Abstract
Objective
The goal of this research was to review the literature from randomized controlled trials (RCTs) on the impacts of moxibustion on cancer-related fatigue (CRF) as well as provide credible evidence to guide clinical practice.
Methods
Three English electronic medical databases (PubMed, Embase, and the Cochrane Library) and two Chinese databases (China National Knowledge Infrastructure and Wanfang) were searched. Only randomized controlled trials on the effect of moxibustion on CRF were included in this systematic review. Study selection, data extraction, and validation were all carried out independently by two reviewers. The revised Cochrane Risk of Bias tool was used to assess the quality of the RCTs (RoB 2.0). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was applied to assess effect sizes in individual RCTs and pooled effect sizes in meta-analyses. Data were meta-analyzed using Stata (version 14.0).
Results
In a random-effects meta-analysis of 24 RCTs with 1894 participants, the aggregated standardized mean difference (SMD) revealed a statistically significant association between moxibustion and alleviation from cancer-related fatigue (SMD = − 1.66, 95% CI = − 2.05, − 1.28, p = 0.000). Pooled results, however, show significant heterogeneity (I2 = 92.5%), and the evidence is insufficient to determine whether this association varies systematically by measuring tools and moxibustion modalities. Furthermore, evidence ranging from very low to low showed that moxibustion had an immediate positive effect on patients with CRF.
Conclusion
Moxibustion may have a therapeutic effect on cancer-related fatigue. However, further large-scale, multicenter, high-quality RCTs on moxibustion for fatigue relief and safety are still needed because of the handful of studies included and the low methodological quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence and impacts of cancer-related fatigue
Cancer-related fatigue (CRF) is an accumulation of subjective feelings caused by chronic stress and pain resulting from cancer and related treatments, such as weakness, activity intolerance, inattention, and loss of motivation or interest [1]. CRF is a common symptom in cancer patients. It persists long term during and after anticancer therapy, lowering one’s quality of life significantly [2]. The prevalence of CRF varies according to the literature; however, it typically ranges from 40 to 100% [3]. CRF is often overlooked despite its high incidence among cancer patients because it is a common symptom that anybody might experience. CRF differs from general tiredness in that it is challenging to alleviate, even with adequate rest, and is unrelated to the level of exercise or the intensity of the condition.
The potential role of moxibustion in the management of cancer-related fatigue
The pathogenesis of CRF is complex, and the possible mechanisms are currently considered to include central and peripheral mechanisms. The central mechanism includes the hypothesis of cytokine imbalance, hypothalamic–pituitary–adrenal axis disorder, circadian rhythm disorder, serotonin disorder, and activation of vagus nerve conduction, and the peripheral mechanism mainly includes the hypothesis of muscle metabolism disorder [4, 5]. Pharmacological approaches to enhance CRF are restricted due to the intricacy of the underlying mechanisms. Consequently, there is a growing body of evidence supporting the use of complementary and integrative initiatives to manage CRF [6,7,8].
Moxibustion is an East Asian traditional external treatment method that uses the heat of burning herbs (primarily Artemisia vulgaris) to stimulate specialized acupoints on the skin. It operates through direct heat stimulus at various temperature levels [9]. Animal studies have shown that thermal stimulation of particular acupoints can be the approach for reducing oxidative stress and improving immunosuppression [10, 11]. Moxibustion has traditionally been thought to be particularly effective at replenishing energy and is commonly used in patients with impaired resistance. Moxibustion has been shown in numerous studies to be advantageous for chronic fatigue [12,13,14]. Moxibustion, like the benefits of treating chronic fatigue syndrome, may be an effective option to treat CRF.
The current state and the central question of moxibustion study for the treatment of cancer-related fatigue
To date, the therapeutic impact of moxibustion on CRF has been studied in a variety of clinical trials, and regardless of the type of moxibustion, positive results have been consistently shown [15,16,17,18]. Three systematic reviews (SRs) and meta-analyses of moxibustion for the treatment of cancer-related fatigue have been published to synthesize the current evidence and reach a more solid conclusion. These reviews’ findings are somewhat consistent, concluding that moxibustion has shown promise in treating CRF. The initial review (2013) [19] focused on studies evaluating acupuncture or moxibustion for cancer-related fatigue. The results showed that despite several inherent defects in the included studies, acupuncture and moxibustion are still effective adjuvant treatments for CRF, but since only 3 RCTs on the effect of moxibustion on CRF were included, the authors could not draw definite conclusions. The second review [20], conducted in Korea in 2014, focused on moxibustion for cancer-related fatigue, but the authors found it hard to conclude as only four RCTs got involved, and each of the four RCTs reviewed had a high risk of bias and poor reporting quality. Notably, all of the studies in this systematic review found that moxibustion treatment improved CRF significantly. It is worth noting, however, that all four studies used daily treatments that differed from the general treatment regimen, and none of these studies used sham as a control group. Moxibustion in treating cancer-related fatigue is also discussed in the third review [21], with 22 trials involving a total of 1628 cancer patients. Moxibustion, according to the authors, can enhance cancer-related fatigue and quality of life while also being safe. However, due to the small sample size and poor study quality, the authors did not perform subgroup analysis or publication bias, and the strength of the evidence was restricted. Meanwhile, no available published reviews of moxibustion for cancer-related fatigue were found using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, and the absence of a grading system for the level of evidence body may lead to an inappropriate interpretation of the pooled effect size (ES). In addition, several new studies on RCTs of moxibustion for cancer-related fatigue have been published since 2019, and regular updates of the meta-analyses are worthwhile [15].
Based on these considerations, we hope to investigate the safety and efficacy of moxibustion for cancer-related fatigue, assist clinicians in making recommendations, and promote moxibustion research progress.
Methods
Study registration
No protocol has been publicly registered. The report, however, was synthesized following the Preferred Reporting Items of the Guidelines for Systematic Reviews and Meta-Analysis (PRISMA 2020) [22].
