the supplemental materials). The five questionnaires were chosen based on previous studies, most notable NCCTG N08C1, which also used five questionnaires, three of which were the same for this study (daily P-APS questionnaire, day-8 P-APS symptom summary, and the EORTC-QLQ CIPN 20 questionnaire). A baseline/pretreatment questionnaire was added to account for any pre-existing neuropathy, and a monthly CIPN questionnaire was added as CIPN can persist months after treatment [31].
The Baseline Pre-Paclitaxel questionnaire has two yes/no questions to assess chronic pain and pain medication use followed by four questions to assess numbness, tingling, burning or shooting pain scored 0–10 to assess baseline chronic pain, pain medication use, and neuropathy. The daily P-APS symptom log has five questions scores 0–10 to assess aches/pain, two yes/no questions asking about non-opioid and opioid pain medications, and one question asking whether the patient believes they were on placebo or not to assess the development of P-APS. The day-8 P-APS symptom summary asks 21 questions to assess pain, numbness, or tingling during the previous week. The CIPN monthly questionnaire consisted of four questions ranked 0–10 assessing numbness, tingling, burning, or shooting. The EORTC QLQ–CIPN-20 is a 20-item CIPN-specific questionnaire that includes three scales assessing sensory (nine items), motor (eight items), and autonomic (three items) symptoms and functioning, with each item measured on a scale of 1 to 4 (1, not at all; 4, very much) and is designed to measure occurrence and severity of CIPN. The EORTC QLQ–CIPN-20 composite scores were compared as described in the data analysis, and the CIPN monthly questionnaire was used to detect any CIPN that developed over time. The maximum pain reported in each time window from the daily P-APS symptom log and the day-8 APS symptom log compared to the baseline pre-paclitaxel questionnaire was used to summarize the self-assessments of the degree of P-APS.
Due to a potential risk for increased low-density lipoprotein cholesterol (LDL) and transaminase levels, LDL levels were determined at baseline, at week 4, and on the last day of paclitaxel treatment. Adverse events (AEs) were assessed according to the National Cancer Institute’s Common Toxicity Criteria for Adverse Events (NCI-CTCAE) version 4.0. Use of over-the-counter pain medications and opioids was collected via patient-reported questionnaires.
Trial objectives
The primary objective was to compare the effect of omega-3 fatty acids, versus placebo, on the incidence of pain (any pain vs. no pain) following paclitaxel treatment over the 12-week treatment cycle. Secondary objectives included assessments of differences in maximum pain score in the week following the first treatment, and following each subsequent paclitaxel treatment cycle over 12 weeks; to compare pain medication use; to compare the effect of the omega-3 fatty acid intervention on the severity of CIPN; and to assess the tolerability of omega-3 fatty acids through comparisons between the two treatment groups.
Sample size
The sample size was selected in order to provide at least 80% power to detect an absolute difference of 40% between the intervention and the placebo groups in the primary outcome of the occurrence of P-APS using a two-sided type 1 error level of 0.05. Considering that P-APS is experienced by 60–80% of those treated, a sample size of 30 per group was selected.
Data analysis
Characteristics of study participants were summarized with medians, and 25th and 75th percentiles, or with counts and percentages. The statistical team performed analysis of the symptom diarrhea and were blinded to the placebo groups initially. We compared baseline characteristics with Wilcoxon rank sum tests or with Fisher’s exact tests [32]. The comparisons of the primary outcomes of interest for this trial, the report of any treatment-associated pain in the first week following initiation of paclitaxel, and separately over the 12-week study period, were made using Fisher’s exact tests. Comparisons of other outcome measures of interest below were made between groups using generalized linear models while accounting for within-person correlations using generalized estimating equations for both daily responses and weekly data over the 12 weeks of the intervention. The maximum pain level reported on the pain inventory on each day following the first paclitaxel cycle was analyzed using a negative binomial distribution, as was the maximum pain level reported for each week following the first paclitaxel cycle. Use of over-the-counter pain medications and opioids was analyzed using logistic regression models. EORTC-QLQ CIPN 20 scores were analyzed on the square root scale using a linear model. Each of these models accounted for within-person correlations using an autoregressive repeated measures structure. The initial comparisons for these outcomes tested whether there were significant differences in trends over time between the two treatment groups. When there was no evidence of trends over time, a single time invariant test was performed to compare the outcomes between the two treatment groups. Estimates of group means, and between-group comparisons, and their 95% confidence intervals were obtained from these models. Further adjustments based on age, race, and ethnicity were not planned for or not done due to small sample size. The type 1 error level in this pilot study was set at a two-sided value of 0.05, unadjusted for multiple comparisons. Initial evaluations of the data suggested that there might be an association between paclitaxel-associated pain scores and reported CIPN scores. We therefore performed a post hoc analysis and estimated the correlation between maximum reported paclitaxel-associated pain levels and self-reported CIPN scores. In this analysis, we used a linear mixed effects model that adjusted for week on study and treatment group, as well as within-person autoregressive correlations, to estimate the correlation between P-APS and CIPN scores. We also examined whether the relationship between P-APS and CIPN scores varied as a function of the study week using linear models that accounted for treatment group and within-person autoregressive correlations. Analyses were performed using the SAS statistical software platform (version 9.4, Cary NC). Data quality was ensured by review of data by the study chairperson following Alliance policies.
