Abstract
Purpose
To systematically examine and summarize the current evidence regarding the effects of virtual reality (VR) on physical, cognitive, and psychological outcomes in cancer rehabilitation.
Methods
Six bioscience and engineering databases were searched. Two independent reviewers screened the titles and abstracts of 2397 records and retrieved 25 full-text articles. Inclusion criteria included patients with a current or previous diagnosis of cancer; VR was used as an intervention for physical, cognitive, or psychological impairments and functional limitations; and clinical trials with at least two arms and with both pre- and post-intervention assessments. Reviewers assessed methodological quality using the Physiotherapy Evidence Database scale.
Results
Seventeen studies including 799 patients with cancer were identified. Within-group pooled analysis indicated that patients demonstrated significant improvement in pain (P < 0.001), fatigue (P < 0.001), anxiety (P < 0.001), upper extremity function (P < 0.001), and quality of life (P = 0.008) after VR intervention. Between-group pooled analysis indicated significant improvements with VR in pain (P = 0.004), anxiety (P < 0.001), and upper extremity function (P < 0.001) compared with the control. Three studies reported the positive effects of VR on cognition.
Conclusions
VR demonstrates promising effects in physical, cognitive, and psychological aspects of patients with cancer. VR can be incorporated into a comprehensive cancer rehabilitation program to alleviate impairments and functional limitations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer has a major impact on human health across the lifespan and creates a serious challenge on society. Cancer is the second-leading cause of death in the USA, accounting for one in every four deaths [1]. There were almost 17 million cancer survivors in 2019 in the USA, and by 2030 this number is estimated to increase to more than 22 million with the growth and aging of the population [2]. Cancer treatments (surgery, chemotherapy, radiotherapy, immunotherapy) impose a variety of negative consequences on patients’ health and function. Cancer survivors or individuals undergoing cancer treatments may suffer from a decline in physical, cognitive, and psychological function. Cancer-related disabilities may further limit their participation in daily activities and quality of life [3].
Cancer rehabilitation comprises an interdisciplinary team, which is dedicated to promoting function, ameliorating symptoms, maximizing independence, and improving quality of life throughout the continuum of cancer care [4]. There is growing empirical evidence supporting the implementation of cancer rehabilitation. Ture et al. [5] reported that an inpatient cancer rehabilitation program demonstrated significant improvement in pain, physical function, mental health, and vitality. Kudre et al. [6] conducted a systematic review and found that a multidisciplinary outpatient cancer rehabilitation program can improve cancer patients’ physical and psychosocial status. Moreover, economic analyses revealed that cancer rehabilitation is potentially cost-effective with related health outcome gains [7, 8].
The integration of innovative technology into clinical practice has greatly enhanced the quality and outcomes of rehabilitation services. Virtual reality (VR) is an emerging technology used in rehabilitation in recent decades. As a computer-generated simulation technology, VR creates a novel environment with multidimensional simulation to enrich an individual experience in rehabilitation, with the features of immersion, imagination, and interaction [9]. VR can elicit enjoyment, motivation, and active participation, engaging the patients to perform meaningful practices and facilitating movement [10]. VR also provides a highly controlled environment where cognitive training can take place [11]. More importantly, VR can render esthetic scenarios and deliver analgesic effects in a nonpharmacological approach [12] for patients with cancer.
Although VR has been rapidly adopted in the rehabilitation field, current research mainly focuses on its effects on neurological [13] and musculoskeletal [14] conditions. There is a knowledge gap regarding the application of VR in cancer rehabilitation. Therefore, the specific aim of this review is to systematically examine the current evidence and summarize the effects of VR on physical, cognitive, and psychological outcomes in cancer rehabilitation.
Methods
This systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline to guarantee high-quality reporting [15]. This review was registered at the International Prospective Register of Systematic Reviews (PROSPERO): CRD42022307007.
Literature search
Reviewers searched six bioscience and engineering databases, including PubMed, Cumulative Index of Nursing and Allied Health, PsycINFO, IEEE Explore, Embase, and Scopus. The search strategy combined controlled vocabulary terms and free-text words in the title or abstract on the concepts of virtual reality and cancer. The search results were limited to clinical trials reported in the English language. No restriction was applied to publication time. The initial search was conducted on January 21, 2022. Records from each database were then exported to RefWorks (ProQuest LLC, Ann Arbor, MI) for duplication removal. The complete search strategy is listed in Appendix. The search was updated on August 21, 2022, with no new articles included.
Eligibility criteria
Articles selected in this review met the following inclusion criteria: (1) participants had a current or previous diagnosis of any type of cancer; (2) VR was used as an intervention for physical or psychological impairments and functional limitations; (3) other forms of VR intervention, conventional rehabilitation, usual care, or waiting list control, were used in control groups; and (4) they were peer-reviewed controlled trials with at least two arms and with both pre- and post-intervention assessments. Articles were excluded if: (1) they were non-human research; (2) VR was used during surgical or other invasive procedures; (3) research outcomes were only measured at one time point; and (4) they were abstract-only papers, reviews, or study protocols.