Data sources and search strategy
From inception to December 31, 2022, the following MeSH terms and text words were searched in three English databases (PubMed, Embase, and the Cochrane Library) and two Chinese databases (China National Knowledge Infrastructure and Wanfang Data): (“Neoplasms” [MeSH Terms] OR “tumor*” [Title/Abstract] OR “neoplas*” [Title/Abstract] OR “cancer*” [Title/Abstract] OR “malignanc*” [Title/Abstract] OR “malignant neoplasm*” [Title/Abstract]) AND (“Fatigue” [MeSH Terms] OR “Lassitude” [Title/Abstract] OR “Tiredness” [Title/Abstract] OR “Weary” [Title/Abstract] OR “cancer related fatigue” [Title/Abstract] OR “CRF” [Title/Abstract]) AND (“Moxibustion” [MeSH Terms] OR “Moxabustion” [Title/Abstract] OR “Moxa” [Title/Abstract] OR “Mugwort” [Title/Abstract] OR “Wormwood” [Title/Abstract]) AND (“Randomized Controlled Trial” [Publication Type] OR “Randomized Controlled Trials as Topic” [MeSH Terms] OR “Controlled Clinical Trial” [Publication Type] OR “Controlled Clinical Trials as Topic” [MeSH Terms] OR “Randomized” [Title/Abstract] OR “Placebo” [Title/Abstract] OR “Trial” [Title/Abstract] OR “Groups” [Title/Abstract] OR “RCT” [Title/Abstract]).
We also went through the reference lists of eligible studies to find any articles that had been missed by the electronic search. EndNote software was used to manage citations. The searching procedures and outcomes for each database are presented in Supplementary Appendix A.
Inclusion and exclusion criteria
Included studies should meet the PICOS elements listed as follows.
-
(1)
P (Population). All patients with CRF were included, irrespective of cancer type, severity, duration of cancer, or fatigue. In addition, the clinical status of the patient (e.g., ongoing treatment, post-treatment, or end-stage) was not limited.
-
(2)
I (Intervention). The control group received routine care, such as health education and psychological support. The experimental group received the moxibustion combined with routine care for cancer-related fatigue. The type of stimulation used in moxibustion (direct, indirect, heat-sensitive, infrared, etc.) was not restricted. During the study, any new treatment used to reduce CRF was not permitted.
-
(3)
C (Comparison). A waiting list/no treatment, sham (placebo) moxibustion, and normal care were given to the control group (e.g., conventional medications, health education, exercise therapy, or cognitive-behavioral intervention).
-
(4)
O (Outcome). The severity of fatigue was the primary outcome of interest. Quality of life, impact on immunity, blood indicators, and adverse events were all secondary outcomes of interest. Studies that assessed only these secondary outcomes were excluded.
-
(5)
S (Study Design). Only randomized controlled trials (RCTs) were considered for inclusion.
Screening procedure
Duplicates were identified and removed using EndNote X9 (Clarivate Analytics (US) LLC). Two reviewers (XQW and YQ) independently reviewed the titles and abstracts of candidate articles for relevance to the topic under review using the inclusion criteria. Each reviewer decides on each article marked “yes” or “no.” The two reviewers compared their opinions once they had finished judging all of the candidate research. PBD, the third reviewer, was in charge of resolving controversial differences through discussion.
To make a final decision on whether to include the study, two reviewers, XQW and YQ, independently retrieved and extensively read the full text of potentially relevant studies. They then went through discussions to resolve their differences. On contentious results, a third reviewer (PBD) will reach a decision.
Assessment of the risk of bias
The revised Cochrane Risk of Bias tool for RCTs was used to assess the risk of bias in included studies (RoB 2.0) [23]. In RoB 2.0, there were five domains for assessing bias risks: (a) bias in the randomization process; (b) bias in deviations from intended interventions; (c) bias in missing outcome data; (d) bias in outcome measurement; and (e) bias in the selection of the reported results. For each domain and overall judgment, each RCT received one of three judgments: “low risk of bias,” “some concerns,” or “high risk of bias.” The RoB 2.0 tool provided by Cochrane was used to complete this process. Two reviewers (XQW and YQ) assessed the work independently, with a third reviewer (PBD) resolving disagreements and making the final decision.
Data extraction
Information was extracted from each original manuscript by 2 reviewers (XQW and YQ) independently using predesigned standardized tables, including study characteristics (author, country, year of publication), participant information (sample size, age, cancer type, cancer stage, TCM syndrome, current anti-tumor treatment, etc.), intervention measures (moxibustion type, intervention frequency, course of treatment, duration, etc.), and controls. In addition, key outcomes such as fatigue level and quality of life were extracted. Following treatment, follow-up data were divided into two categories: short-term effects (less than one month) and long-term effects (more than one month).
Statistical analysis
A quantitative meta-analysis was performed to synthesize the findings of the included studies. When standard errors or ranges were provided, standard deviations were calculated using standard formulae. Because of the diversity of participants and interventions, we used random-effects models. Because the outcomes were all continuous measures, we used standardized mean differences (SMD) when they had different measurement scales.
The Cochran Q test was used to assess study heterogeneity. We also used I2 testing to figure out how much study heterogeneity there was. The Cochrane handbook (www.training.cochrane.org/handbook) was used to determine thresholds for I2 interpretation: 0–40% could be significant or not, 30–60% could indicate moderate heterogeneity, 50–90% could suggest significant heterogeneity, and 75–100% could show considerable heterogeneity. To investigate heterogeneity further, meta-regression was used. Simultaneously, a sensitivity analysis was performed. Besides, in a funnel plot, Egger’s test was used to assess publication bias by plotting the effect size of each trial against the standard error. To estimate the impact of publication bias on the interpretation of the results, trim-and-fill was used. For all statistical analyses, we used Stata (version 14.0).
Evaluation of evidence quality
The GRADE system was used to assess the certainty of the body of evidence for each pooled or individual effect size for cancer-related fatigue. To resolve any discrepancies, two reviewers (XQW and YQ) and a third reviewer (PBD) worked independently. GRADE assesses five domains: design limitations, inconsistency, indirectness, imprecision, and publication bias. As a result, one of four evidence levels (high, moderate, low, or very low) can be obtained. We adopted an operating standard for downgrading GRADE evidence based on Ryan and Hill [24]. The GRADE evidence file was created using GRADEpro GDT (https://gradepro.org/).
Results
Search results
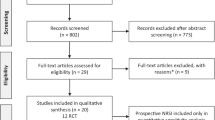
There were a total of 196 potentially relevant records found. Following the removal of duplicates, 158 studies were screened, and 48 were evaluated for eligibility using the PICOS criteria (participants, interventions, comparisons, outcomes, and study design). Finally, 19 reports were rejected for the reasons listed as follows: data were unavailable (n = 6), the main outcome was ineligible (n = 8), the control treatment was moxibustion (n = 2), and the study was not randomized (n = 3). Figure 1 shows a flowchart adapted from the PRISMA diagram that depicts the selection process.