Results
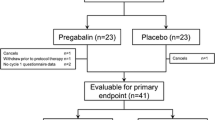
Sixty patients were enrolled between March 7, 2013 and January 16, 2018 and were randomized to intervention versus placebo. Of these, 28 were randomized to the placebo arm and 32 were randomized to the intervention arm. After randomization, four participants were subsequently deemed ineligible. Three of these had been randomized to the intervention arm. A total of 2 additional individuals dropped out of the intervention group, and 3 dropped out of the placebo group due to withdrawal of consent before intervention initiation. Two additional patients randomized to the intervention arm dropped out of the study after initiating treatment. This left a final sample of 49 patients (24 placebo, 25 intervention) with data available for analysis for an attrition rate of 18% (Table 1). Data lock occurred August 3, 2022.
Over the first week of treatment with paclitaxel, 17 (68.0%) of those receiving the omega-3 fatty acid intervention experienced any P-APS based on the daily P-APS symptom log and day 8 P-APS summary questionnaire, compared to 15 (62.5%) of those in the placebo arm (p = 0.77). Over the full 12-week study period, 21 (84.0%) of those receiving the omega-3 fatty acids intervention experienced any P-APS, compared to 21 (87.5%) of those in the placebo arm (p = 1.0).
The maximum reported pain in the first week following the initial treatment showed no significant difference in pain levels over time (p = 0.78) (Fig. 1a). Those in the intervention group reported maximal pain scores during the first week that were 1.53 (95% confidence interval [CI]: 0.81–2.90) times higher than for those in the placebo group (p = 0.20). Similar results were observed when modeling the maximum reported pain scores over the 12 weeks of the observation period (Fig. 1b, p = 0.83), with those in the intervention arm reporting maximal pain scores over the 12 weeks that were not significantly higher (1.21 times, p = 0.48, 95% CI: 0.72-2.02).
Maximum pain scores during omega-3 fatty acid (FA) treatment. a shows daily reported values over the first week following the first cycle of paclitaxel. b shows weekly reported values during the weekly 12 cycles of paclitaxel treatment. Estimated means are shown with 95% confidence intervals. Values are offset to enable better viewing of the overlapping confidence intervals that correspond to the daily or weekly estimates
There were no significant differences in trends over time between the two treatment groups in the use of over-the-counter analgesics or opioids, or in the average CIPN scores including shooting pain, burning pain, numbness, and tingling (Supplemental Table 1, p = 0.66, 0.55, and 0.83, respectively). Accounting for within-person correlations over the course of the study, the estimated proportion of participants who used over-the-counter analgesics was 34.8% (95% CI: 26.1–44.5%). Similarly, the estimated proportion of participants who used opioids was 7.8% (95% CI: 4.4–13.5%), and the average CIPN score was 9.7 (95% CI: 6.6–13.3). When controlling for week-to-week differences, we observed a non-significantly higher odds of over-the-counter analgesic use in the intervention group (OR: 1.65, 95% CI: 0.72–3.78, p = 0.23). Use of opioids was also higher, though not significantly, in the intervention group (OR: 2.06, 95% CI: 0.55–7.75, p = 0.28). Similarly, there were higher average CIPN scores in the treatment group (intervention mean: 12.8, 95% CI: 7.6–19.4 vs. placebo mean: 8.4, 95% CI: 4.6–13.2, p = 0.21), though this difference was not statistically significant.