Data extraction
Two reviewers (J.H. and Y.L.) independently screened the titles and abstracts, then checked the full texts as needed to examine if the articles met the eligibility criteria; they excluded irrelevant articles. The details collected from each article included study type, patient characteristics, group assignment, VR intervention and setting, outcome measures, adverse events, and main findings. Any disagreement during this process was settled by a group discussion, and the final decision was made with a third experienced reviewer (K.-C.S.).
Quality assessment
The Physiotherapy Evidence Database (PEDro) scale was used to evaluate the methodological quality of all included clinical trials. This scale was developed to identify clinical trials that are likely to be internally valid and have sufficient statistical information to guide clinical decision-making [16]. There are 11 items on this scale; item 1 is classified as “yes or no” and counts no score. Items 2–11 are counted for 1 point each, and the total score range is 0–10. Higher scores indicate better study quality, with scores of 9–10 commonly interpreted as reflecting excellent quality, 6–8 as good quality, 4–5 as fair quality, and 0–3 as poor quality [17]. Two reviewers (J.H. and Y.L.) independently scored the included studies; the third experienced reviewer (K.-C.S.) identified discrepancies and solved them with the two reviewers.
Data synthesis
The included studies were analyzed based on the contents of intervention and comparisons groups, the VR interventions, outcome measures, and main findings. Quantitative data across studies that used similar outcome measures were pooled for meta-analysis. Meta-analysis was conducted using Review Manager software, version 5.3 (The Cochrane Collaboration, Copenhagen, Denmark). The within-group analysis compared the results before and after VR intervention, and the between-group analysis compared results of the VR group to the control group. The post-intervention assessment results or the change values, including mean and standard deviation, as well as the sample size in each group were used to compute pooled effect estimates. Heterogeneity was evaluated by calculating the I2 statistic, and a value of more than 50% indicated substantial heterogeneity [18]. Pooled analyses were conducted in a random effects model if there was substantial heterogeneity, or a fixed effects model was used instead. The standardized mean difference (SMD) was used to compute effect sizes and the significance level was set at P = 0.05 for all analyses. The mean effect was indicated as SMD with 95% confidence intervals.
Results
Study identification
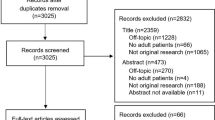
A total of 2751 records were identified from six databases. After removing duplicates, 2397 remaining records were screened by titles and abstracts. Twenty-five full-text articles were assessed for eligibility, and 17 articles were ultimately included in this systematic review. No studies were identified from other methods. The study identification process and reasons for excluding papers are delineated in the PRISMA flow diagram (Fig. 1).
Study characteristics
In the 17 included studies, there were 12 randomized controlled trials (RCT) and five controlled clinical trials (CCT). As for patient population, five studies were for pediatric patients with unspecified cancer types [19,20,21,22,23], and one was for children with brain tumor [24]; in the 11 studies for adult patients with cancer, five of them were breast cancer [25,26,27,28,29], two were brain tumor [30, 31], one was prostate cancer [32], and the other three did not specify types of cancer [33,34,35]. For VR intervention, commercial VR systems were commonly used: six studies used Xbox Kinect [23, 27, 29, 32, 34, 35], three studies used Nintendo Wii [22, 24, 25], one study used Aquasnao videogame [28], and one used PlayMotion system [21]. A specialized VR system for rehabilitation, IREX, was used in two studies [30, 31]. Three studies used immersive head-mounted VR devices [19, 20, 26]. One study used a customized VR system for interactive sensor-based balance training [33]. The intervention duration varied from one single session [19, 26] to 12 weeks [28, 32]. The interventions took place in a research laboratory, the specialized cancer care center of a hospital, the physical therapy/rehabilitation department, or at home. Six studies [22, 27, 29, 31,32,33] recorded adverse events during the intervention, and none of them reported VR-related adverse events, except Villumsen et al. [32] reported one patient in the intervention group underwent chest pain due to surgical clips in the thorax leading to discontinuation from the study. The outcome measures, main findings, and other details of study characteristics are summarized in Table 1.
Quality assessments
According to the PEDro assessment, 12 studies were graded as good quality, two as fair quality, and three as poor quality. Only six RCTs had concealed allocation. As for blinding, one study blinded subjects, one study blinded therapists, and six studies blinded assessors. Five studies had an attrition rate of more than 15%. All studies had comparable baseline measurements in both groups, conducted between-group statistical comparisons, and provided proper reports of data. The PEDro scores of all studies are provided in Table 2.
Effects on pain
Four studies assessed pain using the visual analog scale [19, 26, 27, 29] and one using the McGill Pain Questionnaire [20]. A within-group pooled analysis with four studies found that pain level was significantly lower after VR intervention (P < 0.001; SMD = − 3.86, 95%CI: − 4.84 to − 2.88) (Fig. 2A). A between-group pooled analysis showed that the VR group had significantly lower pain level than control (P = 0.004; SMD = − 1.53, 95%CI: − 2.55 to − 0.50) (Fig. 3A).