Study characteristics
General details
The 2254 participants in the twenty-nine studies included in this systematic review ranged in age from 44.96 to 65.7 years, as shown in Table 1. The twenty-nine trials were all published between 2010 and 2021. Of those, 631 (27.99%) participants were various cancer, 531 (23.56%) participants were breast cancer, 511 (22.67%) participants were lung cancer, 177 (7.9%) participants were gastric cancer, 120 (5.3%) participants were liver cancer, 104 (4.6%) participants were ovarian cancer, 80 (3.5%) participants were cervical cancer, and only one study included (100 participants) to not report the concrete type of cancer. Twenty-five studies (86.21%) included 1948 participants with conventional therapy, whereas three studies (10.34%) included 241 participants with placebo moxibustion. The individual study focused on blank control. Twenty-seven out of 29 studies were conducted in China, one each in Mongolia and South Korea. Considering the involved outcomes, we focused on fatigue, which is the primary outcome. Likewise, there are other secondary outcomes, like the quality of life, immune indicators, blood indicators, inflammatory factors, hormone indicators, sleep quality, anxiety, and depression. Furthermore, three [25,26,27] reported a 4-week follow-up period among twenty-nine RCTs.
Treatment interventions
All of the studies used indirect moxibustion. Two RCTs [33, 45] used moxa sticks, four [30, 41, 47, 52] applied ginger-partitioned moxas, three [26, 28, 49] utilized infrared lasers, three [29, 34, 42] exploited thermal boxes, two [32, 53] employed moxa cones, four [38, 39, 44, 51] availed wheat-grain size cones, eight have access to the governor vessel moxibustion [31, 36, 37], thunder-fire moxibustion [40, 46, 48], herbal cake–separated moxibustion [50], heat-sensitive moxibustion [43], respectively, and the remaining study [27, 35] used a combination of moxibustion techniques. The choice of the acupoint for moxibustion treatment was inspired by traditional Chinese medicine theory across all RCTs, and the explanation for the selection was described in the respective trial. Each session lasted 15 to 30 min, and the time length of moxibustion therapies ranged from 5 to 8 weeks. Both fixed and individual acupoints determined by the practitioner were used in two randomized trials [28, 50], and the others [25,26,27, 29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49, 51,52,53] preferred the fixed one. Besides, we analyzed the acupoints adopted in trials, and the findings are as follows: Zusanli ST36 was the most preferred acupoint (58.62%). Moreover, Qihai RN6 (44.83%), Guanyuan RN4 (44.83%), Zhongwan RN12 (27.59%), Shenque RN8 (24.14%), Sanyinjiao SP6 (10.34%), and Yongquan KI1 (10.34%) were commonly used acupoints. Additionally, the combination of Qihai RN6, Guanyuan RN4, and Zusanli ST36 was the highest frequency acupoints adopted in studies (27.59%). Table 2 displays the specifics of moxibustion regimens.
Risk of bias in eligible studies
Twenty-nine RCTs were assessed according to the RoB 2.0 tool, of which two were regarded as having a “low risk of bias” and the rest rated as “some concerns” (Fig. 2). Overall, the studies’ quality was moderate (Fig. 3). Due to a lack of detail in the studies, the risk of bias from allocation concealment was unclear in nineteen of them [25, 30,31,32, 34, 35, 37, 39,40,41,42, 44,45,46,47,48, 50, 52, 53]. Although these 19 RCTs did not state whether or not participants were aware of the grouping during the trial, none of the studies presented additional interventions that were inconsistent with the trial. Given the lack of participants (the dropout rate in a single study was more than 5 percent) in three RCTs [39, 48, 51], the risk of attrition bias was unclear. Meanwhile, there was scant evidence that missing outcome data did not influence the outcome. The implementation of blinding, on the other hand, was the most difficult domain involved. The blinding of the practitioner was inapplicable given the characteristics of moxibustion, and the results of the ROB finding indicated that the depiction of blinding of participants and personnel seemed to be unclear or low. Besides, twenty RCTs made no mention of whether or not the assessors were aware of the interventions provided to study participants (Fig. 3).
Primary outcome: fatigue
Overall effects of moxibustion on fatigue
An individual study [39] could not be used for the meta-analysis because it only provided scores for each dimension of the fatigue scale, not the overall score. We attempted to contact the study’s author but were unable to obtain the necessary data to conduct the analysis. Finally, we conducted a meta-analysis of 28 articles [25,26,27,28,29,30,31,32,33,34,35,36,37,38, 40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Of these 28 RCTs, 24 RCTs were in the control group using routine care, 3 RCTs were in the sham moxibustion group, and one was in the blank control group. As a result, we pooled data separately based on the control group.
All trials [25,26,27,28,29,30,31,32,33,34,35,36,37,38, 40,41,42,43,44,45,46,47,48,49,50,51,52,53] reported that moxibustion was effective in relieving CRF, and the moxibustion was found to be superior to the control group in a random-effects analysis (Fig. 4 and Fig. 5). The results of the routine care group revealed a large (SMD = − 1.66, 95% CI = − 2.05, − 1.28) and significant (p = 0.000) overall effect size. The degree of heterogeneity among the studies was high (I2 = 92.5%) (Fig. 4). However, the overall effect size of the sham moxibustion group was SMD = − 1.33 (95% CI = − 1.61, − 1.05, p = 0.000, I2 = 0.0%) (Fig. 5).
Lung cancer versus other cancers
Cancer types were divided into two parts, one was other cancers [25,26,27,28,29,30,31,32,33,34,35,36,37, 41, 42, 44, 45, 47,48,49, 52, 53], and the other was lung cancer [38, 40, 43, 46, 50, 51]. Figure 6 reveals that 95% confidence intervals overlapped partially, which concluded that we could not achieve a positive result on whether moxibustion prevails for lung cancer patients or others.
Secondary outcomes: quality of life
As shown in Fig. 7, the pooled data from 16 studies [25, 28, 32,33,34,35,36,37, 39, 42, 44, 46, 48, 50, 52, 53] demonstrated a large effect of moxibustion on quality of life (SMD = 2.15, 95% CI = 1.39, 2.91; p = 0.000). The studies’ heterogeneity was high (I2 = 96.0%).