Evaluation of simultaneous assessment of paclitaxel associated pain scores and reported average CIPN scores revealed a significant association between them which suggested that paclitaxel-associated pain was an early marker for future CIPN (Fig. 2). The simple Spearman’s correlation between the two scores was 0.366 (p < 0.001) which would suggest a low correlation. However, this value did not account for the design of the study. Adjusting for treatment arm, week of study, and within-person correlations resulted in a correlation estimate of 0.215 (95% CI: 0.144–0.286, p < 0.001) which would suggest a weak relationship (Fig. 2). This relationship may suggest that that paclitaxel-associated pain could be an early marker for future CIPN, as the strength of the relationship tended to increase over time (p = 0.005).
Chemotherapy-induced peripheral neuropathy (CIPN) total scores in relationship to weekly reported maximal pain scores. Estimated CIPN scores that are adjusted for treatment arm, week of study, and within-person correlations are shown with 95% confidence intervals. Also shown is the estimated trend in average CIPN scores (heavy line), along with its 95% confidence band, where the CIPN total score was modeled on the square root scale
The maximum grade 3 and 4 adverse events (AEs) were mild in the intervention arm with one episode each thromboembolic event and peripheral motor neuropathy while the placebo arm had one episode each of peripheral motor neuropathy, allergic reaction, and nausea (Supplemental Table 2). Overall AE burden was minimal, and there were no trends toward additional AE burden with the intervention. Cholesterol levels did not display differential changes over the course of treatment between treatment groups (p = 0.44) and did not display significant changes with time overall (p = 0.55) (Fig. 3).
Discussion
In this double-blinded placebo-controlled pilot trial, omega-3 acid supplementation did not reduce (1) the weekly maximum pain score and (2) over-the-counter analgesic use or opioid use in patients receiving weekly paclitaxel. There were no concerning safety signals or changes in cholesterol levels in patients randomized to omega-3 acid supplementation versus placebo.
P-APS and CIPN remain significant sources of morbidity for patients receiving paclitaxel, and its prevention remains a focus of ongoing research. The numerous studies undertaken to date to prevent P-APS have been largely negative, with no one agent emerging as beneficial and worthy of further study or use in clinical practice [33, 34]. Our evaluation of omega-3 acid supplementation was similarly found to be ineffective. Our findings are contrary to other studies that supported a decrease in peripheral neuropathy with omega-3 fatty acid supplementation [35, 36]. All studies used different outcome measures; Ghoreishi et al. used a subjective patient-reported “Reduced Total Neuropathy Score,” and Anoushirvani et al. used neurologist examinations and electrophysiological studies while this study used a different subjective reported patient outcome measure as described in the “Methods” section. We believe patient-reported outcomes are the ideal measurement tool for the measurement of neuropathy as it directly collects the patient’s perspective [37]. Additionally, both of the above-referenced studies used a formulation of fatty acids that was 54% DHA and 10% EPA, while the formulation in this study was 38% DHA and 47% EPA, and this difference in types of omega-3 fatty acids may have impacted results. Lastly, this study evaluated neuropathy symptoms more frequently than in the other trials. Omega-3 fatty acid supplementation may be ineffective for P-APS and CIPN due to current dosing, non-optimal formulation, or insufficient time as the neuroprotective effects may take months to develop, which is not feasible in the setting of active cancer requiring treatment. The American Society of Clinical Oncology recommends against dietary omega acid supplementation for the prevention of CIPN at this time [38].
This study replicates prior data supporting that P-APS correlates with later CIPN. This was first revealed by NCCTG N08C1 demonstrating that those with higher P-APS scores with the first dose of paclitaxel appear to have more chronic neuropathy [2]. This was further elucidated with additional data by the same group in a different publication supporting the concept that P-APS is a form of nerve damage [4]. This study adds support to that literature and highlights P-APS as both a target of further CIPN research and a clinical marker predicting the future development of CIPN which clinicians can monitor for vigilantly.
