Abstract
Purpose
Cancer is a major reason for concurrent prescription of opioids with other sedating medications—particularly benzodiazepines and gabapentinoids—yet population-based assessments of the extent and predictors of concurrent prescribing among clinically and demographically diverse patients with cancer are lacking.
Methods
We conducted a retrospective cohort study of patients with non-metastatic cancer using North Carolina cancer registry data linked with Medicare and private insurance claims (2013–2016). We used modified Poisson regression to assess associations of patient characteristic with adjusted relative risk (aRR) of new concurrent prescribing of opioids with benzodiazepines or gabapentinoids after diagnosis.
Results
Overall, 15% of patients were concurrently prescribed opioids with benzodiazepines or gabapentinoids. Characteristics independently associated with an increased risk of concurrent prescribing included cancer type (e.g., aRR cervical vs. colorectal cancer: 1.55, 95% CI: 1.12–2.14); prior use of opioids (aRR: 2.43, 95% CI:2.21–2.67), benzodiazepines (aRR: 4.08, 95% CI: 3.72–4.48), or gabapentinoids (3.82, 95% CI: 3.31–4.39), and premorbid mental health conditions, including substance use disorder (aRR: 1.27, 95% CI: 1.05–1.54). Black and Hispanic patients were less likely to experience concurrent prescribing (aRR, Black vs. White: 0.35, 95% CI: 0.15–0.83; aRR, Hispanic vs. White: 0.75, 95% CI: 0.66–0.85).
Conclusion
Approximately 1 in 7 patients with cancer was concurrently prescribed opioids with other sedating medications. Associations between patient characteristics and risk of concurrent prescribing highlight predictors of concurrent prescribing and suggest a rationale for systematic assessment of substance use history at diagnosis. Future research could explore inequitable pain and symptom management and investigate risk of adverse medication-related events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies have shown that, in the general population, concurrent use of opioids with other sedating medications is associated with an increased risk of adverse outcomes. In particular, concurrent use of opioids with benzodiazepines [1,2,3] or gabapentinoids [4, 5] has been shown to increase the risk of opioid overdose. Most studies examining the risks associated with concurrent use of opioids and other sedating medications have specifically excluded patients diagnosed with cancer. Yet a cancer diagnosis is a major reason why patients may be newly co-prescribed opioids with benzodiazepines and/or gabapentinoids [6, 7]. Opioid therapy has long been a mainstay of cancer-related pain management, and gabapentinoids are frequently used to specifically help manage treatment-related neuropathic pain [8]. Benzodiazepines are commonly prescribed for the management of other cancer-related symptoms, including anxiety, insomnia, and chemotherapy-related nausea [9].
Although patients with cancer have generally been excluded from prior studies assessing risks associated with concurrent use of opioids with other sedating medications, these risks may be compounded by cancer and its treatment. For example, an estimated 50% of all patients with cancer experience dyspnea or shortness of breath [10]. As a result, they may be particularly vulnerable to co-prescribing effects on respiratory function. Further, due to the neurological effects of chemotherapy—which affect up to 75% of patients [11]—patients with cancer may also be more prone to the effects of these medications and their combinations on postural stability and cognition.
Population-based assessments of the extent and predictors of concurrent prescribing among clinically and demographically diverse patients with cancer are lacking, but are critically needed to inform targeted interventions aimed at reducing avoidable harms for patients most at risk for using opioids with other sedating medications. To address this need, we leveraged unique state cancer registry data linked with multipayer insurance claims to (1) assess the prevalence of concurrent prescribing of opioids with benzodiazepines or gabapentinoids after a cancer diagnosis and (2) identify patient characteristics associated with concurrent prescribing.