Secondary outcomes: others
Blood indicators
The pooled data from 5 studies [35, 37,38,39, 44] demonstrated a moderate (WMD = 0.77, 95% CI = 0.41, 1.13) and significant (p = 0.000) effect size on WBC, with small heterogeneity across studies (I2 = 16.3%), as shown in Supplementary Fig. 1. Furthermore, 2 studies [37, 40] found a moderate (WMD = 0.58, 95% CI = 0.28, 0.87) and significant (p = 0.000) effect size on RBC (Supplementary Fig. 2), and large heterogeneity (I2 = 68.1 percent) across studies. Furthermore, 2 studies [38, 39] found a large (WMD = 33.04, 95% CI = 15.53, 50.55) and significant (p = 0.000) effect size on PLT (Supplementary Fig. 3), with no heterogeneity across studies (I2 = 0.0%). Furthermore, data from 3 studies [35, 42, 43] revealed a large (WMD = 2.89, 95% CI = 0.93, 4.86) and significant (p = 0.004) effect size on NK (Supplementary Fig. 4). There was only a small amount of variation between the studies (I2 = 19.2%). In addition, HGB outcomes were reported in 6 studies [35, 37,38,39,40, 44] (Supplementary Fig. 5). The overall effect size was significant (p = 0.001) and large (WMD = 7.68, 95% CI = 3.05, 12.31). The studies showed significant heterogeneity (I2 = 72.8%). Furthermore, the pooled data from 2 studies [35, 36] revealed a moderate effect (WMD = 0.67, 95% CI = 0.14, 1.19) in favor of the moxibustion intervention on LY, which was significant (p = 0.012) (Supplementary Fig. 6). The studies had a high degree of heterogeneity (I2 = 73.9%). Three studies [35, 37, 44] found a non-significant (p = 0.132) effect size on NEUT (Supplementary Fig. 7).
Immune indicators
The data from 5 studies [35, 37, 42, 43, 46] showed a large (WMD = 4.98, 95% CI = 3.17, 6.79) and significant (p = 0.000) effect size on CD3+ (Supplementary Fig. 8). There was no heterogeneity across the studies (I2 = 0.0%). The pooled data from 3 studies [37, 43, 46] demonstrated a large (WMD = 7.07, 95% CI = 5.78, 8.36) and significant (p = 0.000) effect size on CD4+ (Supplementary Fig. 9), with no heterogeneity across studies (I2 = 0%). Pooling data from 2 studies [37, 43] showed a large (WMD = − 6.13, 95% CI = − 8.64, − 3.63) and significant (p = 0.000) effect size in CD8+ (Supplementary Fig. 10). There was moderate heterogeneity across the studies (I2 = 51.1%). To determine the effect of moxibustion on CD4 + /CD8 + , data from 4 studies [35, 37, 42, 43] were pooled (Supplementary Fig. 11). The magnitude of the overall effect (WMD = 0.38, 95% CI = 0.15, 0.62) was small but significant (p = 0.002). The studies had a high degree of heterogeneity (I2 = 78.5 percent). The combined data from 2 studies [33, 40] revealed that moxibustion had a significant effect on ALB (WMD = 3.54, 95% CI = − 1.48, 8.56; p = 0.167) (Supplementary Fig. 12). The studies’ heterogeneity was significant (I2 = 96.7%). The overall effect sizes of CD3 + CD4 + [35, 42] (Supplementary Fig. 13) and CD3 + CD8 + [35, 42] (Supplementary Fig. 14) were non-significant (CD3 + CD8 + : p = 0.858, CD3 + CD4 + : p = 0.271), tending to favor moxibustion and control interventions equally.
Hormone indicators
For Cor, 4 studies [34, 41, 48, 53] were included (Supplementary Fig. 15). The overall effect size was significant (p = 0.000) and large (WMD = 48.48, 95% CI = 40.67 to 56.28). The studies’ heterogeneity was high (I2 = 80.9%). The meta-analysis of ACTH [34, 41, 48] revealed a large (WMD = − 42.93, 95% CI = − 45.52 to − 40.34) and significant effect size (p = 0.000), and there was no heterogeneity between the studies (I2 = 0.0%) (Supplementary Fig. 16).
Inflammatory factors
The results of 2 studies [30, 53] on TGF-β were combined to determine the effect of moxibustion on TGF-β (Supplementary Fig. 17). The magnitude of the overall effect was large (WMD = − 26.41, 95% CI = − 55.77, 2.94), but not statistically significant (p = 0.078). The studies showed significant heterogeneity (I2 = 97.6%). To determine the effect of moxibustion on TNF-α, data from 3 studies [30, 48, 53] were pooled (Supplementary Fig. 18). The overall effect size was large (WMD = − 38.89, 95% CI = − 83.65, 3.88) and non-significant (p = 0.074). Heterogeneity across the studies was extensive (I2 = 98.8%).
Follow-up period
The majority of trials either did not specify a follow-up period or explicitly limited the assessment to the time of moxibustion treatment. Finally, only 3 RCTs [25,26,27] in the included studies reported a follow-up period, which was 4 weeks. However, the control group in one RCT [26] was given sham moxibustion, while the control groups in the other 2 RCTs [25, 27] were given routine care. Therefore, we only combined 2 RCTs whose control group was routine care. In trials [25, 27] that reported a 4-week follow-up period after the cessation of moxibustion therapy, there was a significant difference in fatigue scores (SMD = − 1.50, 95% CI = − 1.87, − 1.13, p = 0.000, I2 = 0.0%) (Fig. 8).
Meta-regression
Meta-regression analyses were conducted in an attempt to explain some of the observed heterogeneity. Meta-regression analysis in this study showed that the duration of intervention had a significant impact on heterogeneity (p = 0.021). Measuring tools and the modalities of moxibustion did not significantly impact fatigue in these analyses.
Subgroups
To clarify the potential discrepancies, we performed a subgroup analysis based on intervention duration. According to the duration of the intervention (Fig. 9), there were few overlaps in 95% confidence intervals of short-term effects (< 1 month) [25, 28, 31, 32, 34, 35, 38, 42,43,44,45,46,47, 50, 52, 53] and long-term effects (> 1 month) [27, 30, 33, 36, 37, 40, 41, 48]. It was determined that more high-quality studies comparing short-term and long-term interventions should be conducted.