Effects on fatigue
Two studies [32, 35] assessed fatigue by the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), and a within-group analysis were conducted. Since two groups in Alves et al. [35] received VR intervention, the data from both groups were included in the analysis. According to the pooled results, participants after VR intervention demonstrated significant improvement as shown in FACIT-F (P < 0.001; SMD = 0.86, 95%CI: 0.44 to 1.28) (Fig. 2B). Hamari et al. [22] used PedsQL to evaluate fatigue in children with cancer and found that the change in fatigue from the pre- to post-tests did not differ between VR and control groups.
Effects on anxiety
Three studies assessed anxiety, using the State Anxiety Inventory [26], pain anxiety symptoms scale [20], and visual analog scale [19], respectively. All three studies used head-mounted VR devices. A within-group pooled analysis showed that VR intervention significantly reduced anxiety level (P < 0.001; SMD = − 6.99, 95%CI: − 9.73 to − 4.25) (Fig. 2C). A between-group pooled analysis showed that compared to the control group, the VR group demonstrated a lower anxiety level (P < 0.001; SMD = − 3.02, 95%CI: − 5.27 to − 0.77) (Fig. 3B).
Effects on upper extremity function
Three studies for breast cancer rehabilitation [25, 27, 29] assessed upper extremity function using Disability of the Arm, Shoulder, and Hand (DASH) and Arabic version of QuickDASH-9 scales. A within-group pooled analysis showed that the VR intervention significantly reduced DASH score, indicating less disability and better function (P < 0.001; SMD = − 3.45, 95%CI: − 4.77 to − 2.13) (Fig. 2D). A between-group analysis showed that the VR group had a lower score compared to the control group (P < 0.001; SMD = − 0.66, 95%CI: − 1.02 to − 0.31) (Fig. 3C). In addition, Yoon et al. [31] investigated the effect of VR on upper extremity function in patients with brain tumors. They found that VR induced more improvements in all segmental functions except for the hand using the Box and Block Test, the Fugl-Meyer Assessment, and the Manual Functional Test.
Effects on quality of life
Two studies assessed the quality of life, using Functional Assessment of Cancer Therapy (FACT)-General [34] and FACT-Prostate [32] respectively for different cancer populations. A within-group analysis showed significant improvement in the quality of life after VR intervention (P = 0.008; SMD = 0.53, 95%CI: 0.14 to 0.93) (Fig. 2E).
Effects on cognition
Three studies assessed cognition. Bellens et al. [28] reported that VR training significantly improved cognitive failure than a waitlist control. Yang et al. [30] reported that compared to conventional occupational therapy, VR demonstrated greater improvement in a series of neuropsychological tests, including visual and auditory continuous performance tests, backward digit span tests and visual span test, and Trail Making Test-A. Benzing et al. [23] found that working memory training group, but not VR, showed improvement in visual working memory after training and follow-up.
Effects on balance ability and mobility function
Schwenk et al. [33] developed a customized VR balance training for cancer patients with chemotherapy-induced peripheral neuropathy and found that the VR group significantly reduced sway of the hip, ankle, and the center of mass in narrow base standing with eye open and semi-tandem position. However, no significant effects were found in eye closed conditions and gait speed. Villumsen et al. [32] reported that a 12-week unsupervised VR home program induced a significant improvement in the six-minute walk test.
Discussion
This systematic review identified and synthesized evidence regarding the applications of VR in cancer rehabilitation. Seventeen clinical trials published between 2011 and 2022 were included with a total of 799 cancer survivors. Twelve studies were assessed as good quality, two as fair quality, and three as poor quality. Most included studies focused on pediatric cancer and breast cancer. The results of the meta-analysis revealed that VR rehabilitation significantly improved pain, fatigue, anxiety, upper extremity function, and quality of life in cancer survivors; compared to the control group, VR also demonstrated significantly more improvement in pain, anxiety, and upper extremity function. Qualitative synthesis results indicated that VR might be effective in cognition, balance, and mobility functions. Additionally, based on the reports from included studies, VR intervention was overall well tolerated by cancer survivors with minimal adverse events.
VR was considered as an adjunctive intervention for pain and anxiety for cancer survivors, especially during painful medical procedures [36, 37]. Pain is one of the most prevalent experiences among patients with cancer. The significant pain reduction induced by VR compared to control groups revealed in this meta-analysis supports the use of VR as a non-pharmacological regimen in a comprehensive pain management program. Of the five studies in the meta-analysis, three [19, 20, 26] used immersive VR, in which patients watched videos through head-mounted displays. The other two [27, 29] used non-immersive VR, and patients were engaged in various body motion exercises in an interactive environment. Distraction has been identified as a mechanism of VR to relieve pain [38]. VR can be a useful tool to provide patients with access to a novel environment with esthetic features, which can divert their attention of illness or unpleasant experience to the enjoyable scenarios shown in the virtual world. In two included studies [19, 26], significant pain reduction was found after a single session of VR, highlighting the instant analgesic effect. Moreover, pain is commonly a limiting factor for patients with cancer to participate in rehabilitation, especially mobility training and physical exercises. As shown in Basha et al. [27] and Feyzioglu et al. [29], using VR as an alternative approach to deliver therapy is feasible and effective to engage patients in active movement with better pain outcomes.