Methods
Data source and population studied
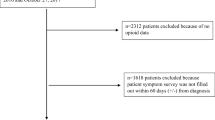
We conducted a retrospective cohort study using the University of North Carolina’s Cancer Information and Population Health Resource (CIPHR) [12]. CIPHR consists of data from the North Carolina Central Cancer Registry (NCCCR) linked with insurance claims from Medicare, North Carolina (NC) Medicaid, and private insurance. The present analysis is limited to Medicare and private insurance beneficiaries only, as, at the time of our analysis, state Medicaid data were only available through 2012, and our analysis starts in 2013, when Medicare part D began to cover benzodiazepines. We used the NCCCR data to select the study cohort, which included cancer survivors diagnosed at age 19 years or older, with a first primary diagnosis of a non-metastatic solid tumor (breast, cervical, colorectal, endometrial, esophageal, head/neck, lung, melanoma, ovarian, prostate, testicular) between April 1, 2013, and December 31, 2015 (most recent data available at the time of analysis). Recognizing that the benefits and risks of concurrent prescribing of sedating, symptom-directed medications may be weighed differently in the context of a life-limiting diagnosis, and we restricted inclusion to patients with non-metastatic disease at diagnosis with the goal of limiting our analytic cohort to patients with longer prognoses.
Patients were required to have continuous medical insurance coverage from 12 months prior to cancer diagnosis (to ascertain comorbidities) through 12 months following cancer diagnosis (to ascertain treatment). They were required to have continuous pharmaceutical coverage from 3 months prior to cancer diagnosis through 12 months following cancer diagnosis to ascertain medication use. We included patients who switched insurance types (e.g., private to Medicare).
Measures
Our primary outcome was a binary indicator of new concurrent prescribing of opioids with 1 of 2 sedating medications (benzodiazepines or gabapentinoids). Because we were focused on new concurrent prescribing after a cancer diagnosis, we excluded cancer survivors who used opioids concurrently with benzodiazepines or gabapentinoids in the 3 months before diagnosis. Consistent with prior research [2, 6, 7], we define concurrent prescribing as having 1 or more days with overlapping opioid/benzodiazepine supplies or overlapping opioid/gabapentinoid supplies during the 12-month period following cancer diagnosis. To ascertain overlapping supplies, we used the dispensing date and days’ supply information from prescription drug claims to create daily indicators for whether a patient possessed an opioid, benzodiazepine, and/or gabapentinoid prescription supply. Secondary outcomes were concurrent prescribing of opioids with benzodiazepines and concurrent prescribing of opioids with gabapentinoids.
Factors evaluated for their associations with concurrent prescribing were the following: (1) demographic characteristics, including age at diagnosis, gender, race/ethnicity, urban/rural residence, area-level measures of income and education, and insurance provider (Medicare or private); (2) cancer- and treatment-related factors, including cancer type and stage (local or regional), receipt of surgery, radiation, and/or chemotherapy [6], and cancer treatment setting (academic or community); (3) other health-related characteristics, including baseline comorbidity burden (Charlson Comorbidity Index) [13], prior mental health and substance use disorder diagnoses, and prior chronic pain diagnoses [14]; and (4) use of opioids, benzodiazepines, or gabapentinoids in the 3 months before cancer diagnosis.
Analysis
We used descriptive statistics to characterize the analytic cohort overall and by any concurrent prescribing. Unadjusted differences between cancer survivors who did and did not experience concurrent prescribing were assessed using chi-squared tests for categorical variables and t-tests for continuous variables. Multivariable analysis used modified Poisson regression [15] to assess the adjusted relative risk (aRR) of concurrent prescribing associated with patient characteristics.
Results
Characteristics of the analytic cohort
A total of 14,376 patients met inclusion criteria, 56% of whom were female and 16% of whom were Black (Table 1). The mean age at diagnosis was 68 years (± 11), and most (76%) cancer survivors were insured by Medicare. The most common cancer diagnoses were breast (34%) and prostate (27%). The most common cancer affecting both men and women was colorectal cancer (13%). Nearly half of patients (46%) had 2 or more medical comorbidities. About 10% of patients had a prior diagnosis of chronic pain. With respect to prior mental health conditions, 12% had a depression diagnosis, 10% had an anxiety diagnosis, and 2% had a substance use disorder diagnosis.