Sensitivity analysis
Sensitivity analysis revealed that when each study was excluded from the analysis, there was no significant change in the pooled data.
Publication bias
A visual examination of the funnel plot on fatigue revealed significant asymmetry (Fig. 10). Egger’s test of publication bias on fatigue yielded a P-value of 0.00. Consequently, we reconducted the meta-analysis via the trim-and-fill computation. As a result, the combined effect size estimates did not change significantly, indicating that the outcomes were relatively robust.
Adverse effects
Only 13 of the included trials mentioned adverse events related to moxibustion, of which 5 trials had no adverse events, and the remaining 8 trials reported a total of 2 mild burns, 3 mild local erythema, 8 mild blisters, 1 instance of skin allergy, and 1 case of systemic fever and sore throat. It was worth noting that none of these adverse reactions was treated with special treatment, and all spontaneously disappeared in 1–2 days.
Evaluating the body of evidence
As summarized in Table 3, we evaluated the body of evidence’s certainty using the criteria proposed by Ryan and Hill (2016), including reasons for downgrading the quality of the evidence. The evidence was all rated as being of low or very low quality.
Discussion
Main findings
This systematic review aimed to see how effective and safe moxibustion is for treating CRF. All trials reported that moxibustion significantly improved CRF. Noteworthily, fatigue, quality of life, blood indicators, and inflammatory factors of cancer patients were statistically significant with substantial heterogeneity. Despite subgroup analysis being conducted, heterogeneity still existed in these comparisons. The various acupoint selection schemes and therapeutic manipulation may lead to unresolved heterogeneity. Moreover, given the potential risk of bias and the tiny proportion of eligible RCTs, conclusive evidence of the efficacy of moxibustion in the treatment of CRF is difficult to come by. In addition, there is no denying that the level of evidence currently available has been assessed as “low” or “very low” by the GRADE system. This dramatically reduces the reliability and power of the evidence, suggesting that these positive findings should be interpreted with caution. As a result, the efficacy of moxibustion in the treatment of CRF needs to be further explored.
As for the safety of moxibustion, only 13 included trials mentioned adverse events related to moxibustion, of which 5 trials had no adverse events, and the remaining 8 trials reported a total of 2 mild burns, 3 mild local erythema, 8 mild blisters, 1 instance of skin allergy, and 1 case of systemic fever and sore throat. It was worth noting that none of these adverse reactions was treated with special treatment, and all spontaneously disappeared in 1–2 days. For this reason, due to insufficient evidence, the conclusion of the safety of moxibustion treatment needs to be confirmed by further research.
Interpretation of the findings
Cancer-related fatigue is a typical symptom of cancer patients caused by a complex combination of factors that occurs during the entire tumor development, treatment, and prognosis phase. CRF differs from regular physical weariness in that it is not greatly eased by rest or sleep and lasts a long period during treatment and rehabilitation. The physical, psychological, and social functions of the patient are seriously harmed, lowering the patient’s quality of life. Moxibustion has attracted extensive attention for its usage in the treatment of a variety of health issues in China. It entails employing the energy of burning moxibustion to trigger acupoints or particular surface areas to alleviate the patient’s ailments. The therapeutic effects of moxibustion, according to descriptions in ancient Chinese literature, are related to improving the symptoms of “weakness” in patients and preventing human diseases. As a consequence, moxibustion can be used to treat a variety of conditions, including CRF.
Moxibustion resulted in a significant improvement in fatigue in all trials that were included, which may be due to the following reasons: First, researchers have attempted to elucidate the mechanism of moxibustion throughout the treatment of fatigue in animal studies [54, 55], which may be associated with the modulation of inflammatory markers and neurotransmitters in the hippocampus, like as 5-hydroxyethyl amine and dopamine, and the decrease of malondialdehyde levels in the blood. Second, patient-centered subjective results are susceptible to patient preferences and expectations. Fatigue is a subjective symptom that is difficult to detect using objective diagnostic methods and is usually measured using patient-centered questionnaires. Patients who favored moxibustion were more likely to volunteer to take part in these studies, and patients in each moxibustion group got the same standard of care as those in the control group. These factors may result in a higher positive response in the moxibustion group compared to the control group. Other trials that include subjective measures such as symptom severity or quality of life tend to suffer from the same problems [56]. In addition, just one trial [29] in this study used a sham-controlled placebo moxibustion device to evaluate the actual effect of moxibustion. It was performed in the same way as the moxibustion group, except that a piece of standard double-thick cardboard (combined thickness of 6 mm) was placed on the bottom of the thermal box to absorb heat and lower the temperature at the trigger point. Several clinical studies [57,58,59,60] have used these devices as placebos that produce the heat sensation of moxibustion. However, appropriate placebo moxibustion is unlikely to be conceived and manufactured since the heating effect of the sham device may not be inert, and sham acupuncture has encountered a similar problem. Perhaps, as a result, the majority of moxibustion experiments employed conventional therapy as a control. Performance bias is unavoidable in moxibustion trials as long as these active controls are used in the absence of an inert placebo. It was simple to show that the effect size was larger in the moxibustion group than in the control group because none of the studies in this study was designed to differentiate the non-specific effects of moxibustion.
Implications for study design
Some trials included in this study did not provide appropriate baseline data. Baseline imbalances are potentially important threats to internal validity and can lead to exaggerated estimates of effectiveness [23]. Baseline data, such as patient variables (e.g., education level, cognition level, or fatigue level) and disease factors (e.g., cancer stage, type of cancer, or length of diagnosis), are more likely to influence CRF’s therapeutic effect [61, 62]. The majority of the studies included in this evaluation, however, did not provide detailed baseline data. It is suggested that more strict and standardized research object inclusion criteria should be formulated, and the tumor types of the research objects should be unified to improve the credibility of the research results.
Moreover, inhalation of smoke from moxibustion may also have certain adverse effects on cancer patients. For example, cough and dyspnea are common complaints among patients with primary or metastatic lung cancer, and moxibustion therapy may negatively affect patients with these cancers [63, 64]. As many of the studies in this review included patients with primary lung cancer, these patients may have pulmonary symptoms, including dyspnea. Of concern, no studies mentioned respiratory-related adverse events. Given the poor quality of the reports in most of the included studies, it is difficult to recommend that each investigator carefully verify the occurrence of adverse events.