In addition to distraction, it was revealed by several studies that the production of neurophysiologic changes might be another mechanism of VR in pain reduction [39]. There were mixed results regarding whether different VR systems impact the degree of pain reduction. Malloy et al. [40] analyzed the VR effect on pain reduction in seven studies and suggested that the use of more sophisticated VR with full immersion was associated with greater pain relief. However, another systematic review [41] indicated that the effects of immersive versus non-immersive VR may differ in children and adults. In our review, given the limited number of clinical studies reporting pain outcomes and the heterogeneity in study design, the subgroup analysis was not able to be conducted to compare the effect of different VR systems. Hence, further studies are warranted to investigate the effects of different VR paradigms on pain relief in patients with cancer.
Individuals during hospitalization or cancer treatment might sustain psychological distress due to a variety of personal and environmental factors, which can undermine their mental health status. Results from this meta-analysis confirmed the effectiveness of VR on reducing anxiety in both within-group and between-group comparisons; all three studies used head-mounted displays. Li et al. [21] reported that children in the VR group demonstrated significantly fewer depression symptoms compared to the control; however, it should be noticed that the quality assessment of this study was fair. The beneficial effects of VR for treating anxiety and depression rely on the engagement of patients’ imagination through a customized and controlled virtual environment [42]. Also, as an emotion-focused distraction intervention, VR serves as an emerging tool to mitigate anxiety and depression along with pain and other psychological distress [43]. Rehabilitation professionals may consider taking advantage of these features of VR to aid in symptom management during practice. Furthermore, a systematic review including five studies by Zeng et al. [44] revealed that performing physical exercise in VR had significant effects on anxiety and depression, resonating part of the results by Li et al. [21] which used VR therapeutic play in hospitalized children with cancer. Nonetheless, it should be recognized that participants in four out of the five included studies in Zeng et al. [44] were healthy individuals, therefore, the generalization of their conclusions to individuals of cancer remains unclear and should be further elucidated.
Three included studies used VR in individuals with breast cancer postmastectomy and found that compared to control groups (proprioceptive neuromuscular facilitation [25], resistance exercises [27], and standard physical therapy [29]), VR demonstrated a superior improvement in upper extremity function. VR interventions used in those studies were commercial VR systems, including Xbox Kinect [27, 29] and Nintendo Wii [25]. Since the above three control groups all adopted a time-matched active control design, the improvement in function should be attributed to the VR training mode rather than additional time spent in rehabilitation. For lymphedema severity, as measured by excessive arm volume, both Atef et al. [25] and Basha et al. [27] reported no significant difference between groups. This non-significant result was not unexpected as both VR and control groups in those two studies also received standard decongestive physiotherapy, and commercial VR systems were not specifically designed for reducing lymphedema. Additionally, although only presented in one study [29], VR significantly reduced the fear of movement compared to standard physical therapy control. It concurs with literature reports regarding the effects of VR on kinesiophobia in patients suffering from chronic pain [45,46,47]. Overall, the above findings corroborate the value of integrating VR into current physical therapy regimens to promote movement and optimize upper extremity functional outcomes in breast cancer survivors.
Two studies reported the effects of VR on balance and mobility functions. Schwenk et al. [33] devised an interactive sensor-based VR balance training paradigm for patients with chemotherapy-induced peripheral neuropathy, and Villumsen et al. [32] used Xbox Kinect in a home exercise program for patients with prostate cancer. In neurological rehabilitation, VR has been commonly used to restore balance and mobility [48], and its effectiveness has been supported by systematic reviews in stroke [49], Parkinson’s disease [50], multiple sclerosis [51], and cerebral palsy [52]. Balance and mobility are also essential considerations in cancer rehabilitation, as patients may experience sensory impairments, muscle weakness, reduced stamina, and limited functional activity tolerance; multiple body systems involved in balance control can be influenced by cancer and treatment. Evidently, chemotherapy negatively affects balance and gait among cancer survivors and is associated with increased fall incidence through chemotherapy-induced peripheral neuropathy [53]. With the ongoing VR research in neurological rehabilitation, VR as a promising intervention for patients with cancer to restore balance and mobility can be further explored.