Factors associated with concurrent prescribing
Roughly 15% of patients experienced any concurrent prescribing (i.e., prescribing of opioids with benzodiazepines and/or gabapentinoids). In unadjusted analysis, demographic factors associated with any concurrent prescribing included younger age, female gender, White race, and private insurance (Table 1). Cancer type was also strongly associated with concurrent prescribing, with incidence reaching 31%, 26%, and 23% for patients with cervical, ovarian, and lung cancer, respectively. The rates of concurrent prescribing were lowest for patients with testicular, prostate, and melanoma skin cancer (10%, 9%, and 8%, respectively) (Fig. 1).
With respect to other health characteristics, more advanced stage, increased comorbidity burden, and prior depression, anxiety, chronic pain, and substance use disorder were all associated with concurrent prescribing, as were receipt of surgery, radiation therapy, or chemotherapy. In addition, the use of opioids, benzodiazepines, or gabapentinoids prior to cancer diagnosis was associated with concurrent prescribing after cancer diagnosis. For example, among the 936 patients with prior benzodiazepine use, 58% (N = 550) received concurrent prescriptions after cancer diagnosis. Cancer treatment setting was not associated with any concurrent prescribing.
Examining each concurrent prescribing outcome (opioids/benzodiazepines and opioids/gabapentinoids) separately, about 12% of patients received concurrent opioid/benzodiazepine prescriptions, and approximately, 5% received concurrent opioid/gabapentinoid prescriptions. Overall, associations of patient characteristics with each separate concurrent prescribing outcome were similar to those observed for the composite outcome of any concurrent prescribing, with some exceptions. Specifically, comorbidity burden was not associated with receipt of concurrent opioid/benzodiazepine prescriptions, nor were area-level measures of education and income. Insurance provider was not associated with receipt of concurrent opioid/gabapentinoid prescriptions.
Multivariable analysis of factors associated with any concurrent prescribing
After adjustment, demographic differences in any concurrent prescribing persisted (Table 2). Specifically, compared to patients aged 60 to 69 years, those aged 19 to 49 years were 47% more likely (95% CI: 1.27–1.70), and those aged 50–59 years were 19% more likely (95% CI: 1.05–1.34) to experience concurrent prescribing. Conversely, patients aged 70–79 years were 23% less likely to experience concurrent prescribing (95% CI: 0.70–0.86). Further, women were 15% or more likely than men to experience concurrent prescribing (95% CI: 1.01–1.31). With respect to race/ethnicity, compared to non-Hispanic White patients, Black patients were 65% less likely (95% CI: 0.15–0.83), and Hispanic patients were 25% less likely (95% CI: 0.66–0.85) to experience concurrent prescribing. In addition, patients insured by Medicare were 14% less likely than those with private insurance to receive concurrent prescriptions (95% CI: 0.77–0.96). Area-level indicators of education and income were no longer associated with concurrent prescribing after adjustment.
Several health characteristics were independently associated with concurrent prescribing after adjustment. These included increased comorbidity burden (aRR, Charlson Comorbidity Index of 2 vs. 0: 1.12, 95% CI: 1.02–1.22), prior diagnosis of anxiety (aRR: 1.18, 95% CI: 1.07–1.32), prior diagnosis of a substance use disorder (aRR: 1.27, 95% CI: 1.05–1.54), and prior diagnosis of chronic pain (aRR: 1.19, 95% CI: 1.06–1.33). With respect to prior medication use, patients who used opioids before being diagnosed with cancer were more than twice as likely to experience concurrent prescribing after diagnosis (aRR: 2.43, 95% CI: 2.21–2.67), and those who used benzodiazepines or gabapentinoids before diagnosis were about 4 times as likely to experience concurrent prescribing after diagnosis (aRR for benzodiazepines: 4.08, 95% CI: 3.72–4.48; aRR for gabapentinoids: 3.82, 95% CI: 3.31–4.39). We also observed differences by cancer type. Compared to patients with colorectal cancer, those with cervical (aRR: 1.55, 95% CI: 1.12–2.14), head/neck (aRR: 1.59, 95% CI: 1.27–1.99), or lung cancer (aRR: 1.34, 95% CI: 1.14–1.57) were more likely to experience concurrent prescribing. Regional vs. local disease (aRR: 1.20, 95% CI: 1.10–1.31), receipt of surgery (aRR: 1.19, 95% CI: 1.05–1.34), and receipt of adjuvant chemotherapy (aRR: 1.53, 95% CI: 1.40–1.67) were also associated positively with concurrent prescribing.