This study has several limitations. While 60 patients were enrolled on the study, only 49 were evaluable, resulting in a smaller than anticipated sample size that may have led to an underpowered study. This study was conducted at a single comprehensive cancer center with a large minority population (40% of patients at our center are of Hispanic or Native American ancestry), so the results may not be applicable to other centers. The main outcomes of patient-reported maximum pain scores, opioid use, and over-the-counter analgesic use relied on patients accurately completing surveys and were subject to limitations including incomplete and missing surveys and recall bias. This study did not take into account intake of dietary arachidonic acid, which negatively affects omega-3 fatty acid metabolism, which is high in the Western diet and may have further regional differences. Also, the omega-3 fatty acid supplementation may have been ineffective due to low dosing, non-optimal formulation, or insufficient time on the supplementation before initiation of chemotherapy. Pain surveys were specifically drawn from well-validated previous research about P-APS, and we included a baseline questionnaire asking about pain, but it is possible that pain caused by other etiologies were counted towards P-APS, and this should have been accounted for by comparing to the control arm.
In conclusion, omega-3 acid supplementation did not significantly reduce or prevent P-APS or CIPN compared to placebo in patients receiving weekly paclitaxel. Despite limitations, this was a well-designed placebo-controlled trial using validated outcome measures. As such, our results do not support further exploration of omega-3 acids as a prevention strategy in breast cancer patients receiving weekly paclitaxel.
References
Garrison JA et al (2003) Myalgias and arthralgias associated with paclitaxel. Oncology (Williston Park) 17(2):271–277; discussion 281-282, 286–288
Loprinzi CL et al (2011) Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. JCO 29(11):1472–1478. https://doi.org/10.1200/JCO.2010.33.0308
Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 52(8):885–897. https://doi.org/10.1002/mnfr.200700289
Reeves BN et al (2012) Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy. Cancer 118(20):5171–5178. https://doi.org/10.1002/cncr.27489
Chiu N et al (2018) A prospective study of docetaxel-associated pain syndrome. Support Care Cancer 26(1):203–211. https://doi.org/10.1007/s00520-017-3836-z
Bindu S, Mazumder S, Bandyopadhyay U (2020) Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 180:114147. https://doi.org/10.1016/j.bcp.2020.114147
Markman M, Kennedy A, Webster K, Kulp B, Peterson G, Belinson J (1999) Use of low-dose oral prednisone to prevent paclitaxel-induced arthralgias and myalgias. Gynecol Oncol 72(1):100–101. https://doi.org/10.1006/gyno.1998.5226
Jacobson SD et al (2003) Glutamine does not prevent paclitaxel-associated myalgias and arthralgias. J Support Oncol 1(4):274–278
Martoni A, Zamagni C, Gheka A, Pannuti F (1993) Antihistamines in the treatment of taxol-induced paroxystic pain syndrome. JNCI 85(8):676–677. https://doi.org/10.1093/jnci/85.8.676
Samuels N, Ben-Arye E (2020) Integrative approaches to chemotherapy-induced peripheral neuropathy. Curr Oncol Rep 22(3):23. https://doi.org/10.1007/s11912-020-0891-2
Yoshida T, Sawa T, Ishiguro T, Horiba A, Minatoguchi S, Fujiwara H (2009) The Efficacy of prophylactic Shakuyaku-Kanzo-to for myalgia and arthralgia following carboplatin and paclitaxel combination chemotherapy for non-small cell lung cancer. Support Care Cancer 17(3):315–320. https://doi.org/10.1007/s00520-008-0508-z
Brami C, Bao T, Deng G (2016) Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: a systematic review. Crit Rev Oncol Hematol 98:325–334. https://doi.org/10.1016/j.critrevonc.2015.11.014
Schloss J, Colosimo M, Vitetta L (2016) New insights into potential prevention and management options for chemotherapy-induced peripheral neuropathy. Asia Pac J Oncol Nurs 3(1):73–85. https://doi.org/10.4103/2347-5625.170977
Cavaletti G, Cavalletti E, Montaguti P, Oggioni N, De Negri O, Tredici G (1997) Effect on the peripheral nervous system of the short-term intravenous administration of paclitaxel in the rat. Neurotoxicology 18(1):137–145
Peters CM et al (2007) Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 203(1):42–54. https://doi.org/10.1016/j.expneurol.2006.07.022
Goldberg RJ, Katz J (2007) A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 129(1–2):210–223. https://doi.org/10.1016/j.pain.2007.01.020