Six studies applied VR to pediatric patients, and three of them reported compromised compliance. Benzing et al. [23] reported less than half of the participants attended the prescribed amount of VR training sessions; Hamari et al. [22] reported although no participants withdrew from the study during the 2.5-year follow-up, most participants did not follow instructions to allow for adequate time spent on VR each week; Li et al. [21] reported the attrition rate of VR group was 13% higher than the control group, in which children received usual care without additional intervention. The insufficient time in active involvement might contribute to the non-significant difference between VR and control group [22]. On the other hand, all participants completed a 10-week home-based VR program and reached the desired exercise dosage in Sabel et al. [24]; the weekly coaching sessions via teleconferencing during the intervention period plays a role in providing encouragement, addressing challenges, and promoting compliance. Therefore, special considerations should be taken in the future design and implementation of using VR in children versus adults to ensure participants’ compliance and the fidelity of study. Motivation is an essential factor in pediatric rehabilitation, and heightened motivation appears to be related to better rehabilitation outcomes [54]. In particular, the interaction between children and specific tasks performed in VR can impact their subject experience as well as their attitude towards VR rehabilitation. In this case, to elicit children’s motivation to participate, the individualization of VR might be of importance to inform task selection and parameter adjustment, to tailor the demands of children with a variety of capacities and interests [55, 56].
Cancer-related cognitive impairment is an area which attracts cumulative attention in the literature in the last decade. It encompasses deficits in memory, attention, concentration, executive function, and other domains of cognition [57]. While most previous studies have focused on those who received chemotherapy and breast cancer survivors, its underlying mechanisms, as well as ramifications in other types of cancer, are gradually revealed by recent multimodal research investigations [58]. In addition to conventional cognitive rehabilitation, which is typically delivered by neuropsychologists and occupational therapists, VR is supported by compelling evidence as an effective approach to promote motivation and participation, and augment cognitive rehabilitation outcomes [59]. As of now, compared to the literature body that inspected the effects of VR on cognitive outcomes in individuals with neurological conditions [60,61,62], there is a limited number of articles within the context of cancer rehabilitation. Our systematic review identified two RCTs and one CCT in this topic, and all three were evaluated as good quality. Two of them reported significant improvement obtained through VR compared to control groups, while one did not find any significant results in VR. The differences in the above findings might be related to different VR apparatus employed in those studies. Yang et al. [30] and Bellens et al. [28] used the IREX system and Aquasnao videogame, respectively, which are all VR systems specifically designed for rehabilitation use; both reported superior cognitive outcomes in the VR group. Benzing et al. [23] used Xbox Kinect, which is a commercially available VR console. It found the working memory training group, rather than the VR group, obtained improvement in visual working memory; and neither of these two groups obtained improvement in executive function. Since the exergames performed in Xbox Kinect were not designed to address the above cognitive domains, these findings implied the significance of task specificity in the selection of VR systems with the aim to ameliorate cancer-related cognitive impairment.
Limitations
This review has several limitations. First, only RCT and CCT were included in this review to control variance in study design and allow for not only within-group but also between-group meta-analysis. However, in doing so, the number of eligible studies were reduced, as single-group trials, case series, and case reports were excluded, although they were also about the application of VR on cancer survivors. Second, patients with all types of cancer were included. And due to the limited number of available studies, a subgroup meta-analysis was not performed for different cancer types. Third, including studies of both pediatric and adult cancer survivors could increase the heterogeneity of this review. Finally, there were other demographic factors, such as age, gender, disease severity, and concurrent treatment, which might potentially impact the outcomes of rehabilitation. A meta-regression is an appropriate approach to synthesize findings while adjusting for the effects of available covariates, while it could not be performed based on the current dataset from included studies.
Conclusions
VR demonstrates promising effects in physical, cognitive, and psychological aspects of patients with cancer. VR rehabilitation significantly improved pain, fatigue, anxiety, upper extremity function, and quality of life in cancer survivors; compared to the control group, VR also demonstrated significantly more improvement in pain, anxiety, and upper extremity function. VR might be also effective in cognition, balance, and mobility functions. VR can be incorporated into a comprehensive cancer rehabilitation program to optimize outcomes in mitigating impairments and functional limitations.
Data Availability
Not applicable.