Overall, results from the secondary model examining associations of patient characteristics with concurrent prescribing of specifically opioids/benzodiazepines were similar (Supplemental Table 1). However, surgery was not independently associated with concurrent opioid/benzodiazepine prescribing (aRR: 1.12, 95% CI: 0.99-–1.28). Esophageal cancer emerged as a statistically significant predictor of concurrent prescribing (aRR, esophageal vs. colorectal: 1.51, 95% CI: 1.04–2.20). For the secondary model examining associations of patient characteristics with concurrent prescribing of opioids with gabapentinoids, results diverged from the primary model in several respects (Supplemental Table 2). First, neither race/ethnicity nor insurance provider was independently associated with the outcome (aRR, Black vs. non-Hispanic White: 0.57, 95% CI: 0.18–1.80; aRR, Hispanic vs. non-Hispanic White: 1.04, 95% CI: 0.86–1.25). Further, cervical and lung cancer were the only cancer diagnoses independently associated with concurrent prescribing (aRR, lung vs. colorectal: 1.34, 95% CI: 1.04–1.74; aRR, cervical vs. colorectal: 1.72, 95% CI: 1.05–2.82). Chronic pain was the only previously diagnosed health condition associated with this outcome (aRR: 1.40, 95% CI: 1.18–1.66). In addition, living in an area with a lower level of education was associated with an increased likelihood of concurrent opioid/gabapentinoid prescribing (aRR lowest vs. highest education: 1:49, 95% CI: 1.14–1.95).
Discussion
In our study, we found that roughly 1 in 7 (15%) of patients received opioid prescriptions that overlapped with benzodiazepine and/or gabapentinoid prescriptions. We also found that concurrent prescribing of opioids with benzodiazepines (12%) was more prevalent than concurrent prescribing of opioids with gabapentinoids (5%). In adjusted analysis, factors associated with our primary outcome of any concurrent prescribing included younger age; female gender; White race; diagnosis of cervical, lung, head/neck, or esophageal cancer; receipt of surgery or chemotherapy; increased comorbidity burden; prior diagnosis of chronic pain, anxiety, or substance use disorder; and the use of any of the medications of interest before cancer diagnosis.
Our study adds to the small evidence base concerning population-level patterns of concurrent opioid/benzodiazepine prescribing. Because concurrent opioid/benzodiazepine prescribing was much more prevalent in our sample than concurrent opioid/gabapentinoid prescribing, results derived from our secondary model focused on concurrent opioid/benzodiazepine were generally consistent with those from our primary model focused on any concurrent prescribing.
These results confirm several findings from two previously published population-based studies of concurrent opioid/benzodiazepine prescribing two conducted among older women with breast cancer [6, 16], and one among older adults with breast, lung, colorectal, or head/neck cancer [17]. Specifically, we found race/ethnicity, receipt of chemotherapy, a prior diagnosis of anxiety, and use of either opioids or benzodiazepines at diagnosis to be associated with concurrent opioid/benzodiazepine prescribing after diagnosis.
Our analysis also provides several new insights. First, previous studies of concurrent opioid/benzodiazepine prescribing in cancer have not accounted for substance use disorder history—a known risk factor for opioid misuse and overdose [18, 19]. In the present analysis, patients with a prior substance use disorder diagnosis were more likely to experience concurrent prescribing of opioids/benzodiazepines, compounding their risk for adverse opioid-related outcomes.