Therefore, a more rigorous study to measure expected adverse events is needed before moxibustion may be judged safe for CRF treatment. In addition, our study found that compared with conventional moxibustion, infrared laser moxibustion has no difference in the effect of cancer-related fatigue. Therefore, for lung cancer patients, we can choose infrared laser moxibustion, which can increase the compliance of participants. To some extent, compliance affects the successful implementation of the experimental scheme, the quality of clinical research, and the reliability of experimental results. The safety of moxibustion is also difficult to determine due to the lack of experimental evidence. Moxibustion treatment requires the operator to be very cautious as it can easily burn the skin. A cross-sectional study found that moxibustion caused 16.8% of adverse outcomes in clinical trials [65]. Therefore, adverse events need to be properly assessed and described to compare the advantages and risks of moxibustion for CRF to recommend clinical guidelines.
Furthermore, we recommend certain methodological improvements for future studies evaluating moxibustion for CRF. First, consideration must be given to appropriate blinding. Due to ethical concerns, it is debatable whether or not placebo controls should be used in cancer experiments, and many trials involving cancer patients do not use placebo controls. Therefore, certain methodological and ethical criteria must be met in placebo-controlled trials involving cancer patients [66]. However, because cancer-related fatigue has a high rate of placebo response, placebo-controlled clinical trials may be acceptable from a methodological standpoint. Notably, ethical concerns would have been reduced if the placebo group received enhanced usual care or provided all groups with the same usual medications and included smaller numbers of individuals than the intervention group. Therefore, if an appropriate sham moxibustion model is designed and developed, placebo-controlled and blinding study reviewers are recommended to determine the actual efficacy of moxibustion for CRF. Atkinson et al. [67], on the other hand, found no clear evidence that patient blinding is a source of bias in patient-reported outcomes (PRO), or at least that bias significantly affects PRO outcomes. They insisted that the use of PRO in clinical studies for cancer patients is essential to directly assess the risk, benefit, and value of treatment in cancer patients and that the use of PRO should be advocated regardless of whether patients are blinded because there is no other implication. It aims to provide information by fully capturing the patient’s experience related to treatment.
In addition, objective indicators need to be used to evaluate the efficacy of moxibustion on cancer-related fatigue. Because personal preferences and expectations can affect a patient’s perception of fatigue levels, therefore, semi-subjective assessments, such as fatigue-induced reductions in working hours, changes in the volume of other routine treatments, economic assessments, as well as subjective assessments, are recommended. Moxibustion has been reported to improve immune responses to treat certain diseases, so biomarkers of immunity can be assessed [68, 69].
Limitations
This review has several limitations. First, 26 of the 29 trials included were from Chinese databases, potentially resulting in linguistic bias. Second, we compared infrared laser moxibustion to conventional moxibustion in the management of cancer-induced fatigue in this study, and the results showed that there was no significant difference in efficacy between the two interventions. However, due to the lack of included infrared laser moxibustion studies, the degree of certainty was not high, which may lead to a false negative. Therefore, the comparison of the efficacy of infrared laser moxibustion and conventional moxibustion in the treatment of cancer-induced fatigue needs further exploration. Third, follow-up data were lacking to assess long-term efficacy, and only 2 studies included in this study followed up with patients. Finally, the meta-analysis results were highly heterogeneous (PFS-R, CFS, PFS-12, and BFI), which could be connected to the type of moxibustion therapy regimen used in the experimental group and the control group. All of the aforementioned issues may restrict the accuracy of this study’s conclusions.
Conclusion
Moxibustion may help patients suffering from cancer-related fatigue. However, given the small sample size, low methodological quality, and large inter-study heterogeneity of the involved studies, more high-quality, large-scale, multicenter controlled trials are needed to validate that moxibustion can treat fatigue symptoms while also ensuring patient safety and providing more reliable evidence.
Data availability
The data utilized to support the study’s findings can be found in the supplemental material.
References
Berger AM, Mooney K, Alvarez-Perez A et al (2015) Cancer-related fatigue, Version 2.2015. J Natl Compr Canc Netw. 13(8):1012–1039
Bower JE (2014) Cancer-related fatigue mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 11(10):597
Zaydiner B, Savina S (2019) Cancer-related fatigue: some clinical aspects. Asia Pac J Oncol Nurs 6(1):7
Saligan LN, Olson K, Filler K et al (2015) The biology of cancer-related fatigue: a review of the literature. Support Care Cancer 23(8):2461–2478
Yang S, Chu S, Gao Y et al (2019) A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells 8(7):738
Zeng Y, Luo T, Finnegan- John J, Cheng ASK (2014) Meta-analysis of randomized controlled trials of acupuncture for cancer-related fatigue. Integr Cancer Ther 13(3):193–200
Finnegan-John J, Molassiotis A, Richardson A, Ream E (2013) A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 12(4):276–290
Mohandas H, Jaganathan SK, Mani MP, Ayyar M, Rohini Thevi GV (2017) Cancer-related fatigue treatment: an overview. J Cancer Res Ther 13(6):916–929
Kim JI, Choi JY, Lee H, Lee MS, Ernst E (2010) Moxibustion for hypertension: a systematic review. BMC Cardiovasc Disord 10:33
Hua JS, Li LP, Zhu XM (2008) Effects of moxibustion pretreating on SOD and MDA in threat of global brain ischemia. J Tradit Chin Med 28(4):289–292