References
Centers for Disease Control and Prevention (2018) United States Cancer Statistics: data visualizations. https://www.cdc.gov/cancer/uscs/dataviz/index.htm. Accessed 15 Apr 2022
American Cancer Society (2019) Cancer treatment & survivorship facts & figures 2019–2021. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html. Accessed 15 Apr 2022
Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB (2015) The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J Geriatr Oncol 6:194–201. https://doi.org/10.1016/j.jgo.2015.01.004
Silver JK, Raj VS, Fu JB, Wisotzky EM, Smith SR, Kirch RA (2015) Cancer rehabilitation and palliative care: critical components in the delivery of high-quality oncology services. Support Care Cancer 23:3633–3643. https://doi.org/10.1007/s00520-015-2916-1
Ture M, Angst F, Aeschlimann A, Renner C, Schnyder U, Zerkiebel N, Perseus J, Barth J, Bredell M, Soelch CM (2017) Short-term effectiveness of inpatient cancer rehabilitation: a longitudinal controlled cohort study. J Cancer 8(10):1717. https://doi.org/10.7150/jca.19564
Kudre D, Chen Z, Richard A, Cabaset S, Dehler A, Schmid M, Rohrmann S (2020) Multidisciplinary outpatient cancer rehabilitation can improve cancer patients’ physical and psychosocial status—a systematic review. Curr Oncol Rep 22:1–17. https://doi.org/10.1007/s11912-020-00979-8
Silver JK, Baima J, Newman R, Galantino ML, Shockney LD (2013) Cancer rehabilitation may improve function in survivors and decrease the economic burden of cancer to individuals and society. Work 46:455–472. https://doi.org/10.3233/WOR-131755
Mewes JC, Steuten LM, IJzerman MJ, Van Harten WH (2012) Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist 17:1581–1593. https://doi.org/10.1634/theoncologist.2012-0151
Howard MC (2017) A meta-analysis and systematic literature review of virtual reality rehabilitation programs. Comput Hum Behav 70:317–327. https://doi.org/10.1016/j.chb.2017.01.013
Rohrbach N, Chicklis E, Levac DE (2019) What is the impact of user affect on motor learning in virtual environments after stroke? A scoping review. J NeuroEng Rehab 16(1):1–14. https://doi.org/10.1186/s12984-019-0546-4
Faria AL, Andrade A, Soares L (2016) Benefits of virtual reality based cognitive rehabilitation through simulated activities of daily living: a randomized controlled trial with stroke patients. J NeuroEng Rehab 13:1–12. https://doi.org/10.1186/s12984-016-0204-z
Tao G, Garrett B, Taverner T, Cordingley E, Sun C (2021) Immersive virtual reality health games: a narrative review of game design. J NeuroEng Rehab 18:1–21. https://doi.org/10.1186/s12984-020-00801-3
Słyk S, Zarzycki MZ, Kocwa-Karnaś A, Domitrz I (2019) Virtual reality in the diagnostics and therapy of neurological diseases. Expert Rev Med Devices 16:1035–1040. https://doi.org/10.1080/17434440.2019.1693892
Gumaa M, Rehan Youssef A (2019) Is virtual reality effective in orthopedic rehabilitation? A systematic review and meta-analysis. Phys Ther 99:1304–1325. https://doi.org/10.1093/ptj/pzz093
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Verhagen AP, De Vet HC, De Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51:1235–1241. https://doi.org/10.1016/S0895-4356(98)00131-0
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M (2003) Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 83:713–721. https://doi.org/10.1093/ptj/83.8.713
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Tennant M, Youssef GJ, McGillivray J, Clark T, McMillan L, McCarthy MC (2020) Exploring the use of immersive virtual reality to enhance psychological well-being in pediatric oncology: a pilot randomized controlled trial. Euro J Oncol Nurs 48:101804. https://doi.org/10.1016/j.ejon.2020.101804
Sharifpour S, Manshaee GR, Sajjadian I (2021) Effects of virtual reality therapy on perceived pain intensity anxiety catastrophising and self-efficacy among adolescents with cancer. Counsel Psychother Res 21:218–226. https://doi.org/10.1002/capr.12311
Li WH, Chung JO, Ho EK (2011) The effectiveness of therapeutic play, using virtual reality computer games, in promoting the psychological well-being of children hospitalised with cancer. J Clin Nurs 20:2135–2143. https://doi.org/10.1111/j.1365-2702.2011.03733.x
Hamari L, Järvelä LS, Lähteenmäki PM, Arola M, Axelin A, Vahlberg T, Salanterä S (2019) The effect of an active video game intervention on physical activity, motor performance, and fatigue in children with cancer: a randomized controlled trial. BMC Res Notes 12:1–7. https://doi.org/10.1186/s13104-019-4821-z
Benzing V, Spitzhüttl J, Siegwart V, Schmid J, Grotzer M, Heinks T, Roebers CM, Steinlin M, Leibundgut K, Schmidt M (2020) Effects of cognitive training and exergaming in pediatric cancer survivors—a randomized clinical trial. Med Sci Sports Exerc 52:2293. https://doi.org/10.1249/MSS.0000000000002386