There are several possible explanations for this finding. First, patients with substance use disorders often have co-occurring anxiety associated with pain [20]; when necessary, providers may opt to prescribe both opioids and benzodiazepines for co-occurring pain and anxiety with increased monitoring and/or additional risk mitigation efforts (e.g., prescribing naloxone). A second potential explanation is that providers may lack knowledge about a patient’s substance use history. While the American Society of Clinical Oncology recommends that oncologists systematically assess patients’ substance use disorder history to inform risk-stratified monitoring [21], based on prior research, providers frequently do not ask their patients about their substance use history [22]. A third potential explanation is that providers may know of a patient’s substance use history, but lack information about their concurrent medication use, particularly in cases where opioids and benzodiazepines are prescribed by different providers and/or in different health systems. Our findings highlight potential opportunities to strengthen routine assessment of substance use history and routine use of state prescription drug monitoring program (PDMP) databases in oncology. Both are included in oncology guidelines [21] as strategies to inform risk-based prescribing and monitoring.
Our study also provides new information about the association of cancer type with concurrent opioid/benzodiazepine prescribing. In our cohort, patients with cervical, head/neck, lung, and esophageal cancers had an increased likelihood of concurrent opioid/benzodiazepine prescribing. Given behavioral factors (e.g., smoking, alcohol use) that may contribute to the development of cervical, head/neck, lung, and esophageal cancers, patients diagnosed with these cancers may experience a greater burden of substance use disorder and related mental health conditions; they may also be at increased risk for harms associated with concurrent prescribing [21].
Further, treatment for each of these cancers is often multimodal and may include surgery, radiation therapy, and chemotherapy, which can result in additional physical and psychological symptom burden from treatment-related adverse effects and, correspondingly, increased need for symptom-directed medications such as opioids and benzodiazepines. In the case of esophageal and some head/neck cancers, high morbidity and shorter median survival may also contribute to higher rates of co-prescribing. It may not be feasible or clinically beneficial to avoid overlap in opioid/benzodiazepine prescriptions for patients who require both medications to better tolerate anti-cancer treatment and/or to improve quality of life.
When both medications are required at the same time, oncology practice guidelines recommend prescription of naloxone. Research on uptake of naloxone prescribing for patients with cancer who are at increased risk of overdose is lacking; however, available data indicate that uptake is extremely limited among both oncology and palliative care providers [23]. Providing naloxone in the context of concurrent opioids/benzodiazepine prescribing seems particularly important for patients who have additional risk factors, including a history of substance use disorder. Assessing and improving uptake of targeted naloxone prescribing in cancer care represent an important area for future research.
In the context of new evidence suggesting that gabapentinoids exacerbate the risk of opioid overdose [4, 5], our population-based study also provides new information about the prevalence of and factors associated with the practice of prescribing gabapentinoids concurrently with opioids among patients diagnosed with cancer. Based on our results, it is relatively uncommon for patients with cancer to be prescribed opioids and gabapentinoids at the same time, potentially because gabapentinoids are increasingly prescribed as an alternative to opioid therapy, particularly for neuropathic pain [24].
We are aware of only one previously published study that specifically examined concurrent prescribing of opioids with gabapentinoids in the cancer care context. That study was conducted within the supportive care clinic of a comprehensive cancer center, and the authors observed a much higher prevalence of concurrent opioid/gabapentinoid prescribing than we did (49% vs. 5%) [25]. Our differential findings are likely partly explained by our different study denominators. The previous study was restricted to patients prescribed opioids, whereas our denominator included all patients who met diagnostic and continuous insurance enrollment eligibility criteria. In addition, our focus was on non-metastatic cancer, whereas the cohort for the previous study was primarily composed of patients with metastatic cancer. Patients with metastatic cancer often have more complex pain management needs and, as a result, may require medication for both nociceptive pain (opioids) and neuropathic pain (gabapentinoids) simultaneously.
Across outcomes, we observed differences in concurrent prescribing related to demographics—specifically, age and correspondingly, insurance provider—likely reflecting increased caution in prescribing to older adults, consistent with guideline recommendations. Further, we observed racial differences in concurrent prescribing. Specifically, in our study, Black and Hispanic patients were less likely to experience concurrent prescribing. While avoiding concurrent prescribing is consistent with available evidence and guideline recommendations, it is unlikely that the racial differences we observed are reflective of higher quality care for Black and Hispanic patients. Rather, these differences may indicate under-treatment of symptoms among Black and Hispanic patients due to implicit bias and systemic racism in healthcare [26,27,28]. Future studies should investigate potentially modifiable factors (e.g., implicit bias, cultural competence, communication) contributing to racial disparities in pharmacological symptom management in cancer in an effort to improve equity in symptom and quality of life outcomes.