Kwiatkowska B, Maślińska M (2020) The place of omega-3 and omega-6 acids in supplementary treatment of inflammatory joint diseases. Reumatologia 58(1):34–41. https://doi.org/10.5114/reum.2020.93511
Silva RV et al (2017) Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front Pharmacol 8:723. https://doi.org/10.3389/fphar.2017.00723
Hong S, Gronert K, Devchand PR, Moussignac R-L, Serhan CN (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. J Biol Chem 278(17):14677–14687. https://doi.org/10.1074/jbc.M300218200
Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0021925820826696?via%3Dihub. Accessed 20 Dec. 2021
Ji R-R, Xu Z-Z, Strichartz G, Serhan CN (2011) Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci 34(11):599–609. https://doi.org/10.1016/j.tins.2011.08.005
Bradberry JC, Hilleman DE (2013) Overview of omega-3 fatty acid therapies. P T 38(11):681–691
Dyall SC, Michael-Titus AT (2008) Neurological benefits of omega-3 fatty acids. NeuroMolecular Med 10(4):219–235. https://doi.org/10.1007/s12017-008-8036-z
Bazan NG, Molina MF, Gordon WC (2011) Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr 31:321–351. https://doi.org/10.1146/annurev.nutr.012809.104635
King VR, Huang WL, Dyall SC, Curran OE, Priestley JV, Michael-Titus AT (2006) Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci 26(17):4672–4680. https://doi.org/10.1523/JNEUROSCI.5539-05.2006
Lim S-N, Huang W, Hall JCE, Ward RE, Priestley JV, Michael-Titus AT (2010) The acute administration of eicosapentaenoic acid is neuroprotective after spinal cord compression injury in rats. Prostaglandins Leukot Essent Fat Acids 83(4–6):193–201. https://doi.org/10.1016/j.plefa.2010.08.003
Gladman SJ et al (2012) Improved outcome after peripheral nerve injury in mice with increased levels of endogenous ω-3 polyunsaturated fatty acids. J Neurosci 32(2):563–571. https://doi.org/10.1523/JNEUROSCI.3371-11.2012
Kyle DJ, Schaefer E, Patton G, Beiser A (1999) Low serum docosahexaenoic acid is a significant risk factor for Alzheimer’s dementia. Lipids 34 Suppl:S245. https://doi.org/10.1007/BF02562306
Barberger-Gateau P, Letenneur L, Deschamps V, Pérès K, Dartigues J-F, Renaud S (2002) Fish, meat, and risk of dementia: cohort study. BMJ 325(7370):932–933
Chemotherapy-induced peripheral neuropathy - EORTC - Quality of Life: EORTC – Quality of Life. https://qol.eortc.org/questionnaire/qlq-cipn20/, https://qol.eortc.org/questionnaire/qlq-cipn20/. Accessed 18 Jun. 2023
Quasthoff S, Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249(1):9–17. https://doi.org/10.1007/PL00007853
Altman DG (1990) Practical Statistics for Medical Research. CRC Press
Ibrahim EY, Ehrlich BE (2020) Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol 145:102831. https://doi.org/10.1016/j.critrevonc.2019.102831
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol 81(6):772–781. https://doi.org/10.1002/ana.24951
Ghoreishi Z et al (2012) Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer 12(1):355. https://doi.org/10.1186/1471-2407-12-355
Anoushirvani AA, Poorsaadat L, Aghabozorgi R, Kasravi M (2018) Comparison of the effects of omega 3 and vitamin E on palcitaxel-induced peripheral neuropathy. Open Access Maced J Med Sci 6(10):1857–1861. https://doi.org/10.3889/oamjms.2018.333
Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M (2018) The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas 9:353–367. https://doi.org/10.2147/PROM.S156279
Loprinzi CL et al (2020) Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. JCO 38(28):3325–3348. https://doi.org/10.1200/JCO.20.01399
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA232760, and partially supported by the UNM Comprehensive Cancer Center Support Grant NCI P30CA118100, and the Biostatistics shared resource. https://acknowledgments.alliancefound.org.
Author information
Authors and Affiliations
Contributions
Z.D., J.L., L.C., and D.B. conceived the initial trial design. V.P. performed data analysis and prepared figures and tables. B.T. wrote the manuscript with significant edits from U.B. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the University of New Mexico Health Sciences Center Institutional Review Board [UNM HRPO] (#19-562) and monitored by the Data Safety Monitoring Board.
Informed consent
Was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tawfik, B., Dayao, Z.R., Brown-Glaberman, U.A. et al. A pilot randomized, placebo-controlled, double-blind study of omega-3 fatty acids to prevent paclitaxel-associated acute pain syndrome in breast cancer patients: Alliance A22_Pilot2. Support Care Cancer 31, 637 (2023). https://doi.org/10.1007/s00520-023-08082-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-08082-x