Sabel M, Sjölund A, Broeren J, Arvidsson D, Saury J, Blomgren K, Lannering B, Emanuelson I (2016) Active video gaming improves body coordination in survivors of childhood brain tumours. Disabil Rehabil 38:2073–2084. https://doi.org/10.3109/09638288.2015.1116619
Atef D, Elkeblawy MM, El-Sebaie A, Abouelnaga WAI (2020) A quasi-randomized clinical trial: virtual reality versus proprioceptive neuromuscular facilitation for postmastectomy lymphedema. J Egypt Nat Cancer Inst 32:1–9. https://doi.org/10.1186/s43046-020-00041-5
Mohammad EB, Ahmad M (2019) Virtual reality as a distraction technique for pain and anxiety among patients with breast cancer: a randomized control trial. Palliat Support Care 17:29–34. https://doi.org/10.1017/S1478951518000639
Basha MA, Aboelnour NH, Alsharidah AS, Kamel FH (2022) Effect of exercise mode on physical function and quality of life in breast cancer–related lymphedema: a randomized trial. Support Care Cancer 30:2101–2110. https://doi.org/10.1007/s00520-021-06559-1
Bellens A, Roelant E, Sabbe B, Peeters M, Van Dam PA (2020) A video-game based cognitive training for breast cancer survivors with cognitive impairment: a prospective randomized pilot trial. The Breast 53:23–32. https://doi.org/10.1016/j.breast.2020.06.003
Feyzioğlu Ö, Dinçer S, Akan A, Algun ZC (2020) Is Xbox 360 Kinect-based virtual reality training as effective as standard physiotherapy in patients undergoing breast cancer surgery? Support Care Cancer 28:4295–4303. https://doi.org/10.1007/s00520-019-05287-x
Yang S, Chun MH, Son YR (2014) Effect of virtual reality on cognitive dysfunction in patients with brain tumor. Ann Rehab Med 38:726. https://doi.org/10.5535/arm.2014.38.6.726
Yoon J, Chun MH, Lee SJ, Kim BR (2015) Effect of virtual reality–based rehabilitation on upper-extremity function in patients with brain tumor: controlled trial. Amer J Phys Med Rehab 94:449–459. https://doi.org/10.1097/PHM.0000000000000192
Villumsen BR, Jorgensen MG, Frystyk J, Hordam B, Borre M (2019) Home-based ‘exergaming’ was safe and significantly improved 6-min walking distance in patients with prostate cancer: a single-blinded randomised controlled trial. BJU Int 124:600–608. https://doi.org/10.1111/bju.14782
Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B (2016) Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology 62:553–563. https://doi.org/10.1159/000442253
da Silva Alves R, Iunes DH, de Carvalho JM, Menezes FdS, Silva AM, Borges JBC, Carvalho LC (2018) Effects of exergaming on quality of life in cancer patients. Games Health J 7:385–392. https://doi.org/10.1089/g4h.2017.0174
da Silva Alves R, Iunes DH, Pereira IC, Borges JBC, Nogueira DA, Silva AM, Lobato DFM, Carvalho LC (2017) Influence of exergaming on the perception of cancer-related fatigue. Effects of exergaming on quality of life in cancer patients. Games Health J 6:119–126. https://doi.org/10.1089/g4h.2016.0051
Chow H, Hon J, Chua W, Chuan A (2021) Effect of virtual reality therapy in reducing pain and anxiety for cancer-related medical procedures: a systematic narrative review. J Pain Symptom Manage 61:384–394. https://doi.org/10.1016/j.jpainsymman.2020.08.016
Ahmad M, Mohammad EB, Anshasi HA (2020) Virtual reality technology for pain and anxiety management among patients with cancer: a systematic review. Pain Manag Nurs 21:601–607. https://doi.org/10.1016/j.pmn.2020.04.002
Kenney MP, Milling LS (2016) The effectiveness of virtual reality distraction for reducing pain: a meta-analysis. Psychol Conscious Theor Res Pract 3:199. https://doi.org/10.1037/cns0000084
Gupta A, Scott K, Dukewich M (2018) Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain Med 19:151–159. https://doi.org/10.1093/pm/pnx109
Malloy KM, Milling LS (2010) The effectiveness of virtual reality distraction for pain reduction: a systematic review. Clin Psychol Rev 30:1011–1018. https://doi.org/10.1016/j.cpr.2010.07.001
Shahrbanian S, Ma X, Aghaei N, Korner-Bitensky N, Moshiri K, Simmonds MJ (2012) Use of virtual reality (immersive vs. non-immersive) for pain management in children and adults: a systematic review of evidence from randomized controlled trials. Eur J Exp Biol 2:1408–1422. https://doi.org/10.1111/jan.14607
Baghaei N, Chitale V, Hlasnik A, Stemmet L, Liang H, Porter R (2021) Virtual reality for supporting the treatment of depression and anxiety: scoping review. JMIR Mental Health 8:e29681. https://doi.org/10.2196/29681
Ioannou A, Papastavrou E, Avraamides MN, Charalambous A (2020) Virtual reality and symptoms management of anxiety, depression, fatigue, and pain: a systematic review. SAGE Open Nurs 6:2377960820936163. https://doi.org/10.1177/2377960820936163
Zeng N, Pope Z, Lee JE, Gao Z (2018) Virtual reality exercise for anxiety and depression: a preliminary review of current research in an emerging field. J Clin Med 7:42. https://doi.org/10.3390/jcm7030042