Our study has several limitations. We used claims-based measures of overlapping prescriptions, which do not necessarily indicate concurrent use (i.e., taking both prescribed drugs at the same time). Relatedly, opioids and benzodiazepines are sometimes prescribed as-needed, meaning patients may use them beyond the claims’ minimum days’ supply; the implication is that we may have underestimated overlapping days for some patients. In addition, estimating the prevalence of concurrent prescribing in the year following cancer diagnosis required that we exclude patients who died during that period, including those who may have died from overdose. It is also important to acknowledge that we did not assess overdose or other potential harms (e.g., falls) resulting from co-prescribing. Future research should examine the risk of overdose and other potential harms among patients with cancer.
To summarize, in our retrospective cohort study of North Carolina adults diagnosed with non-metastatic cancer, we found that 15% of patients were prescribed opioids alongside benzodiazepines or gabapentinoids during the year following diagnosis. The associations we observed between patient characteristics and risk of concurrent prescribing highlight several potential opportunities for future research, including implementation of systematic and patient-centered assessments of prior substance use disorder, and assessing the use of state prescription drug monitoring program databases and uptake of targeted naloxone prescribing in oncology. In addition, in the context of existing evidence on racial disparities in cancer-related symptom burden, observed racial differences in concurrent prescribing may indicate inadequate symptom management or care access among Black and Hispanic patients. Understanding and addressing multilevel barriers to equitable symptom management represent an important area for future research.
Availability of data
Not applicable.
Code availability
Not applicable.
References
Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS (2015) Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ 350:h2698. https://doi.org/10.1136/bmj.h2698
Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S (2017) Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 356:j760. https://doi.org/10.1136/bmj.j760
Hernandez I, He M, Brooks MM, Zhang Y (2018) Exposure-response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in Medicare part d beneficiaries. JAMA Netw Open 1(2):e180919. https://doi.org/10.1001/jamanetworkopen.2018.0919
Gomes T et al (2018) Pregabalin and the risk for opioid-related death: a nested case-control study. Ann Intern Med 169(10):732–734. https://doi.org/10.7326/M18-1136
Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W (2017) Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med 14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396
Check DK, Winn AN, Fergestrom N, Reeder-Hayes KE, Neuner JM, Roberts AW (2020) Concurrent Opioid and Benzodiazepine Prescriptions Among Older Women Diagnosed With Breast Cancer. J Natl Cancer Inst 112(7):765–768. https://doi.org/10.1093/jnci/djz201
Moyo P et al (2019) Opioid prescribing safety measures in Medicaid enrollees with and without cancer. Am J Prev Med 57(4):540–544. https://doi.org/10.1016/j.amepre.2019.05.019
"National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN guidelines): adult cancer pain. Version 2.2021. NCCN. https://www.nccn.org/. Accessed September 7 2021."
"National Comprehensive Cancer Network (NCCN). https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Antiemesis (Version 1.2021). Accessed September 7, 2021. ."
Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K (2001) Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manag 21(2):95–102. https://doi.org/10.1016/s0885-3924(00)00258-x
Janelsins MC, Kesler SR, Ahles TA, Morrow GR (2014) Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 26(1):102–113. https://doi.org/10.3109/09540261.2013.864260
Meyer AM et al (2014) Big data for population-based cancer research: the integrated cancer information and surveillance system. N C Med J 75(4):265–9. https://doi.org/10.18043/ncm.75.4.265
Klabunde CN, Potosky AL, Legler JM, Warren JL (2000) Development of a comorbidity index using physician claims data. J Clin Epidemiol 53(12):1258–1267. https://doi.org/10.1016/s0895-4356(00)00256-0
"Centers for Medicare and Medicaid services chronic conditions warehouse." https://www2.ccwdata.org/web/guest/condition-categories (accessed November 19, 2020).