Pei J, Wei H, Liu ZD, Yu YM, Ni CR, Wu HG (2010) Effects of moxibustion on the expression of IL-1beta, IL-2, IL-6 mRNA and protein in the cerebral cortex in tumor-bearing mice. Zhen Ci Yan Jiu 35(4):243–249
Kim HG, Yoo SR, Park HJ, Son C-G (2013) Indirect moxibustion (CV4 and CV8) ameliorates chronic fatigue: a randomized, double-blind, controlled study. J Altern Complement Med 19(2):134–140
Wang T, Zhang Q, Xue X, Yeung A (2008) A systematic review of acupuncture and moxibustion treatment for chronic fatigue syndrome in China. Am J Chin Med 36(01):1–24
Lu C, Yang XJ, Hu J (2014) Randomized controlled clinical trials of acupuncture and moxibustion treatment of chronic fatigue syndrome patients. Zhen ci yan jiu Acupuncture Res 39(4):313–317
Han K, Kim M, Kim EJ et al (2021) Moxibustion for treating cancer-related fatigue: a multicenter, assessor-blinded, randomized controlled clinical trial. Cancer Med 10(14):4721–4733
Mao H, Mao JJ, Chen J et al (2019) Effects of infrared laser moxibustion on cancer-related fatigue in breast cancer survivors. Medicine 98(34):e16882
Zhang X, Huang W, Tianshu XU (2016) Effect of grain-sized moxibustion on cancer-related fatigue and quality of life in patients with a malignant tumor. Shanghai J Acupunct Moxibustion 35(6):659–662
Yinxia J, Hongyan L, Shuijie S (2018) Effect of electronic moxibustion combined with music therapy on cancer-related fatigue in patients with lung cancer. Chin J Integrative Nurs 4(5):77
He XR, Wang Q, Li PP (2013) Acupuncture and moxibustion for cancer-related fatigue: a systematic review and meta-analysis. Asian Pac J Cancer Prev 14(5):3067–3074
Lee S, Jerng UM, Liu Y, Kang JW, Nam D, Lee JD (2014) The effectiveness and safety of moxibustion for treating cancer-related fatigue: a systematic review and meta-analyses. Support Care Cancer 22(5):1429–1440
Ma H-L, Lou L-F, Sun Z-H, Lv B-L, Yang B (2019) The effectiveness of moxibustion for cancer-related fatigue: an updated systematic review and meta-analysis. Eur J Integr Med 30(1):100960
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Cumpston M, Li T, Page MJ et al (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:142
Ryan R, Hill S (2016) How to GRADE the quality of the evidence, version 3.0. December, Retrieved July 08, 2020, from. http://cccrg.cochrane.org/author-resources
Li LC, Hu CH, Jiang ZY (2021) Observation of moxibustion Zusanli in treating advanced cancer patients with cancer-related fatigue. Inn Mong J Tradit Chin Med 40(08):117–119
Mao HJ, Mao JJ, Guo M, Cheng K, Wei J, Shen X, Shen X (2016) Effects of infrared laser moxibustion on cancer-related fatigue: a randomized, double-blind, placebo-controlled trial. Cancer 122(23):3667–3672
Han K, Kim M, Kim EJ, Park YC, Kwon O, Kim AR, Park HJ, Park YC, Cho JH, Kim JH, Lee JH (2021) Moxibustion for treating cancer-related fatigue: a multicenter, assessor-blinded, randomized controlled clinical trial. Cancer Med 10(14):4721–4733
Chen MG (2019) Clinical study of far infrared moxibustion in the treatment of cancer-related fatigue in breast cancer patients. (Ph.D. dissertation) Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Cao Y (2017) Study on the effect of acupoints moxibustion on cancer-related fatigue in patients with cervical cancer after concurrent chemoradiotherapy. (MA thesis) Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Chen D, Chi M, Yan YT (2019) Clinical observation of moxibustion at Guanyuan and Qihai points in patients with advanced liver cancer. Chin J Integr Tradit West Med Liver Dis 29(01):35–37
Chen L, Kong TD, Wang LY, Duan FF, Wang T, Zhou HL, Zhang YJ, Zhang YH (2021) Effect of governor’s vessel moxibustion on cancer-related fatigue during chemotherapy in patients with tumor. Shanghai J Acupunct Moxibustion 40(01):44–48
Enkhtuya (2010) Clinical and experimental studies of moxa at Guanyuan on effects of the chemotherapy patients’ vital signs. (Ph.D. dissertation) Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Li J, Chen J (2013) Clinical research on treating advanced breast cancer-related fatigue by moxibustion. Clin J Chin Med 5(22):1–4
Sun C (2018) Clinical research on moxibustion in treating cancer-related fatigue. (MA thesis) Anhui University of Chinese Medicine, Hefei, Anhui, China
Qin XY (2012) Clinical research on the effect of moxibustion on cancer-related fatigue. (MA thesis) Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Wang YY (2019) Clinical study on intervention effect of moxibustion with ginger on cancer-related fatigue of breast cancer patients undergoing chemotherapy. (MA thesis) Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Ma L (2020) The clinical observation on treatment of breast cancer-related fatigue with moxibustion. (MA thesis) Anhui University of Chinese Medicine, Hefei, Anhui, China
Xie BY (2017) Clinical observation on the effect of wheat grain moxibustion for non-small cell lung cancer cancer-related fatigue relevant to chemotherapy. (MA thesis) Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
Zhang M (2014) Clinical observation of wheat moxibustion adjuvant chemotherapy for breast cancer-related fatigue and hematology change interventions carcinogenic effect. (MA thesis) Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Zhao YL, Huang Y, Zhou YQ, Huo JN, Lv T (2021) Observation on the effect of constant temperature thunder and fire moxibustion on cancer-related fatigue in patients with lung cancer. Guangxi Med J 43(4):498–502
Song N, Zhao YY, Xu HJ, Wang J, Lai ZL, Yu X, Wu Y (2021) Clinical observation of cancer-related fatigue treated with ginger-isolated moxibustion in the patients with gastric cancer. World J Acupunct-Moxibustion 31(1):1–5
Xu XZ (2017) Clinical study on the effect of moxibustion Shenque Zusanli on quality of life in patients with a malignant tumor. (MA thesis) Anhui University of Chinese Medicine, Hefei, Anhui, China
Wu HY, Guo HF, Xu T, Chen ML (2016) Effect of heat-sensitive moxibustion intervention on cancer-induced fatigue in patients with advanced lung cancer. J Clin Acupunct Moxibustion 32(7):52–54
Zhang X, Huang WJ, Xu TS (2016) Effect of grain-sized moxibustion on cancer-related fatigue and quality of life in patients with a malignant tumor. Shanghai J Acupunct Moxibustion 35(6):659–662