Nambi G, Abdelbasset WK, Alrawaili SM, Alsubaie SF, Abodonya AM, Saleh AK (2021) Virtual reality or isokinetic training; its effect on pain, kinesiophobia and serum stress hormones in chronic low back pain: a randomized controlled trial. Technol Health Care 29:155–166. https://doi.org/10.3233/THC-202301
Gulsen C, Soke F, Eldemir K, Apaydin Y, Ozkul C, Guclu-Gunduz A, Akcali DT (2020) Effect of fully immersive virtual reality treatment combined with exercise in fibromyalgia patients: a randomized controlled trial. Assist Technol 34(3):256–263. https://doi.org/10.1080/10400435.2020.1772900
Fowler CA, Ballistrea LM, Mazzone KE, Martin AM, Kaplan H, Kip KE, Ralston K, Murphy JL, Winkler SL (2019) Virtual reality as a therapy adjunct for fear of movement in veterans with chronic pain: single-arm feasibility study. JMIR Format Res 3:e11266. https://doi.org/10.2196/11266
Porras DC, Siemonsma P, Inzelberg R, Zeilig G, Plotnik M (2018) Advantages of virtual reality in the rehabilitation of balance and gait: systematic review. Neurology 90:1017–1025. https://doi.org/10.1212/WNL.0000000000005603
Li Z, Han X, Sheng J, Ma S (2016) Virtual reality for improving balance in patients after stroke: a systematic review and meta-analysis. Clin Rehabil 30:432–440. https://doi.org/10.1177/0269215515593611
Sarasso E, Gardoni A, Tettamanti A, Agosta F, Filippi M, Corbetta D (2021) Virtual reality balance training to improve balance and mobility in Parkinson’s disease: a systematic review and meta-analysis. J Neurol 269:1873–1888. https://doi.org/10.1007/s00415-021-10857-3
Casuso-Holgado MJ, Martín-Valero R, Carazo AF, Medrano-Sánchez EM, Cortés-Vega MD, Montero-Bancalero FJ (2018) Effectiveness of virtual reality training for balance and gait rehabilitation in people with multiple sclerosis: a systematic review and meta-analysis. Clin Rehabil 32:1220–1234. https://doi.org/10.1177/0269215518768084
Warnier N, Lambregts S, Port IVD (2020) Effect of virtual reality therapy on balance and walking in children with cerebral palsy: a systematic review. Dev Neurorehab 23:502–518. https://doi.org/10.1080/17518423.2019.1683907
Wechsler S, Wood L (2021) The effect of chemotherapy on balance, gait, and falls among cancer survivors: a scoping review. Rehab Oncol 39:6–22. https://doi.org/10.1097/01.REO.0000000000000238
Meyns P, Roman de Mettelinge T, van der Spank J, Coussens M, Van Waelvelde H (2018) Motivation in pediatric motor rehabilitation: a systematic search of the literature using the self-determination theory as a conceptual framework. Dev Neurorehab 21:371–390. https://doi.org/10.1080/17518423.2017.1295286
Harris K, Reid D (2005) The influence of virtual reality play on children’s motivation. Can J Occup Ther 72:21–29. https://doi.org/10.1177/000841740507200107
Reid DT (2002) Benefits of a virtual play rehabilitation environment for children with cerebral palsy on perceptions of self-efficacy: a pilot study. Pediatr Rehabil 5:141–148. https://doi.org/10.1080/1363849021000039344
Janelsins MC, Kesler SR, Ahles TA, Morrow GR (2014) Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatr 26:102–113. https://doi.org/10.3109/09540261.2013.864260
Lange M, Joly F, Vardy J, Ahles T, Dubois M, Tron L, Winocur G, De Ruiter MB, Castel H (2019) Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol 30:1925–1940. https://doi.org/10.1093/annonc/mdz410
Maggio MG, Maresca G, De Luca R, Stagnitti MC, Porcari B, Ferrera MC, Galletti F, Casella C, Manuli A, Calabrò RS (2019) The growing use of virtual reality in cognitive rehabilitation: fact, fake or vision? A scoping review. J Natl Med Assoc 111:457–463. https://doi.org/10.1016/j.jnma.2019.01.003
Shin H, Kim K (2015) Virtual reality for cognitive rehabilitation after brain injury: a systematic review. J Phys Ther Sci 27:2999–3002. https://doi.org/10.1589/jpts.27.2999
Maggio MG, Latella D, Maresca G, Sciarrone F, Manuli A, Naro A, De Luca R, Calabrò RS (2019) Virtual reality and cognitive rehabilitation in people with stroke: an overview. J Neurosci Nurs 51:101–105. https://doi.org/10.1097/JNN.0000000000000423
Alashram AR, Annino G, Padua E, Romagnoli C, Mercuri NB (2019) Cognitive rehabilitation post traumatic brain injury: a systematic review for emerging use of virtual reality technology. J Clin Neurosci 66:209–219. https://doi.org/10.1016/j.jocn.2019.04.026
Author information
Authors and Affiliations
Contributions
J.H. and K.-C.S. wrote the initial version of the main content. All authors revised, finalized, and approved the last version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hao, J., Li, Y., Swanson, R. et al. Effects of virtual reality on physical, cognitive, and psychological outcomes in cancer rehabilitation: a systematic review and meta-analysis. Support Care Cancer 31, 112 (2023). https://doi.org/10.1007/s00520-022-07568-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-022-07568-4