G. Zou, "A modified poisson regression approach to prospective studies with binary data," Am J Epidemiol, vol. 159, no. 7, pp. 702–6, Apr 1 2004. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/15033648.
Sakamoto MR et al (2021) “New persistent opioid and benzodiazepine use after curative-intent treatment in patients with breast cancer,” (in eng). J Natl Compr Canc Netw 19(1):29–38. https://doi.org/10.6004/jnccn.2020.7612
Salz T et al (2022) Safety of opioid prescribing among older cancer survivors. Cancer 128(3):570–578. https://doi.org/10.1002/cncr.33963
Michna E et al (2004) Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manag 28(3):250–258. https://doi.org/10.1016/j.jpainsymman.2004.04.007
Matzger H, Weisner C (2007) Nonmedical use of prescription drugs among a longitudinal sample of dependent and problem drinkers. Drug Alcohol Depend 86(2–3):222–229. https://doi.org/10.1016/j.drugalcdep.2006.06.010
Weisner CM et al (2009) Trends in prescribed opioid therapy for non-cancer pain for individuals with prior substance use disorders. Pain 145(3):287–293. https://doi.org/10.1016/j.pain.2009.05.006
Paice JA et al (2016) Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34(27):3325–3345. https://doi.org/10.1200/JCO.2016.68.5206
Edlund MJ, Unutzer J, Wells KB (2004) Clinician screening and treatment of alcohol, drug, and mental problems in primary care: results from healthcare for communities. Med Care 42(12):1158–1166. https://doi.org/10.1097/00005650-200412000-00002
Roberts AW (2021) Naloxone prescribing among frequent opioid prescribers in medicare part d from 2013 to 2017: a retrospective study. J Gen Intern Med 36(2):543–545. https://doi.org/10.1007/s11606-020-05872-5
Fauer AJ, Davis MA, Choi SW, Wallner LP, Friese CR (2020) Use of gabapentinoid medications among US adults with cancer, 2005–2015. Support Care Cancer 28(1):5–8. https://doi.org/10.1007/s00520-019-05100-9
Madden K et al (2020) Frequency of concomitant use of gabapentinoids and opioids among patients with cancer-related pain at an outpatient palliative care clinic. J Palliat Med. https://doi.org/10.1089/jpm.2019.0614.
Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ (1997) Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 127(9):813–816. https://doi.org/10.7326/0003-4819-127-9-199711010-00006
John DA, Kawachi I, Lathan CS, Ayanian JZ (2014) Disparities in perceived unmet need for supportive services among patients with lung cancer in the Cancer Care Outcomes Research and Surveillance Consortium. Cancer 120(20):3178–3191. https://doi.org/10.1002/cncr.28801
McNeill JA, Reynolds J, Ney ML (2007) Unequal quality of cancer pain management: disparity in perceived control and proposed solutions. Oncol Nurs Forum 34(6):1121–1128. https://doi.org/10.1188/07.ONF.1121-1128
Acknowledgements
The work on this study was supported by the Cancer Information and Population Health Resource, UNC Lineberger Comprehensive Cancer Center, with funding provided by the University Cancer Research Fund via the state of North Carolina.
Funding
This project is included as part of the Duke University School of Medicine Opioid Collaboratory portfolio, grant funded by the Duke Endowment and administered through the Duke Department of Population Health Sciences. The collaboratory’s mission is to save lives and reduce the harmful impact of opioids in North Carolina through the development, implementation, and evaluation of system-level interventions.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Development of the statistical analysis plan was performed by Devon Check, Christopher Baggett, and Michaela Dinan. Data analysis was performed by KyungSu Kim. All authors contributed to data interpretation. The first draft of the manuscript was written by Devon Check, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was granted exemption from the Institutional Review Boards at Duke University and the University of North Carolina at Chapel Hill.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Check, D.K., Baggett, C.D., Kim, K. et al. Concurrent prescribing of opioids with other sedating medications after cancer diagnosis: a population-level analysis. Support Care Cancer 30, 9781–9791 (2022). https://doi.org/10.1007/s00520-022-07439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07439-y