Li HM, Han Y, Liu XY, Wu T, Song JR (2019) Nursing effect of moxibustion nursing on cancer-related fatigue of Qi deficiency type of patients with advanced ovarian cancer undergoing postoperative chemotherapy. Clin Res Pract 4(32):163–165
Su J (2020) Effect of thunder-fire moxibustion on cancer-related fatigue in patients with non-small-cell lung cancer due to Qi deficiency. Shanghai J Acupunct Moxibustion 39(3):325–329
Xie TT (2019) Effect of ginger-partitioned moxibustion Zusanli on cancer-induced fatigue in patients with breast cancer after chemotherapy. Chron Pathematol J 20(11):1678–1679+1682
Zhang ZX, Xia H, Qiao PR, Luo XL (2019) Thunder-fire moxibustion for cancer-related fatigue in patients with gastric cancer undergoing chemotherapy. China J Chin Med 34(12):2682–2686
Shen XN (2018) A randomized controlled clinical trial of 10.6μm laser moxibustion in the treatment of cancer fatigue. Shanghai Med Pharm J 39(12):16–18
Liang H, Yang LY, Yi Z, Ben DY (2018) Clinical study of herbal cake moxibustion at back Shu point on lung cancer patients with cancer-related fatigue. Lishizhen Med Mater Med Res 29(5):1117–1119
Zhang MX, Guan L (2016) Impact on neutrophil-to-lymphocyte ratio and quality of life in the patients of non-small-cell lung cancer treated with grain-size moxibustion: a randomized controlled trial. Chin Acupunct Moxibustion 36(04):342–346
Zhu JY, Xu W, Chen C, Ou J, Mao SF, Mo XL, Lu DL (2017) Clinical effect of ginger-partitioned moxibustion combined with transarterial chemoembolization in the treatment of primary liver cancer with the stagnation of liver Qi and Spleen deficiency. J Clin Hepatol 33(1):87–90
Jiang SP, Xiao ZW (2018) Clinical study on the treatment of cancer-related fatigue in patients of breast cancer with systematic nursing intervention combined with “Four Flowers” moxibustion. Guiding J Tradit Chin Med Pharmacol 24(13):111–113
Liang YL, Xu XK, Gao F et al (2019) Effects of moxibustion on central fatigue in rats subjected to different degrees of exhaustive exercise. Phys Med Rehabilitationsmedizin Kurortmedizin 29(01):39–44
Zhou LG, Liang YL, Zhou XH et al (2017) Effect of moxibustion at Shénquè (CV 8) on anti-exercise-induced fatigue in exhaustive rats in varying degrees. World J Acupunct-Moxibustion 27(4):35–40
Manheimer E, Wieland LS, Cheng K et al (2012) Acupuncture for irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 107(6):835–848
Zhao B, Wang X, Lin Z, Liu R, Lao L (2006) A novel sham moxibustion device: a randomized, placebo-controlled trial. Complement Ther Med 14(1):53–61
Jang MK, Yoon EH, Jung CY, Byun H, Kim EJ, Kim KH, Kim KS, Lee SD (2010) Credibility of a newly developed sham moxibustion. J Korean Acupunct Moxibustion Soc 27(1):117–127
Kim SY, Yi SH, Cho JH, Yin CS, Lee H, Park HJ (2011) Heat stimulation on the skin for medical treatment: can it be controlled? J Altern Complement Med 17(6):497–504
Park JE, Sul JU, Kang K, Shin BC, Hong KE, Choi SM (2011) The effectiveness of moxibustion for the treatment of functional constipation: a randomized, sham-controlled, patient blinded, pilot clinical trial. BMC Complement Altern Med 11(1):124
Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J (2004) Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr 32:40–50
Kim DI, Choi MS (2013) Guidance for prospective acupuncture treatment on cancer-related fatigue (CRF). Ann Palliat Med 2(1):7–10
Bruera E, Schmitz B, Pither J, Neumann CM, Hanson J (2000) The frequency and correlates of dyspnea in patients with advanced cancer. J Pain Symptom Manag 19(5):357–362
Temel JS, Pirl WF, Lynch TJ (2006) Comprehensive symptom management in patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer 7(4):241–249
Jung HJ, Park JE, Liu Y, Kim AR, Choi SM (2012) The analysis of incidence and type of adverse events in acupuncture & moxibustion clinical trials. Korean J Acupunct 29(3):421–430
Daugherty CK, Ratain MJ, Emanuel EJ, Farrell AT, Schilsky RL (2008) Ethical, scientific, and regulatory perspectives regarding the use of placebos in cancer clinical trials. J Clin Oncol 26(8):1371–1378
Atkinson TM, Wagner JS, Basch E (2017) Trustworthiness of patient-reported outcomes in unblinded cancer clinical trials. JAMA Oncol 3(6):738–739
Mac Pherson H, Bland M, Bloor K, Cox H, Geddes D, Kang’ombe A, Reynolds J, Stamuli E, Stuardi T, Tilbrook H, Torgerson D, Whorwell P (2010) Acupuncture for irritable bowel syndrome: a protocol for a pragmatic randomized controlled trial. BMC Gastroenterol 10:63
Xue CC, Helme RD, Gibson S, Hogg M, Arnold C, Somogyi AA, Da Costa C, Wang Y, Lu SC, Zheng Z (2012) Effect of electroacupuncture on opioid consumption in patients with chronic musculoskeletal pain: protocol of a randomized controlled trial. Trials 13:169
Funding
This study was funded by the Jiangsu Traditional Chinese Medicine Bureau’s Scientific Research Projects (no. ZD202005) and the Jiangsu Hospital of Traditional Chinese Medicine’s Special Project of Innovation and Development Fund (Y2021CX38).
Author information
Authors and Affiliations
Contributions
Creation of ideas (provided the idea for the research): X.Q.W., P.B.D.
Create a design (planned the methods to generate the results): X.Q.W., Y.Q.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): X.Q.W., Y.Q.
Data gathering and processing (responsible for experiments, organization, or reporting data): X.Q.W., Y.Q.
Interpretation/analysis (responsible for statistical analysis, evaluation, and presentation of the results): L.H.Y., P.B.D.
Search the literature (performed the literature search): X.Q.W., L.H.Y.
Writing (responsible for writing a substantive part of the manuscript): X.Q.W., Y.Q.
A critical examination (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): S.Z.D.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was co-written by Xiao-Qing Wang and Yue Qiao.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, XQ., Qiao, Y., Duan, PB. et al. Efficacy and safety of moxibustion on cancer-related fatigue: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 31, 508 (2023). https://doi.org/10.1007/s00520-023-07977-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-023-07977-z